Abstract
Chronic insomnia and memory impairment are both common complaints among older adults. Even so, only a few studies to date have examined the effects of chronic insomnia on memory processes among older people, and the results of these studies are contradictory. Therefore, in the current study we examined whether late-life insomnia is associated with the memory status of older adults. The study population comprised two groups: 50 older adult subjects without sleep disorders, and 23 older adult insomniacs. Memory processing for each of the two groups was evaluated using the Rey Auditory Verbal Learning Test (AVLT). The results demonstrate that chronic insomnia in older adults is associated with impairment in memory. Specifically, we found that older people suffering from late-life insomnia exhibit significantly reduced performance in learning rate and in temporal order judgment as well as significantly reduced resistance to proactive interference. The present findings suggest that late-life insomnia may be one of the factors contributing to the decline in memory processing seen among older people.
Keywords: Ageing, Cognitive impairment, Insomnia, Memory
Introduction
It is generally accepted that sleep disturbances constitute one of the most ubiquitous health problems among older adults. Whereas only 9% of individuals between the ages of 20 and 30 complain of insomnia, some 35–50% of those over the age of 65 voice this complaint. Compared to young adults, healthy older people require more time to fall asleep, awaken more frequently during the night, have difficulty returning to sleep after mid-sleep awakenings, and do not feel rested in the morning (Ancoli-Israel 2004; Ancoli-Israel and Cooke 2005; Foley et al. 1999; Ohayon 2002). These alterations in sleep structure often lead to daytime sleepiness, fatigue, and frequent daytime napping (Carskadon et al. 1982). Late-life insomnia among older adults can have a significant negative impact on quality of life, may be a risk factor for poor health, depression, and mortality, and is associated with increased cardiovascular risk (Kripke et al. 2002; Kryger et al. 2004).
In addition to primary insomnia, complaints of insomnia in the older adult population can be secondary to five other factors. These include medical and psychiatric illnesses (Benca et al. 1992; Foley et al. 2004; Gillin et al. 1981), medication use (Foley et al. 2004), changes in circadian rhythm (Czeisler et al. 1992; Gillin et al. 1981), specific sleep disorders [i.e., sleep-disordered breathing (sleep apnea) (Cohen-Zion et al. 2004), restless legs syndrome (RLS) and periodic limb movements during sleep (PLMS) (Hornyak and Trenkwalder 2004; Rothdach et al. 2000)], and psychosocial factors (Ancoli-Israel 2000; Naylor et al. 2000; Tanaka and Sirakawa 2004).
It is well documented that along with the changes in sleep structure accompanying the ageing process, ageing is also associated with deteriorating performance on various cognitive tasks: speed of processing information, perceptual speed, executive functioning, concentration and attention, inhibition functioning, and memory. Nearly half of persons aged 60 years and older dwelling in the community express concern about declining mental abilities (Jolles et al. 1995; Park 2000).
Empirical evidence indicates that one of the most prominent impairments in the cognitive performance of older adult persons is a decline in memory processes (Light 1991; Verhaeghen and Salthouse 1997; Vakil et al. 1997, Lindenberger and Baltes 1997; Nilsson et al. 1997; Craik and Anderson 1999; Park et al. 2002). Studies evaluating the effects of ageing on memory abilities have shown that ageing is associated with deteriorating performance in declarative memory, particular in episodic memory (Light 1991). Age-related episodic memory changes have been reported on a variety of measures, including learning rate, free recall, cued recall, and recognition of verbal and nonverbal material (Burke-Light 1981; Light 1991). Naveh-Benjamin et al. (2000) have reported that temporal order tests also present a special challenge to older adults.
In conjunction with studies demonstrating the effects of ageing on memory abilities, it is generally accepted that there are individual differences in memory impairment among older adults (Schaie 1990). These individual differences have been explained by various psychosocial variables, such as education (Anstey et al. 1993; Avolio and Waldman 1994), good health (Perlmutter and Nyquist 1990), visual and auditory abilities (Baltes and Lindenberger 1997; Salthouse et al. 1996, Asplund 2004), and depression (Fuhrer et al. 1992; Devanand et al. 1996; Kliegel and Zimprich 2005). Taken together, these findings argue for the great potential benefit derived from identifying risk factors contributing to cognitive decline, especially those that are potentially malleable. A better understanding of the risk factors contributing to cognitive decline among older adults can facilitate more effective intervention strategies.
A recent and growing body of evidence has indicated that sleep is associated with cognition abilities (Empson and Clarke 1970; Fishbein et al. 1974; Salzarulo and Cipolli 1979; Smith 1985; Tilley and Warren 1984; Szelenberger and Niemcewicz 2000; Wetzel et al. 2003). Moreover, studies of young adults and animals have demonstrated a specific facilitating effect of sleep on memory (Roehrs and Roth 2000; Steenary et al. 2003: Hornung et al. 2005; Walker and Stickgold 2006). Much of the early work investigating sleep and memory in humans focused on declarative memory tasks. A substantial body of evidence indicates that both Slow Wave Sleep and REM sleep contribute to the consolidation of declarative memories embedded in networks of previously existing associative memories. For example, De Koninck et al. (1989) demonstrated significant increases in post-training REM sleep after intensive foreign language learning, where the degree of successful learning correlated with the percentage increase of REM sleep. Such findings suggest that REM sleep plays an active role in memory consolidation and that the post-training increases in REM sleep reflect a homeostatic response to increased demands for REM-dependent consolidation. In addition, Gais et al. (2000) demonstrated that both early (mainly Slow Wave Sleep) and late (mainly REM) sleep are needed to achieve optimal performance. Likewise, the reliance of procedural memory on sleep is a robust and consistent finding across a wide variety of functional domains, including visual, auditory, and motoric systems (for a review see Walker and Stickgold 2004). Taken as a whole, these studies suggest that sleep is crucial for the acquisition of new memories and that the role of sleep in the consolidation of memory traces is obligatory rather than secondary (Maquet 2001).
In addition, studies of young adults found that compared with good sleepers, young insomniacs reported decreased daytime functioning, low performance, attention difficulties, greater difficulty with concentration and memory, and diminished ability in coping with minor problems and accomplishing important tasks during the day (Golan et al. 2004; The Gallup Organization 1991; Salzarulo 1995; Sadeh et al. 2002).
Although the relationship between sleep and memory has been investigated extensively in young adults over the past two decades, the interaction between sleep disturbances and cognitive functioning among older adults remains unclear, and the results of studies are controversial. Mazzoni et al. (1999) demonstrated a correlation between sleep structure and reduction in memory processes among older people. Jelicic et al. (2002) found a correlation in older adults between sleep complaints and decreased cognitive abilities. In a longitudinal study of seven thousand older adult participants, Cricco et al. (2001) showed that chronic insomnia independently predicts incident cognitive decline in older adults. The results of the Cohen-Zion et al. (2004) study indicate that among older patients with mild to moderate Sleep Breathing Disorders, reductions in neurocognitive performance are associated with increasingly severe Sleep Breathing Disorders over time. In contrast, results of other studies showed no significant association between sleep and performance on cognitive tests among older adults (Dealberto et al. 1996; Hayward et al. 1992).
Despite the importance of the finding that ageing is associated with deteriorating memory performance, particularly episodic memory, and despite the many implications of this finding for daily life, little is known about the mechanism underlying these age-related changes. The present study aims to assess whether late-life insomnia, independent of its underlying etiology, is associated with the memory status of older adults, when other factors known to influence cognition are controlled (e.g. education, chronic medical condition, vision ability, hearing ability, major depression and dementia). We hypothesized that late-life insomnia, which may be a chronic condition existing over the course of many years, affects episodic memory capacity. As a result, older adult non-insomniac subjects would demonstrate better performance on episodic memory tests than older adult insomniac subjects. Additionally, we evaluated which stages of episodic memory are particularly affected by disturbed sleep among older adults. Based on adult sleep insomnia studies and the existing knowledge on sleep we further hypothesized that only highly demanding tasks requiring a high level of attention resources would show association with late-life insomnia as opposed to the more simple memory tasks.
Method
Participants
The study population comprised 73 older adult participants: 45 males and 28 females, mean age 70.4 years, SD=5.8. All of the participants were living independently in the community and were in good clinical condition. Likewise, all participants had been living in Israel for at least 10 years, and all spoke Hebrew fluently.
These 73 participants were recruited through advertisements and talks given at community senior centers. An initial phone interview was used to eliminate volunteers who exhibited visual or hearing impairments. Applicants were then asked to complete several questionnaires. Based on a standard clinical history questionnaire, subjects were eliminated if they had a significant medical, neurological, or psychiatric illness that might bring about cognitive change. More specifically, subjects were excluded from the study for any of the following reasons: (a) They had significant major medical diseases including cancer; diabetes; liver, kidney, heart, or lung disease; alcoholism or other drug abuse. (b) They were taking any medication known to affect central nervous system functioning. (c) They had historical evidence suggesting significant psychiatric disease such as depression or psychosis or neurologic disease. In order to rule out sleep apnea syndrome or periodic leg movement (PLM), participants completed the Mini Sleep Questionnaire (MSQ; Zomer et al. 1985) and the Technion sleep questionnaire (Haimov et al. 2006). Applicants were also eliminated if they complained of sleep apnea syndrome or PLM. In order to eliminate subjects exhibiting dementia or depression, the older adult participants completed the mini-mental state examination (MMSE; Folstein et al. 1975) and the geriatric depression scale—short form (GDS; Zalsman et al. 1998). None of the older adult subjects who participated in the study met any criteria for dementia (none had a mini-mental score less that 26 out of 30) (mean MMSE=28.5, SD=1.1) or depression (mean GDS=2.4, SD=1.5).
For the purpose of evaluating participant sleep, all subjects were asked to complete two questionnaires that recorded the subject’s subjective evaluation of his/her sleep patterns: (1) a qualitative questionnaire—the Mini Sleep Questionnaire (MSQ; Zomer et al. 1985), and (2) a quantitative questionnaire—the Technion Sleep Questionnaire (Haimov et al. 2006). According to their responses to the sleep questionnaires, the 73 participants were divided into two groups: (1) older adult non-insomniac subjects who did not complain of sleep disorders according to their results on the Technion sleep questionnaire and who scored less than four on the insomnia sub-scale of the Mini Sleep Questionnaire (with a grade of four representing the median on the questionnaire scale), and (2) older adult insomniac subjects who met DSM- IV criteria for chronic insomnia according to their results on both Sleep Questionnaires. That is, on the Technion sleep questionnaire they reported that they had difficulties in initiating and maintaining sleep at least three nights per week and that their insomnia had lasted for a minimum of 6 months. Volunteers also had to report that their insomnia was not caused by chronic pain or by any known medical disease and that they did not use either alcohol or any sedative medication. Likewise, participants had to score 4 and above on the insomnia sub-scale in the Mini Sleep Questionnaire. After the assessments were completed, 23 older adult participants were assigned to the insomniac group (32% of the subjects, similar to the percentage of insomniacs in the older adult population), and 50 older adult participants were assigned to the non-insomniac group (68% of the study subjects). The older adult insomniac group consisted of 15 males and 8 females between the ages of 65 and 83 (mean age=70.8, SD=5.8; mean education=11.6, SD=2.9; mean MMSE=28.1, SD=1.3; mean GDS=2.9, SD=1.4), and the older adult non-insomniac group consisted of 30 males and 20 females between the ages of 65 and 86 (mean age=70.2, SD=5.9; mean education=12.4, SD=3.2; mean MMSE=28.6, SD=1.0; mean GDS=2.2, SD=1.5).
Procedure
The Rey Auditory Verbal Learning Test (AVLT) (Lezak 1983; Vakil and Blachstein 1993) seems to be an ideal tool to aid in clarifying the effect of late-life insomnia on various episodic memory processes. The Rey AVLT test is widely used for clinical and research purposes (Vakil and Blachstein 1997). One of its major advantages is that it simultaneously provides several measures of learning and memory. These measures include immediate and delay recall, learning rate, recognition, proactive interference (the ability to learn a new list of words despite the interference with the earlier list of word), and retroactive interference (the ability to learn a list of words despite the interference with a new list of word), and temporal order.
The Hebrew version of the Rey AVLT (Vakil and Blachstein 1993) was used. Administration was standard, as described by Lezak (1983). Each participant was tested individually, at home, during the afternoon hours (16:00–19:00). This enabled us to monitor cognitive performance under natural circumstances, with minimal distortions, while the effect of the circadian clock on cognitive performance was controlled.
The test consisted of 15 common nouns, which were first read to the subject at the rate of one word per second in five consecutive trials (Trials 1–5); each reading was followed by a free recall task. In Trial 6, an interference list of 15 new common nouns was presented, followed by free recall of these new nouns. In Trial 7, participants were asked to again recall the first list without an additional reading. Twenty minutes later, and again without an additional reading, participants were once again asked to recall the first list (Trial 8). Next, in Trial 9, they were given a list of 50 words (15 from the first list, 15 from the second list, and 20 new common nouns) and were asked to identify the 15 words from the first list. An extra trial (Trial 10) was added to the standard administration to measure ability to remember temporal order (Vakil et al. 1991). In this extra trial, which followed the recognition task, participants were presented with a written list of the 15 words in List A, but with the presentation order differing from the one used in the oral trials. Participants were asked to rewrite the word list to match the order of words in the original list as they heard them.
Results
General data. Preliminary analyses revealed no significant differences between the older adult insomniacs and the older adult non-insomniacs in participant age [t(71)=1.68, P=0.09]; gender [χ2(1)=0.18, P=0.67]; education [t(71)=1.43, P=0.32]; mean MMSE [t(71)=1.88, P=0.07]; and mean GDS [t(71)=1.85, P=0.07]. A significant difference was, however, found between the older adult insomniacs and the older adult non-insomniacs on the insomnia scale [t(71)=11.3, P=0.001].
Rey AVLT task analysis. To test whether late-life insomnia may account for the disproportionate decline in memory among older people, performance of the two groups (i.e. older adult insomniacs and older adult non-insomniacs) on the Rey AVLT was compared. Ten different scores were derived from the Rey AVLT for further analyses. These scores are frequently used in the literature to reflect different aspects of episodic memory (Vakil and Blashtein 1993, 1994). The results are presented in five sections, with each section representing a different category of memory. The tests were administered in the following order: learning, interference, delayed recall, recognition, and temporal order judgment. Means and standard deviation for the raw scores on the nine test trials and for the additional three scores reflecting Trial 10 for each group are presented in Table 1.
Table 1.
Means and standard deviation of the raw memory scores for each experimental group
| Trial | Older people without sleep disorders (N=50) | Older insomniacs (N=23) |
|---|---|---|
| T1 (List A) | 4.72 (1.88) | 4.70 (1.26) |
| T2 | 7.12 (2.26) | 6.61 (1.83) |
| T3 | 7.89 (2.62) | 7.22 (2.61) |
| T4 | 9.28 (2.89) | 8.30 (2.60) |
| T5 | 10.24 (2.72) | 9.22 (2.94) |
| T6 (List B) | 4.64 (1.74) | 3.74 (1.57) |
| T7 (List A) | 7.86 (3.43) | 7.30 (2.90) |
| T8 (DR) | 7.67 (3.66) | 7.39 (3.06) |
| T9 (RC) | 11.76 (2.63) | 11.91 (2.63) |
| T10 (Hits) | 2.41 (1.84) | 2.35 (1.72) |
| T10 (CO) | 0.55 (0.20) | 0.44 (0.27) |
| T10 ( AD) | 10.97 (0.87) | 10.4 (1.20) |
DR delayed recall; RC recognition; CO correlation score; AD absolute deviation
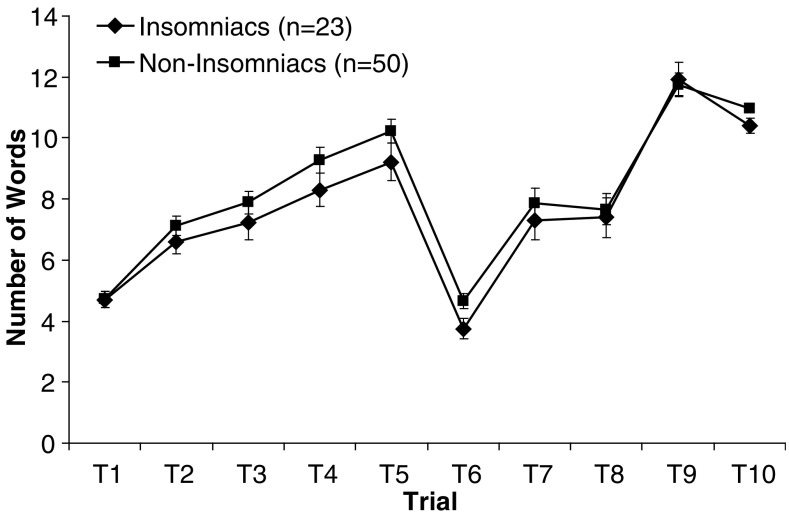
In addition, graphical display of the Rey AVLT raw scores for each experimental group is presented (Fig. 1).
Fig. 1.
Means of the raw memory scores for each experimental group. Error bars indicate SEM
Learning
In this section, the different learning measures extracted from the Rey AVLT are analyzed. These measures involve recall scores on the first five learning trials expressed as: learning curve (Trial 1 score to Trial 5 score), total learning (sum of the scores of Trials 1–5) representing the capacity to recall and accumulate words across learning trials, and immediate memory (Trial 1).
Immediate memory (Trial 1)
For the immediate memory measure, a two-tailed t test for independent samples revealed no significant difference in immediate memory score between older adult insomniacs and older adult non-insomniacs [t(71)<1].
Learning curve (Trials 1–5)
Learning curve represents the learning ability of the participants (Mitrushina et al. 1991). A mixed design ANOVA was conducted with sleep group (older adult insomniacs and non-insomniacs) as a between-subjects factor and learning trials (Trials 1–5) as within-subjects factor. A significant effect was found for learning [F(1, 66)=7.89, P=0.004], indicating that there is an overall increase in the number of words recalled from trial to trial. Group effect did not reach significance [F(1, 66)<1]. The learning × sleep group interaction was significant [F(1, 66)=3.01, P=0.043], suggesting that the learning rate of older adult non-insomniacs is steeper than that of older adult insomniacs. However, the clinical relevance of this result is restricted since the older adult insomniacs recall one word less than the older adult non-insomniac subjects on the fifth trail.
Total learning (Trials 1–5)
The total learning measure consists of the sum of words recalled in all five learning trials, and it reflects the capacity to recall and accumulate words throughout learning trials (Moses 1989). A two-tailed t test for independent samples revealed that although older adult non-insomniacs tended to recall more words than insomniacs, this difference was only marginally significant [t(71)=1.31, P=0.09].
Interference
In this section, the different interference measures extracted from the Rey AVLT are analyzed. These measures include the number of words recalled from the interference list (i.e., Trial 6) and from the first list following the interference (i.e., Trial 7). In addition, the recall of this list as compared to the recall of the first list, enable the derivation of proactive and retroactive interference measures.
Proactive interference (List B, interference list; Trial 6)
A mixed-design ANOVA was conducted with sleep group (older adult insomniacs and non-insomniacs) as a between-subjects factor and proactive interference (Trial 1 score minus Trial 6 score) as a within-subjects factor. No significant effect was found for proactive interference or for sleep group {[F(1, 66)<1; F(1, 66)=1.07, P=0.15], respectively}. However, the proactive interference × sleep group interaction was significant, [F(1, 66)= 2.89, P=0.047], suggesting that resistance to proactive interference among older adult non-insomniacs is stronger than among older adult insomniacs. That is, despite the interference of the first list, older adult non-insomniacs recalled more words from the new list than did older adult insomniacs.
Retroactive interference (List A, following the interference list; Trial 7)
A mixed-design ANOVA was conducted with sleep group (older adult insomniacs and non-insomniacs) as a between-subjects factor and retroactive interference as within-subjects factor (Trial 5 score minus Trial 7 score). The main retroactive effect did not reach significance [F(1, 66)=2.29, P=0.07]. Group effect also did not reach significance [F(1, 66)<1], nor did the retroactive interference × sleep group interaction [F(1, 66)<1].
Delayed recall (Trial 8)
Delayed recall performance (Trial 8) was compared with performance on Trial 5. A mixed-design ANOVA was conducted to analyze the effect of sleep group (older adult insomniacs and older adult non-insomniacs) and delayed recall (Trial 5 score minus Trial 8 score). The main effect of delayed recall did not reach significance [F(1, 66)=2.32, P=0.07]. Group effect also did not reach significance [F(1, 66)<1]. The delayed recall × sleep group interaction did not reach significance [F(1, 66)=1.77, P=0.09].
Recognition (Trial 9)
Two-tailed t test for independent samples revealed no significant difference between the groups on the number of recognition words [t(71)<1]. However, some investigators have argued that success on a memory test requires effective inhibition of related, but non-target, memories (Zacks et al. 2000). Indeed, failure to achieve such inhibition may result in production of false memories, a problem prevalent among older adults (Norman and Schacter 1997; Tun et al. 1998). In order to correct the recognition score for a positive criterion (the tendency to give a positive answer), we used an additional measure, comprised of the proportion of words correctly identified from list A (hit rate) and the number of false positive responses. The t test showed no significant differences on this score between the groups [t(71)=1.16, P=0.12]. In addition, we compared recognition score (Trial 9) to delayed recall score (Trial 8). A mixed-design ANOVA was conducted with sleep group (older adult insomniacs and non-insomniacs) as a between-subjects factor and recognition (Trial 9 score minus Trial 8 score) as within-subjects factor. The main effect of recognition reached significance [F(1, 66)=5.09, P=0.02]. Group effect did not reach significance [F(1, 66)<1]. The recognition × sleep group interaction did not reach significance [F(1, 66)<1], suggesting that no significant difference in recognition was found between non-insomniacs and insomniacs. That is, older adult insomniacs and non-insomniacs exhibit a similar increase in the number of words recognized compared to words recalled.
Temporal order (Trial 10)
A recently introduced supplementary measure to the Rey AVLT for assessing temporal order (Vakil et al. 1997) proposes three alternative scores for measuring temporal order. These measures are: (1) Hits: the number of words correctly placed at their original serial position; (2) Correlation: the Pearson correlation, calculated for each participant, between the listed order and the true order; (3) Absolute deviation: a score calculated by summing the absolute deviation of each word from its original position. The score for each deviation ranges from 0 to 14 (Vakil 1985). Table 1 presents the results for the two groups on these temporal order measures. The hits score showed no significant difference between the groups on the number of words that were written in the correct place [t(70)<1]. The correlation score was found to approach significance [t(44)=1.44, P=0.08], while the absolute deviation score reach significance [t(43)=1.79, P=0.04], suggesting that the temporal order judgment of older non-insomniacs is more accurate than that of older insomniacs.
Taken together, the two groups (i.e. older adult subjects without insomnia and older adult insomniacs) were found to differ significantly from one another on their scores for learning rate, proactive interference and temporal order judgment. Furthermore, although significance was found on these measures only, a tendency of older adult non-insomniacs to remember more words than older adult insomniacs can be found for all memory categories. A brief summary of the test results for the different memory measures is presented in Table 2.
Table 2.
Comparison between the two experimental groups in performance across the different episodic memory measures
| Measure | Test | P |
|---|---|---|
| Immediate memory | t(71)<1 | NS |
| Learning curve | F(1,66)=3.01 | 0.043 |
| Total learning | t(71)=1.31 | NS |
| Proactive interference | F(1,66)=2.89 | 0.047 |
| Retroactive interference | F(1,66)<1 | NS |
| Delayed recall | F(1,66)=1.77 | NS |
| Recognition | t(71)<1 | NS |
| Temporal order | ||
| Hits score | t(70)<1 | NS |
| CO score | t(44)=1.44 | NS |
| AD score | t(43)=1.79 | 0.04 |
NS not significant; CO correlation score; AD absolute deviation
Discussion
The present study demonstrates that chronic insomnia in older adults is associated with their episodic memory status. The results reveal significant differences between older adult insomniac subjects and older adult non-insomniac subjects on three memory processes—learning rate, resistance to proactive interference, and temporal order judgment—all of which have many implications for daily life. The findings of the present study are consistent with results of other studies that show a particularly pronounced age-related decline in the ability to learn (Kessels et al. 2003), the ability to inhibit irrelevant information (Persad et al. 2002) and the ability to temporally code information (Naveh-Benjamin et al. 2000).
In order to determine whether the relation that we found between sleep quality and memory performance is a causal relation and not due to other factors, we sought to establish a dose–response analysis between sleep quality and memory performance for the 73 participants. Unfortunately, we found the sample size insufficient to establish dose–response between the different Rey AVLT measures and insomnia scale.
Some aspects of the memory tests did not reveal significant differences between insomniac and non-insomniac older adult subjects. For example, there was no significant effect of insomnia on the number of recalled words in resistant to retroactive interference task and in delayed recall task. A possible explanation for these results may lie in the finding that older adult insomniacs showed significantly lower resistance to proactive interference, and as a result were less successful in learning a second list of words. Therefore, information from the second list was less intrusive to the older adult insomniacs comparing to the non-insomniac subjects. As a consequence, there was no significant difference between the two groups when asked to recall the first list of words immediately after learning the second list (i.e. resistant to retroactive interference task) or when they had to recall the first list of words 20 min later (delayed recall task).
Likewise, on immediate memory and recognition tasks no significant differences were found between the groups. These results are consistent with those of Szelenberger and Niemcewisz (2000), which show a correlation between insomnia score and impaired learning but did not show a correlation between insomnia score and immediate memory. In their study, Szelenberger and Niemcewisz revealed that although insomniacs did not differ from non-insomniacs on immediate recall tasks, insomniacs required a greater number of repetitions to learn all the test items. A possible reason for our findings that insomnia does not affect immediate memory ability and recognition performance may lie in the relative simplicity of these tasks. Perhaps only highly demanding tasks requiring a high level of attention resources, such as learning, resistance to interference, and temporal order judgment, will more directly underscore the restorative value of sleep. Another explanation for the result that insomnia does not affect immediate memory ability and recognition performance may lie in the finding that those two tasks do not decline in older adults as drastically as do other cognitive aspects (Craik 1977; Howe 1988; Poon 1985; Verhaeghen et al. 1997).
The association between chronic insomnia and the episodic memory status of older adults may be the result of interrelationships between age-related changes in sleep and memory probably involving mechanisms of memory consolidation during sleep, i.e., chronic insomnia in the older adults can be a risk factor for memory decline. Changes in sleep characteristics in late-life insomnia may lead to impaired memory consolidation during sleep, thereby affecting daytime memory performance in older adults with insomnia. Many sleep parameters of relevance for sleep-related memory processing in young adults decline in late-life insomnia, such as REM sleep and SWS percentages (Vitiello et al. 2004). These age-related changes in sleep may affect sleep-related memory processing in older adults suffering from insomnia, leading to impaired daytime memory performance in these patients.
Another potential explanation for the decline in memory functioning with late-life insomnia is that disturbed sleep may result in fatigue and decreased alertness during the day, which in turn may lead to disturbed memory functioning. This decreased alertness may reduce performance in attention-demanding processes. An additional consequence of the decreased alertness during the day is that it can lead to reduced exposure to cognitively challenging situations which help maintain cognitive abilities (Ball et al. 2002). For instance, fatigue can result in decrease interest in reading, hobbies, social involvement, physical activity, and other engagement with one’s environment (Bassuk et al. 1999; Simonsick et al. 1999).
The fact that risk factors contributing to cognitive decline (i.e., education, physical health status, depression and auditory and visual ability) were controlled in the present study supports the conclusion that sleep may be one of the factors contributing to the decline of episodic memory functioning in older adults. Since our findings suggest that poor-quality sleep may contribute to the episodic memory status of older adults, and as a result make the insomniac elderly population more susceptible to cognitive deficiency, it is important that insomnia symptoms be taken seriously by practitioners and treated appropriately. The findings of this study offer hope that treatment of insomnia in older adults could have beneficial effects in improving cognitive functioning in these patients.
Thus, attention to and effective treatment of chronic insomnia in older persons may not only improve the quality of their nighttime sleep, but conceivably may also help maintain their cognitive function, thus improving their overall quality of life. Both the safety and the effectiveness of sleeping pills for treatment of insomnia in chronic older adult insomniacs are questionable (Endeshaw 2001; Morin et al. 1991; Ray et al. 2000). Daytime carryover effects observed with longer-acting sleep medications (Johnson and Chernik 1982) are likely to produce additional, potentially serious, decrements in daytime function among chronic older adult insomniacs. Thus, it is recommended that emphasis be placed on non-pharmacological interventions to improve sleep hygiene among chronic older adult insomniacs (Morin et al. 1991, 1999).
Acknowledgment
The author would like to thank Alina Akselrod for her invaluable assistance in data collection and data processing.
References
- Ancoli-Israel S. Insomnia in the elderly: a review for the primary care practitioner. Sleep. 2000;23(1):S23–S30. [PubMed] [Google Scholar]
- Ancoli-Israel S. A primary care guide to assessing 4 common sleep problems in geriatric patients. Geriatrics. 2004;59(1):37–41. [PubMed] [Google Scholar]
- Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in the elderly populations. J Am Geriatr Soc. 2005;53:S264–S271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- Anstey K, Stankov L, Lord S. Primary aging, secondary aging and intelligence. Psychol Aging. 1993;8:562–570. doi: 10.1037/0882-7974.8.4.562. [DOI] [PubMed] [Google Scholar]
- Asplund R. Nightmares in relation to sleep and sensory organ functions in the elderly. Sleep Hypn. 2004;6(1):1–7. [Google Scholar]
- Avolio BJ, Waldman DA. Variation in cognitive, perceptual and psychomotor abilities across the working life span: examining the effects of race, sex, experiences, education, and occupational type. Psychol Aging. 1994;9:430–442. doi: 10.1037/0882-7974.9.3.430. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch D, Helmers K, Jobe J, Leveck M, Marsiske M, Morris J, Rebok G, Smith D, Tennstedt S, Unverzagt F, Willis S. Effects of cognitive training interventions with older adults. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037/0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling older adult persons. Ann Intern Med. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Burke DM, Light LL. Memory and aging: the role of retrieval processes. Psychol Bull. 1981;90:513–546. doi: 10.1037/0033-2909.90.3.513. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Brown ED, Dement WC. Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging. 1982;3:321–327. doi: 10.1016/0197-4580(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Cohen-Zion M, Stepnowsky C, Johnson S, Marler M, Dimsdale JE, Ancoli-Israel S. Cognitive changes and sleep disordered breathing in elderly: differences in race. J Psychosom Res. 2004;56:549–553. doi: 10.1016/j.jpsychores.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Age differences in human memory. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. New York: Van Nostrand; 1977. [Google Scholar]
- Craik FIM, Anderson ND. Applying cognitive research to problem of aging. In: Gopher D, Koriat A, editors. Attention and performance XVII. Cognitive regulation of performance: interaction of theory and application. Cambridge: MIT; 1999. pp. 583–615. [Google Scholar]
- Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49(9):1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richadson GS, Brown EN, Sanchez R, Rios CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-Y. [DOI] [PubMed] [Google Scholar]
- Dealberto MJ, Pajot N, Courbon D, Alperovitch A. Breathing disorders during sleep and cognitive performance in an older community sample: the EVA study. J Am Geriatr Soc. 1996;44:1287–1294. doi: 10.1111/j.1532-5415.1996.tb01397.x. [DOI] [PubMed] [Google Scholar]
- De Koninck J, Lorrain D, Christ G, Proulx G, Coulombe D. Intensive language learning and increases in REM sleep: evidence of a performance factor. Int J Psychophysiol. 1989;8:43–47. doi: 10.1016/0167-8760(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern, Mayeux R. Depressed mood and the incidence of the Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- Empson J, Clarke P. Rapid eye movements and remembering. Nature. 1970;227:287–288. doi: 10.1038/227287a0. [DOI] [PubMed] [Google Scholar]
- Endeshaw Y. The role of benzodiazepines in the treatment of insomnia. J Am Geriatr Soc. 2001;49:824–826. doi: 10.1046/j.1532-5415.2001.49161.x. [DOI] [PubMed] [Google Scholar]
- Fishbein W, Katsaniotis C, Chattman D. Paradoxical sleep: Prolonged augmentation following learning. Brain Res. 1974;79:61–75. doi: 10.1016/0006-8993(74)90566-6. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: and epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–S372. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fuhrer R, Antonucci TC, Gagnon M, Dartigues JF, Barberger-Gateau P, Alperovitch A. Depressive symptomatology and cognitive functioning: an epidemiological survey in an elderly community sample in France. Psychol Med. 1992;22(1):159–172. doi: 10.1017/S0033291700032815. [DOI] [PubMed] [Google Scholar]
- Gais S, Pllhal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3(12):1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Duncan WC, Murphy DL, Post RM, Wehr TA. Age-related changes in sleep in depressed and normal subjects. Psychiatry Res. 1981;4:73–78. doi: 10.1016/0165-1781(81)90010-X. [DOI] [PubMed] [Google Scholar]
- Golan N, Shahar E, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep. 2004;27(2):261–6. doi: 10.1093/sleep/27.2.261. [DOI] [PubMed] [Google Scholar]
- Haimov I, Breznitz N, Shiloh S (2006) Sleep in healthy elderly: sources of discrepancy between self-report and recorded sleep. In: Kumar VM, Mallick HN (eds) Clinical and neurophysiological aspects of sleep. Medimond International Proceedings
- Hayward L, Mant A, Eyland A, Hewitt H, Purcell C, Turner J, Goode E, Le Count A, Pond D, Saunders N. Sleep disorders breathing and cognitive function in a retirement village population. Age Ageing. 1992;21(2):121–128. doi: 10.1093/ageing/21.2.121. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Danker-Hopfe H, Heuser I. Age-related changes in sleep and memory: commonalities and interrelationships. Exp Gerontol. 2005;40:279–285. doi: 10.1016/j.exger.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hornyak M., Trenkwalder C. Restless legs syndrome and periodic leg movement disorder in the elderly. J Psychosom Res. 2004;56:543–548. doi: 10.1016/S0022-3999(04)00020-0. [DOI] [PubMed] [Google Scholar]
- Howe ML. Measuring memory development in adulthood: a model-based approach to disentangling storage-retrieval contribution. In: Howe ML, Brainerd CJ, editors. Cognitive development in adulthood: progress in cognitive development research. Berlin Heidelberg New York: Springer; 1988. pp. 39–64. [Google Scholar]
- Jelicic M, Bosma H, Ponds R, Van-Boxtel M, Houx P, Jolles J. Subjective sleep problems in later life as predictor of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17(1):73–77. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- Johnson LC, Chernik DA. Sedative-hypnotics and human performance. Psychopharmacology. 1982;76:101–113. doi: 10.1007/BF00435262. [DOI] [PubMed] [Google Scholar]
- Jolles J, Verhey FRJ, Riedel WJ, Houx PJ. Cognitive impairment in elderly people. Drugs Aging. 1995;7(Suppl 6):459–479. doi: 10.2165/00002512-199507060-00006. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Haan EHFD. Mnemonic strategies in older people: a comparison of errorless and errorful learning. Age Aging. 2003;32(5):529–533. doi: 10.1093/ageing/afg068. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Zimprich D. Predictors of cognitive complaints in older adults: a mixture regression approach. Eur J Ageing. 2005;2:13–23. doi: 10.1007/s10433-005-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Garfinked L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Kryger M, Monjan AA, Bliwise D, Ancoli-Israel S. Bridging the gap between science and clinical practice. Geriatrics. 2004;59(1):24–30. [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1983. [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annu Rev Psychol. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychol Aging. 1997;12:410–432. doi: 10.1037/0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294(5544):1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Mazzoni G, Gori S, Formicola G, Gneri C, Massetani R, Murri L, Salzarulo P. Word recall correlates with sleep cycles in elderly subjects. J Sleep Res. 1999;8:185–188. doi: 10.1046/j.1365-2869.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- Mitrushina M, Satz P, Chervinsky A, D`Elia L. Performance of four age groups of normal elderly on the Rey Auditory-Verbal Learning Test. J Clin Psychol. 1991;47:351–357. doi: 10.1002/1097-4679(199105)47:3<351::AID-JCLP2270470305>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late life insomnia. A randomized controlled trial. J Am Med Assoc. 1991;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. Sleep. 1999;22(Suppl 8):1134–1156. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- Moses JA. Replicated factor structure of Benton`s tests of visual retention, visual construction and visual form discrimination. Int J Clin Neuropsychol. 1989;11(1):30–37. [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik FI, Perretta JG, Tonev ST. The effects of divided attention on encoding and retrieval processes: the resiliency of retrieval processes. Q J Exp Psychol. 2000;53(3):609–625. doi: 10.1080/027249800410454. [DOI] [PubMed] [Google Scholar]
- Naylor E, Penev PD, Orbeta L, Janssen I, Ortiz R, Colecchia EF, Keng M, Finkel S, Zee PC. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87–95. [PubMed] [Google Scholar]
- Nilsson LG, Backman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G. The Betula prospective cohort study: memory, health, and aging. Aging Neuropsychol Cogn. 1997;4:1–32. doi: 10.1080/13825589708256633. [DOI] [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: exploring the characteristics of illusory memories. Mem Cognit. 1997;25(6):838–48. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Sleep and the elderly. J Psychosom Res. 2002;56(5):463–464. doi: 10.1016/j.jpsychores.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Park DC. The basic mechanisms accounting for age-related decline in cognitive function. In: Park DC, Schwarz N, editors. Cognitive aging: a primer. Philadelphia: Psychology Press; 2000. pp. 3–21. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. doi: 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Perlmutter M, Nyquist L. Relationships between self-reported physical and mental health and intelligence performance across adulthood. J Gerontol. 1990;45:145–155. doi: 10.1093/geronj/45.4.p145. [DOI] [PubMed] [Google Scholar]
- Persad CC, Abeles N, Zacks RT, Denburg NL. Inhibitory changes after age 60 and their relationship to measures of attention and memory. J Gerontol B Psychol Sci Soc Sci. 2002;57B(3):223–232. doi: 10.1093/geronb/57.3.p223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LW. Differences in human memory with aging: nature, causes and clinical implications. In: Birren JE, Schaie KW, editors. Handbook of psychology of aging. New York: Van Nostrand Reinhold Company; 1985. pp. 427–462. [Google Scholar]
- Ray WA, Purushottan BT, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48:682–685. doi: 10.1111/j.1532-5415.2000.tb04729.x. [DOI] [PubMed] [Google Scholar]
- Roehrs T, RothT Sleep-wake state and memory function. Sleep. 2000;23:64–68. [PubMed] [Google Scholar]
- Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population. The MEMO study. Neurology. 2000;54:1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73(2):405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol. 1996;51:317–330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Salzarulo P. Opening remarks—perspectives on the relationship between cognitive processes and sleep disturbances. J Sleep Res. 1995;4:1. doi: 10.1111/j.1365-2869.1995.tb00142.x. [DOI] [Google Scholar]
- Salzarulo P, Cipolli C. Linguistic organization and cognitive implications of REM and NREM sleep related reports. Percept Mot Skills. 1979;49:767–777. doi: 10.2466/pms.1979.49.3.767. [DOI] [PubMed] [Google Scholar]
- Schaie KW. The optimization of cognitive functioning in old age: predictions based on cohort-sequential and longitudinal data. In: Baltes PB, Baltes MM, editors. Successful aging: perspectives from the behavioural sciences. Cambridge: Cambridge University Press; 1990. pp. 94–117. [Google Scholar]
- Simonsick EM, Guralnik JM, Fried LP. Who walks? Factors associated with walking behavior in disabled older women with and without self-reported walking difficulty. J Am Geriatr Soc. 1999;47:672–680. doi: 10.1111/j.1532-5415.1999.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Smith C. Sleep states and learning: a review of the animal literature. Neurosci Biobehav Rev. 1985;9:157–168. doi: 10.1016/0149-7634(85)90042-9. [DOI] [PubMed] [Google Scholar]
- Steenary MR, Vuontela V, Paavonen EP, Carlson S. Working memory and sleep in 6- to 13-year-old schoolchildren. J Am Acad Child Adolesc Psychiatry. 2003;42(1):85. doi: 10.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Szelenberger W, Niemcewicz S. Severity of insomnia correlates with cognitive impairment. Acta Neurobiol Exp. 2000;60(3):373. doi: 10.55782/ane-2000-1356. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sirakawa S. Sleep health, lifestyle and mental health in the Japanese elderly-ensuring sleep to promote a healthy brain and mind. J Psychosom Res. 2004;56:465–477. doi: 10.1016/j.jpsychores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- The Gallup Organization (1991) Sleep in America. National Sleep Foundation, Los Angeles
- Tilley AJ, Warren P. Retrieval from semantic memory during night without sleep. J Exper Psychol. 1984;36:281–289. [Google Scholar]
- Tun PA, Wingfield A, Rosen MJ, Blanchard L. Response latencies for false memories: gist-based processes in normal aging. Psychol Aging. 1998;13(2):230–241. doi: 10.1037/0882-7974.13.2.230. [DOI] [PubMed] [Google Scholar]
- Vakil E (1985) Encoding of frequency of occurrence, temporal order, and spatial location information by closed head injured and elderly subjects: is it automatic? Doctoral dissertation, City University of New York
- Vakil E, Blachstein H. Rey auditory verbal learning test; structure analysis. J Clin Psychol. 1993;48:883–890. doi: 10.1002/1097-4679(199311)49:6<883::AID-JCLP2270490616>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H. A supplementary measure in the Rey AVLT for assessing incidental learning of temporal order. J Clin Psychol. 1994;50(2):240–245. doi: 10.1002/1097-4679(199403)50:2<240::AID-JCLP2270500215>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H. Rey AVLT: development norms for adults and the sensitivity of different memory measures to age. Clin Neuropsychol. 1997;11(4):356–369. doi: 10.1080/13854049708400464. [DOI] [Google Scholar]
- Vakil E, Blachstein H, Hoofien D. Automatic temporal order judgment: the effect of intentionality of retrieval on closed-head-injured patients. J Clin Exp Neuropsychol. 1991;13:291–298. doi: 10.1080/01688639108401044. [DOI] [PubMed] [Google Scholar]
- Vakil E, Weise M, Enbar S. Direct and indirect memory measures of temporal order: younger versus older adults. Int J Aging Hum Dev. 1997;45(3):195–206. doi: 10.2190/N54R-9Q1M-27F3-GTRY. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: sex and estrogen effects on the subjective—objective sleep quality relationships of healthy, non-complaining older men and women. J Psychosom Res. 2004;56:503–510. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;14(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Wagner T, Balschun D. REM sleep enhancement induced by different procedures improves memory retention in rats. Eur J Neurosci. 2003;18:2611–2617. doi: 10.1046/j.1460-9568.2003.02890.x. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition, 2nd. Mahwah: Erlbaum; 2000. pp. 293–357. [Google Scholar]
- Zalsman G, Aizenberg D, Sigler M, Nahshoni E, Weizman A. Geriatric depression scale-short form—validity and reliability of the Hebrew version. Clin Gerontol. 1998;18(Suppl 3):3–9. doi: 10.1300/J018v18n03_02. [DOI] [Google Scholar]
- Zomer J, Peled R, Rubin AHE, Lavie P. Mini Sleep Questionnaire (MSQ) for screening large populations for EDS complaints. Sleep. 1985;84:467–469. [Google Scholar]