Abstract
Social support is an important factor for exercise among cancer patients, but too much control might elicit reactance and lead to detrimental effects. In this pilot study, 56 dyads (cancer patient + relative) filled out a questionnaire assessing social support, social control, and reactance. After 4 weeks (T2), patients’ exercise was assessed with a 7-day recall. About half of the patients did not engage in any self-reported exercise behavior. Relative-reported support was the only variable associated with exercise behavior at T2. Perceived control (r = .4) but not perceived support was significantly correlated with reactance. Male patients reported more support, but were also more prone to reactance.
Keywords: cancer, gender differences, physical activity, reactance, social control, social support
Introduction
Exercise has been identified to reduce side effects and improve quality of life in cancer patients both during and post medical treatment (e.g. Mishra et al., 2012a, 2012b; Speck et al., 2010). Furthermore, exercise has the potential to prevent and reduce clinically relevant side effects like pain, nausea, fatigue, lymphedema, and various others (Irwin et al., 2015; Kwan et al., 2011; Meneses-Echávez et al., 2015; Rief et al., 2014; van Waart et al., 2015). Based on these findings, the “Roundtable on Exercise Guidelines for Cancer Patients” recommends a weekly activity of 150 minutes of moderate-intensity exercise (Schmitz et al., 2010). However, it has been shown that only about one-third of adult cancer patients meet these recommended exercise guidelines (Bellizzi et al., 2005; Blanchard et al., 2008). Furthermore, exercise levels decline after cancer diagnosis (Courneya and Friedenreich, 1998; Huy et al., 2012). Patients are confronted with various barriers like fatigue, nausea, or other side effects making it difficult (yet feasible and advisable) for them to engage in exercise (Blaney et al., 2013; Courneya et al., 2008). Therefore, the question arises how cancer patients can be supported in setting up or maintaining a physically active lifestyle.
Social support is an important factor influencing health outcomes (e.g. Berkman et al., 2000; Holt-Lunstad et al., 2010). According to the theory by Uchino (2006)—describing the links between social support and physical health—one pathway for this relation is that supportive others help to increase health behaviors. Social support involves attempts to aid and reinforce someone’s own efforts to positively change his or her health behavior (Franks et al., 2006). In contrast, social control refers to interactions that involve influence, regulation, and constraints (Helgeson et al., 2004; Lewis and Rook, 1999) and comprises attempts to change someone’s health behavior who has been unable or unwilling to make such changes (Franks et al., 2006). Thus, both social support and social control behaviors are conducted with the intention to protect the recipient’s health (Khan et al., 2013), but represent two distinct constructs (Helgeson et al., 2004).
In the context of exercise and cancer, social support includes, for example, being physically active together with the cancer patient, encouraging the patient to exercise, or assisting the patient in carrying out exercise (Khan et al., 2013; Sallis et al., 1987). On the other hand, examples of social control behaviors aimed at increasing exercise levels are criticizing the patient for his or her insufficient physical activity, prompting the patient to exercise more, or observing whether the recipient really is exercising (Khan et al., 2013).
Social support has already been identified as a positive determinant of exercise in various populations (e.g. Anderson-Bill et al., 2011; Bauman et al., 2012; Franks et al., 2006; Khan et al., 2013; Van Dyck et al., 2011). A review by Barber (2012) revealed a positive relationship between social support and cancer patients’ physical activity in about 50 percent of the included 22 studies. Most studies of this review focused on perceived social support from family and friends, but a few also examined other aspects of social support like having another cancer survivor as role model for exercise (Rogers et al., 2008, 2011) or environmental support from the neighborhood or community (Coups et al., 2009). However, the samples consisted mostly (59%) of breast cancer patients.
In contrast, the findings regarding the direction of the relationship between social control and exercise are inconsistent (see Knoll et al., 2012). The use of pressure (e.g. criticizing, nagging), which can be classified as a social control strategy, was associated with better health behavior (including physical activity) in patients suffering from osteoarthritis (Stephens et al., 2009). Other research findings, however, suggest that social control has a negative impact on exercise in adults suffering from diabetes (Khan et al., 2013; Thorpe et al., 2008). Franks et al. (2006) also found reduced health behavior, including physical activity, as a result of social control in patients participating in cardiac rehabilitation.
To the authors’ knowledge, no study in the exercise and cancer domain has focused on social support and social control, and so far only one study analyzed the impact of social control on cancer patients’ physical activity levels so far (Helgeson et al., 2004). Helgeson and colleagues investigated the influence of spouse social control on several health behaviors in men with prostate cancer in a longitudinal study. Results showed that spouse social control was ineffective in producing changes in health-enhancing behaviors such as physical activity. Social control related to health-comprising behavior (such as smoking) and health-restorative behavior (such as sleeping) was even associated with poor health behaviors and greater psychological distress (Helgeson et al., 2004).
Overall, the evidence for a negative or no impact of social control on exercise behavior predominates. A construct that could explain the missing positive effect of social control on health behavior or even “boomerang effects” is psychological reactance. Reactance is described as an aversive motivational state that arises when an individual perceives his or her behavioral freedoms as threatened or lost (Brehm, 1966). In order to reduce reactance, individuals try to engage in behaviors that are able to reestablish the freedom that has been threatened. Such behaviors can be contrary to the behavior that was originally aimed by the person who evoked reactance. Reactance can be regarded as a stable personality trait (persons tending to be reactant across many situations) or as a situation-specific state. According to reactance theory, social control should—if perceived as a threat to the personal freedom—evoke reactance, whereas social support should be unrelated to reactance. Empirically, it has been shown among people with diabetes and healthy adults that social control attempts of a spouse can evoke resistance and emotional distress (Rook et al., 2011; Tucker and Anders, 2001; Tucker et al., 2006). To our knowledge, no studies have examined the role of reactance in the field of exercise among cancer patients so far.
When examining the associations between social support, social control, reactance, and exercise, it is important to consider gender aspects. Previous research has investigated differences between men and women regarding health behaviors (Gough, 2013; Helgeson, 2012) and supporting behaviors within a marriage (Neff and Karney, 2005). There are gender differences in how much women and men facilitate their spouses’ health behavior: typically, women take more care of their spouses’ health, nutrition, and exercise and constrain health risk behavior more than men do toward their wives (Allen et al., 2013; Miller and Wortman, 2002; Zhu et al., 2006). In line with these findings, women are often regarded as “health promotion agents” (Marcell et al., 2010) for their partners. Furthermore, large cross-sectional surveys among (culturally diverse) undergraduates have shown that men had a significantly higher level of trait reactance than women (Seemann et al., 2004; Woller et al., 2007). Therefore, the question arises whether female and male cancer patients differ in the amount of received social support and control by their relatives to engage in exercise and whether they react with a varying extent of reactance.
A limitation of previous research on social influences on physical activity among cancer patients—for example, included in the review by Barber (2012)—is that social support was only assessed by self-reports of cancer patients. According to the conceptual framework by Dunkel-Schetter describing elements of social interactions, three different perspectives of social support should be considered: the recipient’s, the provider’s, and an outside observer’s perspective (Dunkel-Schetter et al., 1992). As relatives, friends, or other persons who actually provide social support did not take part in previous studies, only one perspective could be gained. An exception is a study by Gilliam et al. (2012) which questioned both child and adolescent patients and their caregivers about predictors of physical activity, including family support. They found that the strength of predictors varied dependent on caregiver and patient reports. To our knowledge, in adult cancer patients, the perspective of relatives has not been included so far.
The first aim of this pilot study was to examine associations between social support and control as perceived by the patient and relative-reported social support and control (research question 1). Additionally, we wanted to explore the associations between social control and social support with reactance (research question 2). A further focus was on possible gender differences within these social factors (research question 3). Finally, we investigated whether social support, social control, and reactance are predictors of cancer patient’s self-reported exercise behavior which was assessed 4 weeks later (research question 4).
Methods
The pilot study consisted of two assessment points. At the first measurement point (T1), cancer patients and their relatives took part. After 4 weeks (T2), cancer patients were recontacted. Inclusion criteria for the patients were an age of at least 18 years, currently receiving outpatient treatment or follow-up care, and being accompanied by a relative or a partner who also agreed to participate in the study. Exclusion criteria were inability to follow the study instructions, inpatient treatment, and severe physical constraints which made exercise impossible (i.e. inability to walk or stand). The study was approved by the ethics committee of the medical faculty from Heidelberg University.
All in all, 90 cancer patients accompanied by a person were personally approached by the study personnel (M.W. and A.K.); of which 56 patients (62.2%) met the inclusion criteria and agreed to participate. Reasons for not participating were as follows: lack of interest, anticipation of inpatient treatment in the near future, not speaking sufficiently German, and being accompanied by a friend but not a relative.
Four weeks after T1, cancer patients who had participated in the first assessment were again contacted by phone and interviewed (T2). In total, 47 patients (83.9%) completed the study. Of the nine persons who dropped out at T2, seven persons could not be contacted, one person could not be interviewed because of a hospital stay, and one person did not want to take part in the survey anymore. One further patient could not be included in the analyses of exercise behavior due to missing values for this variable.
Procedures
At T1, cancer patients who were accompanied by a relative were approached (at random) in the waiting areas in the outpatient care unit of the National Center for Tumor Diseases Heidelberg/Germany. If they were interested in participating in the study, they provided informed consent prior to receiving instructions for study procedures. Cancer patients at first indicated their exercise behavior within an interview. Thereafter, cancer patients completed a self-administered paper questionnaire that assessed perceived social support and perceived social control for exercise received from the accompanying relative, reactance as well as sociodemographic and medical information. At the same time, relatives completed a paper questionnaire independently from the patient (relatives were told not be in contact with the patient while filling out the questionnaire) regarding social support and social control for cancer patients’ exercise and sociodemographic information.
Approximately 4 weeks after T1 (M = 25.9 days, standard deviation (SD) = 4.6 days), cancer patients were recontacted by phone. In the second interview, only exercise and some medical information were assessed.
Measures
Self-reported exercise behavior
At both assessment time points, cancer patient’s current physical activity behavior was measured with the Seven-Day Physical Activity Recall (Sallis et al., 1985, 1997). They were asked for the frequency and duration of light, moderate and vigorous physical activity, as well as type of physical activity they had carried out on each of the last 7 days. Behavioral descriptors and examples were provided for all three intensities. Unlike the original procedure, we only asked for physical activity behavior during the whole day and not for physical activity at specific times of the day (morning, afternoon, evening). The Seven-Day Physical Activity Recall has demonstrated good reliability and validity in multiple studies (see Sallis et al., 1997 for an overview) and has been used in cancer populations (e.g. Pinto et al., 2005).
Self-reported exercise (in minutes per week) was calculated by adding up exclusively moderate and vigorous exercise behavior. According to Caspersen et al. (1985), we regard exercise as “physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of one or more components of physical fitness is an objective” (p. 128). This variable was of major interest as it mirrors the exercise guidelines of at least 150 minutes moderate-to-vigorous exercise per week (Schmitz et al., 2010).
In additional analyses, self-reported exercise plus walking was used comprising a wider range of physical activity. Beside moderate-to-vigorous exercise, it additionally includes walking for leisure during the last week. As this variable comprises activities with a wide range of different intensities (from light to vigorous), all activities were weighted with its energy expenditure. Therefore, the time spent in an activity was multiplied with the metabolic equivalents (METs) of the activity according to the compendium by Ainsworth et al. (2011) before summing up all activities per week. The final unit of this “leisure time activity” variable was MET-hours per week.
Social support and social control
At T1, social support and social control for exercise were measured with the Spousal Involvement in Patient Exercise Scale developed by Khan et al. (2013) based on research on spousal involvement in illness management (Franks et al., 2006; Trief et al., 2003). The items were translated into German by native speakers through forward–backward translation. One additional item (“Exercised with me”), which was taken from the Family Support for Exercise Habits Scale (Sallis et al., 1987), was added to the social support scale. The social support scale thus consisted of eight items (in the questionnaire for patients, for example: “He/she listened to my concerns about maintaining an exercise routine”) and the social control scale of seven items (in the questionnaire for relatives, for example: “I tried to influence him/her to do more physical exercise”). All items were rated on a scale from 1 (not at all) to 4 (very much) and referred to the last month. Khan et al. (2013) reported good reliabilities for both the social support scale (α = .90, daily test–retest α = .72) and the social control scale (α = .90, daily test–retest α = .67). These questionnaires were completed both by cancer patients (perceived social support and control from the accompanying relative) and relatives (relative-reported social support and control) so that reports on social support and social control were independently obtained from two perspectives. In the current sample, Cronbach’s alpha for perceived (patient-reported) social support was α = .91, and for relative-reported social support, it was α = .77. Cronbach’s alpha for perceived and relative-reported social control scale were α = .90 and α = .87, respectively.
State reactance
State reactance was measured with four items of a modified short scale that had been developed for an intervention study on fruit and vegetable intake (Ungar et al., 2013, 2015). The items for this study were adapted to reactions to the behavior of the relatives and asked for cognitions that have been described as typical indicators of reactance (e.g. Quick and Stephenson, 2007; Traut-Mattausch et al., 2008). An example item used in this study is, “Through my relative’s behavior concerning my exercise during the last month, I felt very restricted in my personal freedom”. Each statement was rated on a scale from 1 (does not apply at all) to 7 (applies completely). Cronbach’s alpha of the short reactance scale with four items was α = .71.
Statistical analyses
Descriptive statistics were used to examine demographic, medical, and psychological variables as well as exercise for all participants (n = 56 patients and n = 56 relatives). Participants and non-participants at T2 were compared using t-tests (for metric variables) and chi-square tests (for non-metric variables) including any demographic, medical, or psychological variables.
To investigate the research questions, bivariate correlations and t-tests were calculated. Pearson correlations were used to analyze associations between perceived and relative-reported social support/social control (research question 1) and between social support/social control and reactance (research question 2). Gender differences regarding perceived and relative-reported social support and social control as well as reactance were analyzed based on t-tests (research question 3). To investigate the associations between psychological variables at T1 and exercise behavior at T2 (research question 4), Spearman correlations were used, as the exercise variable had a highly zero-inflated distribution. Because of the clumping at zero, no linear regression could be calculated. Instead, for an additional analysis of research question 4, a linear regression was conducted with exercise plus walking (instead of exercise) as dependent variable (without clumping at zero). All sociodemographic and medical variables which correlated significantly with the dependent variable were included as covariates in a first step. Perceived and relative-reported social support and social control as well as reactance were included as predictors of exercise plus walking in the second step and the adjusted R2 were compared. Analyses were carried out with IBM SPSS Statistics 21 and employed a significance level of p < .05.
Results
Participants
The sample consisted of N = 56 cancer patients (53.6% female) with a mean age of 53.6 years (SD = 12.7 years, range: 27–75 years) and N = 56 relatives (51.8% female, Mage = 52.8 years, SD = 13.4 years). In 89 percent of the dyads, the accompanying relative was the spouse or life partner of the patient. The sociodemographic and medical variables are listed in Table 1. All sociodemographic and medical variables were unrelated to exercise behavior and the psychological variables investigated in this study (all ps > .05).
Table 1.
Sample characteristics (n = 56 cancer patients; n = 56 relatives).
| Variable | Mean (SD) | % |
|---|---|---|
| Patient report | ||
| Demographic variables | ||
| Female | 53.6 | |
| Age (years) | 53.58 (12.72) | |
| BMI (kg/m2) | 25.56 (4.65) | |
| Marital status | ||
| Married | 94.6 | |
| Single | 3.6 | |
| Divorced/widowed | 1.8 | |
| Currently not working | 76.8 | |
| Degree of relationship | ||
| Couples | 89.3 | |
| Parent–child | 7.1 | |
| Siblings | 3.6 | |
| Living in one household | 89.3 | |
| Medical variables | ||
| Type of cancer | ||
| Breast | 39.3 | |
| Skin | 14.3 | |
| Colorectal | 12.5 | |
| Gastric | 5.4 | |
| Hepatic | 5.4 | |
| Other | 23.2 | |
| Time since diagnosis in months | 26.31 (33.78) | |
| Current chemotherapy | 57.4 | |
| Current radiation therapy | 0.0 | |
| Previous chemotherapy | 20.4 | |
| Previous radiation therapy | 35.2 | |
| Physical activity | ||
| Moderate-to-vigorous exercise at T1a | 85.98 (181.84) | |
| Moderate-to-vigorous exercise at T2a | 90.65 (154.66) | |
| Leisure time physical activity at T1b | 16.73 (15.94) | |
| Leisure time physical activity at T2b | 18.70 (15.80) | |
| Psychological variables | ||
| Social supportc | 2.91 (0.83) | |
| Social controlc | 2.06 (0.84) | |
| Reactanced | 1.61 (0.98) | |
| Relative report | ||
| Demographic variables | ||
| Female | 51.8 | |
| Age (years) | 52.75 (13.42) | |
| BMI (kg/m2) | 25.58 (3.96) | |
| Psychological variables | ||
| Social supportc | 3.07 (0.54) | |
| Social controlc | 2.23 (0.73) | |
MET: metabolic equivalent of task; SD: standard deviation; BMI: body mass index.
In minutes per week.
In MET-hours per week, including light, moderate, and vigorous leisure time activities.
On a scale from 1 to 4.
On a scale from 1 to 7.
Dropout-analysis: The only significant difference between participants and non-participants at T2 emerged for relative-reported social support, t(54) = 2.34, p < .05. At T1, relatives of dropouts indicated more social support (M = 3.44, SD = 0.37) than relatives of participants (M = 3.00, SD = 0.54). For all other demographic, medical, or psychological variable, no differences were found between patients who did or did not participate at T2 (all ps < .05).
Research question 1. Associations between patient-perceived and relative-reported social support and control
Overall, there were significant positive correlations between perceived and relative-reported social support (r = .431, p = .001) as well as social control (r = .490, p < .001). Analyzing female and male patients separately, for women we found moderate and significant correlations, whereas for men the correlations between perceived and relative-reported measures were lower and not significant (see Table 2).
Table 2.
Intercorrelations of study variables for the whole sample (above the diagonal) and separated by sex (below diagonal: men—bold, women—italic).
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
|---|---|---|---|---|---|---|---|---|
| 1. | Exercise at T2a | – | .32* | .06 | .32* | .05 | .04 | −.13 |
| 2. | Exercise at T1a | .34 | – | −.03 | .09 | −.18 | −.23° | −.10 |
| .30 | ||||||||
| 3. | Perceived social supportb | .04 | −.27 | – | .43** | .65** | .40** | .20 |
| .24 | .30 | |||||||
| 4. | Relative-reported social supportb | .05 | .30 | .19 | – | .25° | .60** | .30* |
| .53 ** | .07 | .43 * | ||||||
| 5. | Perceived social controlb | −.11 | −.53** | .44 * | <.01 | – | .49** | .38** |
| .36 ° | .29 | .65 ** | .20 | |||||
| 6. | Relative-reported social controlb | −.12 | −.06 | .11 | .68 ** | .24 | – | .41** |
| .23 | −.29 | .35 | .44 * | .39 * | ||||
| 7. | Reactancec | −.39° | .05 * | .15 | .36 ° | .37 ° | .37 ° | – |
| .10 | −.08 | −.08 | .02 | −.02 | <.01 |
Pearson correlations were conducted for all variables except exercise at T1 and exercise at T2. For the exercise variables, Spearman correlations were used because of strong deviation from normal assumption.
In minutes per week derived from the 7-day recall.
On a scale from 1 to 4.
On a scale from 1 to 7.
p < .10, *p < .05, **p < .01.
Research question 2. Associations between social support, social control, and reactance
Analyses revealed significant positive correlations between social control and reactance. This was true for perceived social control (r = .375, p = .004) as well as for relative-reported social control (r = .407, p = .002). Perceived social support was not significantly correlated with reactance, but we found a significant association between relative-reported social support and reactance (r = .303, p = .023).
Research question 3. Gender differences
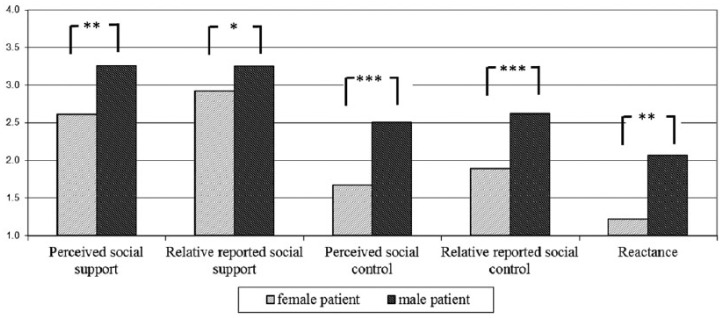
Gender differences in main study variables are shown in Figure 1. Male cancer patients perceived significantly more social support (p = .003) and control (p < .001) compared to female patients. Their (mostly female) relatives also reported to support and control them more (p = .024). Men reported a higher amount of reactance (p = .001).
Figure 1.
Gender differences in psychological variables (assessed at T1). Social support and social control were assessed on a scale from 1 to 4, and reactance was assessed on a scale from 1 to 7; t-tests were calculated. *p < .05, **p < .01, ***p < .001.
Comparisons of relative-reported and perceived support and control for male and female relatives separately revealed that male relatives reported significantly higher amounts of social support and social control than the related (mostly female) patients perceived. For female relatives, there were no such differences between relative-reported and perceived social support or social control (see Table 3).
Table 3.
Comparison of relative-reported and perceived social support and social support separately for male and female relatives.
| Relative-reported |
Perceived by patient |
p | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Male relative | |||||
| Social support | 2.98 | 0.48 | 2.65 | 0.85 | .047 |
| Social control | 1.93 | 0.56 | 1.62 | 0.65 | .040 |
| Female relative | |||||
| Social support | 3.16 | 0.58 | 3.16 | 0.73 | .998 |
| Social control | 2.50 | 0.76 | 2.47 | 0.80 | .836 |
SD: standard deviation.
Research question 4. Predictors of exercise at T2
Self-reported exercise at T2 turned out to have a highly zero-inflated distribution, as 63 percent of participants did not engage in any moderate-to-vigorous exercise at T2. Because of this highly skewed distribution, the clumping of zeros, and the resulting missing normality assumption, no linear regression analyses could be performed. Spearman correlations show that self-reported exercise at T2 was positively associated with relative-reported social support at T1 (r = .324, p = .028). There were no associations with all other psychological variables (see Table 2). Analyzing male and female patients separately yielded different patterns. Among women, relative-reported social support at T1 was highly (r = .533, p = .004) and perceived social control marginally (r = .359, p = .066) related to exercise at T2. For men, on the other hand, no variable could be identified as significant predictor of exercise at T2. For men, reactance at T1 was marginally significant (r = −.392, p = .097): the more men felt restricted in their freedom the less they exercised at T2.
Additional analyses for research question 4
To get around the shortcoming of the “exercise” variable, a further analysis with the broader physical activity variable was calculated to answer research question 4. The associations between psychological variables at T1 and physical activity at T2 were additionally examined using self-reported exercise plus walking (instead of exercise) as a broader indicator of physical activity. As most of the participants engaged in (some amount of) exercise plus walking (only 11% did not engage in any exercise plus walking), this variable could be used as dependent variable in a hierarchical linear regression analyses. The Spearman correlation between exercise and exercise plus walking was r = .532 (p < .001). Exercise plus walking at T1 and body mass index (BMI) were included as covariates in a first step, as they were the only sociodemographic/medical control variables that significantly correlated with the dependent variable. In the first step of the regression, both variables were significant determinants and explained 56 percent of the variance in exercise plus walking at T2 (exercise plus walking at T1: β = .702, p < .001; BMI: β = .315, p = .003). In a second step, all psychological variables (assessed at T1) were included. The regression analysis revealed that relative-reported social support was the only psychological variable being a significant predictor (β = .312, p = .012) over and above the control variables exercise plus walking at T1 and BMI confirming the above-described correlational analyses. Additional 9 percent of the variance could be explained in the second step.
Discussion
Social support can help cancer patients to increase their exercise level (see review by Barber, 2012), but social control can have detrimental effects (Helgeson et al., 2004; Khan et al., 2013; Knoll et al., 2012). This pilot study adds to the previous literature in the exercise and cancer domain by focusing on social support and social control and examining the role of reactance. By including not only the patients but also a relative, two different perspectives could be gained and compared: patient-perceived versus relative-reported support and control. Furthermore, gender aspects were explored.
Results yielded that perceived social support and social control were moderately associated with relative-reported support and control. These moderate relations between patient and relative reports are consistent with prior research in the context of social exchange processes among couples dealing with chronic diseases (Benyamini et al., 2007; Hagedoorn et al., 2000; Kuijer et al., 2000; Stephens et al., 2010). Interestingly, moderate associations were only found for female patients. It would be interesting for future research to find reasons for the missing associations between perceived and relative-reported social support and control among male patients.
We also investigated how psychological variables at T1 were related to self-reported exercise at T2. Relative-reported social support was the only variable that was significantly related to physical activity across two different measurements of physical activity (self-reported exercise and exercise plus walking). This is in line with previous studies from other domains, showing that social support has positive effects on physical activity (e.g. Barber, 2012; Bauman et al., 2012; Franks et al., 2006; Fraser and Rodgers, 2012; Khan et al., 2013; Van Dyck et al., 2011), whereas the results regarding social control were inconsistent (e.g. Helgeson et al., 2004; Khan et al., 2013; Stephens et al., 2009; Tucker and Anders, 2001).
Surprisingly, in our study, only relative-reported and not patient-perceived social support revealed to be a significant predictor of patient’s exercise plus walking at T2. According to the conceptual framework of Dunkel-Schetter et al. (1992), different perspectives of social support should be considered (the recipient, the provider, and outside observer), but the highest priority has perceived social support of the recipient (Dunkel-Schetter and Bennett, 1990). Nevertheless, our results are congruent with evidence from prior research that found that spouses’ perceptions of their influence, and not patients’ reports, explained patients’ dietary adherence (Stephens et al., 2010). Furthermore, Franks et al. (2006) and Khan et al. (2013) have shown that relative-reported social support had a positive effect on health behavior and Grange et al. (2007) reported that practical assistance is perceived as especially supportive. This gap between the theoretical assumption that the perception of the recipient is most influential and empirical findings highlighting the effects of providers’ reports has to be further investigated.
Results of this study revealed that male cancer patients felt more supported by their partners than female patients did. Additionally, their (female) relatives reported to support them more in comparison to the report of the (male) relatives of female patients. This result is in line with findings of a study examining the course of spousal support in the context of mainly gastrointestinal cancer surgery (Luszczynska et al., 2007). Another recent study has not only focused on the help by a relative but differentiated between support received by a significant other and support received by friends (Coleman et al., 2014). Results revealed that walking for exercise was only associated with greater friend support. The support by friends might be especially important for women and this might compensate for the lower support of their partners, as buffering effects of family and friend support have been shown among women with breast cancer (Manne et al., 2003). Previous research has shown that women have a wider range of sources of their support (Fuhrer and Stansfeld, 2002) and that they do not nominate their spouse as closest person as much as men do (women: 79.6%, men: 92.4%) (Fuhrer et al., 1999).
Male patients were not only more supported but also more controlled by their female partners. In line with this result, male patients reported a higher amount of reactance than female patients did. In prior research, such a gender difference had emerged as well (Seemann et al., 2004; Woller et al., 2007). Additionally, we found a positive association between social control and reactance in male patients only. For female cancer patients, there was no association between social control and reactance.
Several limitations of the pilot study have to be mentioned. The study consisted of a small and heterogeneous convenience sample, which threatens external validity. Especially the analyses separately for men and women were based on very small sample sizes. Due to the small sample size, possible analysis options were restricted (e.g. testing moderation effects of gender and other variables of interest; calculating regression analyses and including (more) covariates). The study can be regarded as pilot study and the reported associations have to be investigated in bigger and representative samples. A further limitation of this study is that the analyses of research questions 1–3 are based on cross-sectional data and do not allow any causal assumption. Changes in psychological variables across time could not be explored.
Additionally, more than half of participants did not engage in any moderate-to-vigorous self-reported exercise. This made the analysis of the exercise variable difficult. As the sample size was too small for appropriate regression models accounting for this zero-inflated distribution (e.g. by calculating a Poisson–Gamma regression; Brown and Dunn, 2011), only Spearman correlations were conducted. The limitations of bivariate correlations have to be kept in mind, as they cannot control for any covariates and do not allow any causal interpretation. However, an additional analysis with exercise plus walking (no clumping at zero) instead of exercise was conducted allowing to use a linear regression. The finding of such a high proportion of sedentary cancer patients is in line with previous research. For example, in a study by Speed-Andrews et al. (2012), 46 percent of colorectal cancer patients were classified as completely sedentary (i.e. 0 min/week physical activity).
Furthermore, there are some limitations regarding the measurements of study variables. It has to be considered that several versions of the 7-day questionnaire have been used in past research. We applied a version that was more refined than the original one but which was also less accurate with regard to the time windows during the day than other/more recent versions (Sallis et al., 1985). Good reliability and validity for several versions of the questionnaire have been shown in multiple studies (see Sallis et al., 1997 for an overview). The calculation of MET values from self-reported exercise is accompanied by some inaccuracies, although interviewers asked for detailed descriptions of the activities. Regarding the measurement of state reactance, it has to be acknowledged that the used scale has not been validated so far. However, it has shown good internal consistency in other contexts (Ungar et al., 2013, 2015).
Finally, the fact that we cannot report the stage of disease is a serious limitation, as we could not analyze possible associations to relative’s support or control. However, recent research has shown evidence that exercise interventions are also feasible in advanced cancer patients undergoing chemotherapy treatment (Kuehr et al., 2014; Lowe, 2011).
A strength of this pilot study was the dyadic design. In contrast to previous research regarding exercise behavior among cancer patients, not only the patients but also a close relative (mostly the husband or wife) who accompanied the patient to treatment was included in the study. Thereby, two different perspectives of social support and control could be gained and compared. Additionally, this study was a first attempt to examine the role of reactance regarding exercise behavior in cancer patients.
Future studies should investigate social support and control and the role of reactance with a bigger and more representative sample and (accordingly) more detailed analyses. Additionally, research should broaden its view and also look at the whole family, friends, physicians, and further parts of patients’ networks (Wesley et al., 2013). Different sources of social support and social control should be compared. Thereby, it would be necessary to include relationship satisfaction or the quality of the relationship (Cousson-Gélie et al., 2013) in future studies as it has been shown to be a relevant moderator between social control and health behavior (Knoll et al., 2012). Additionally, it has been shown that sharing similar health behavior values within couples leads to increased health behavior in healthy adults (Skoyen et al., 2013). This should be investigated within couples, in which one partner has cancer, as it might interact with cancer-specific relationship awareness (Manne et al., 2014). Furthermore, future research should not exclusively focus on social support, social control, and reactance but put them in the context to other factors which have found to be important to predict cancer patients’ physical activity (social cognitions, environmental factors, etc.). Finally, research should test the relations found in this correlational study within experimental designs. For example, one randomly chosen group of relatives could be coached how they can support the patient to become physically active without evoking reactance.
Our study has direct practical implications. Results support the need to integrate relatives in the promotion of exercise among cancer patients. It was shown that especially relative-reported support—not perceived social support—was a predictor of engaging in physical activity. If this result of our pilot study is confirmed in other studies, relatives should be reinforced to support their partners. For example, an information event addressing relatives of cancer patients could inform about basic rules regarding exercise during cancer treatment like exercise guidelines and contraindications. Furthermore, information on psychological mechanisms should be provided (e.g. support vs. control) and relatives should be made aware of the danger to evoke reactance. A study by Aymanns et al. (2013) has shown that higher self-ascribed competence to help was associated with an increased provision of social support. All in all, it is important that patients and relatives should not feel an obligation to exercise (this could create distress and reactance) but see physical activity as a possibility to actively deal with their disease by their own choice.
In conclusion, this study showed that only a minority of participants were reporting engagement in meaningful levels of exercise despite its positive effects on well-being during active treatment (Mishra et al., 2012b; Speck et al., 2010). The study examined how patients can be supported to increase their exercise level by integrating two perspectives: the view of the patients as well as their relatives. The distinction between social support and social control seems promising as only support was positively related to exercise. Interesting gender differences emerged and revealed that reactance might play a role especially for male cancer patients.
Acknowledgments
The authors thank the Ernst Ludwig Ehrlich Scholarship Programme for supporting Nadine Ungar with a dissertation scholarship.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported by the Deutsche Krebshilfe e.V. (German Cancer Aid) (Grant Nos 110512, 110551, and 111223). The authors acknowledge the financial support of the Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing.
References
- Ainsworth BE, Haskell WL, Herrmann SD, et al. (2011) 2011 compendium of physical activities: A second update of codes and MET values. Medicine & Science in Sports & Exercise 43: 1575–1581. [DOI] [PubMed] [Google Scholar]
- Allen JO, Griffith DM, Gaines HC. (2013) “She looks out for the meals, period”: African American men’s perceptions of how their wives influence their eating behavior and dietary health. Health Psychology 32: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Bill ES, Winett RA, Wojcik JR. (2011) Social cognitive determinants of nutrition and physical activity among web-health users enrolling in an online intervention: The influence of social support, self-efficacy, outcome expectations, and self-regulation. Journal of Medical Internet Research 13: e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymanns P, Filipp S.-H, Winkeler M. (2003) Age differences in supportive reactions toward a person in need: A quasi-experimental study. International Journal of Behavioral Development, 27: 232–242. DOI: 10.1080/01650250244000308. [DOI] [Google Scholar]
- Barber FD. (2012) Social support and physical activity engagement by cancer survivors. Clinical Journal of Oncology Nursing 16: E84–E98. [DOI] [PubMed] [Google Scholar]
- Bauman AE, Reis RS, Sallis JF, et al. (2012) Correlates of physical activity: Why are some people physically active and others not? The Lancet 380: 258–271. [DOI] [PubMed] [Google Scholar]
- Bellizzi KM, Rowland JH, Jeffery DD, et al. (2005) Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. Journal of Clinical Oncology 23: 8884–8893. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Medalion B, Garfinkel D. (2007) Patient and spouse perceptions of the patient’s heart disease and their associations with received and provided social support and undermining. Psychology & Health 22: 765–785. [Google Scholar]
- Berkman LF, Glass T, Brissette I, et al. (2000) From social integration to health: Durkheim in the new millennium. Social Science & Medicine 51: 843–857. [DOI] [PubMed] [Google Scholar]
- Blanchard CM, Courneya KS, Stein K. (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. Journal of Clinical Oncology 26: 2198–2204. [DOI] [PubMed] [Google Scholar]
- Blaney JM, Lowe-Strong A, Rankin-Watt J, et al. (2013) Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire–survey. Psycho-Oncology 22: 186–194. [DOI] [PubMed] [Google Scholar]
- Brehm JW. (1966) A Theory of Psychological Reactance. New York: Academic Press. [Google Scholar]
- Brown JE, Dunn PK. (2011) Comparisons of Tobit, linear, and Poisson–Gamma regression models: An application of time use data. Sociological Methods & Research 40: 511–535. [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. (1985) Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports 100: 126–131. [PMC free article] [PubMed] [Google Scholar]
- Coleman S, Berg CJ, Thompson NJ. (2014) Social support, nutrition intake, and physical activity in cancer survivors. American Journal of Health Behavior 38: 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coups EJ, Park BJ, Feinstein MB, et al. (2009) Correlates of physical activity among lung cancer survivors. Psycho-Oncology 18: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM. (1998) Relationship between exercise during treatment and current quality of life among survivors of breast cancer. Journal of Psychosocial Oncology 15: 35–57. [Google Scholar]
- Courneya KS, McKenzie DC, Reid RD, et al. (2008) Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Annals of Behavioral Medicine 35: 116–122. [DOI] [PubMed] [Google Scholar]
- Cousson-Gélie F, De Chalvron S, Zozaya C, et al. (2013) Structural and reliability analysis of quality of relationship index in cancer patients. Journal of Psychosocial Oncology 31: 153–167. [DOI] [PubMed] [Google Scholar]
- Dunkel-Schetter C, Bennett TL. (1990) Differentiating the cognitive and behavioral aspects of social support. In: Sarason BR, Sarason IG, Pierce GR. (eds) Social Support: An Interactional View. Oxford: John Wiley & Sons, pp. 267–296. [Google Scholar]
- Dunkel-Schetter C, Blasband DE, Feinstein LG, et al. (1992) Elements of supportive interactions: When are attempts to help effective? In: Spacapan S, Oskamp S. (eds) Helping and Being Helped: Naturalistic Studies (The Claremont Symposium of Applied Psychology). Thousand Oaks, CA: SAGE, pp. 83–114. [Google Scholar]
- Franks MM, Stephens MAP, Rook KS, et al. (2006) Spouses’ provision of health-related support and control to patients participating in cardiac rehabilitation. Journal of Family Psychology 20: 311. [DOI] [PubMed] [Google Scholar]
- Fraser SN, Rodgers WM. (2012) The influence of general and exercise specific social support on self-efficacy for overcoming barriers to cardiac rehabilitation. Journal of Applied Social Psychology 42: 1811–1829. [Google Scholar]
- Fuhrer R, Stansfeld SA. (2002) How gender affects patterns of social relations and their impact on health: A comparison of one or multiple sources of support from “close persons.” Social Science & Medicine 54: 811–825. [DOI] [PubMed] [Google Scholar]
- Fuhrer R, Stansfeld SA, Chemali J, et al. (1999) Gender, social relations and mental health: Prospective findings from an occupational cohort (Whitehall II study). Social Science & Medicine 48: 77–87. [DOI] [PubMed] [Google Scholar]
- Gilliam MB, Madan-Swain A, Whelan K, et al. (2012) Social, demographic, and medical influences on physical activity in child and adolescent cancer survivors. Journal of Pediatric Psychology 37: 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough B. (2013) The psychology of men’s health: Maximizing masculine capital. Health Psychology 32: 1–4. [DOI] [PubMed] [Google Scholar]
- Grange CM, Matsuyama RK, Ingram KM, et al. (2007) Identifying supportive and unsupportive responses of others: Perspectives of African American and Caucasian cancer patients. Journal of Psychosocial Oncology 26: 81–99. [DOI] [PubMed] [Google Scholar]
- Hagedoorn M, Kuijer RG, Buunk BP, et al. (2000) Marital satisfaction in patients with cancer: Does support from intimate partners benefit those who need it most? Health Psychology 19: 274. [PubMed] [Google Scholar]
- Helgeson VS. (2012) Gender and health: A social psychological perspective. In: Baum A, Revenson TA, Singer J. (eds) Handbook of Health Psychology. 2nd ed. New York: Psychology Press, pp. 519–537. [Google Scholar]
- Helgeson VS, Novak SA, Lepore SJ, et al. (2004) Spouse social control efforts: Relations to health behavior and well-being among men with prostate cancer. Journal of Social and Personal Relationships 21: 53–68. [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. (2010) Social relationships and mortality risk: A meta-analytic review. PLoS Medicine 7: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huy C, Schmidt ME, Vrieling A, et al. (2012) Physical activity in a German breast cancer patient cohort: One-year trends and characteristics associated with change in activity level. European Journal of Cancer 48: 297–304. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Cartmel B, Gross CP, et al. (2015) Randomized exercise trial of aromatase inhibitor–induced arthralgia in breast cancer survivors. Journal of Clinical Oncology 33: 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan CM, Stephens MAP, Franks MM, et al. (2013) Influences of spousal support and control on diabetes management through physical activity. Health Psychology 32: 739–747. [DOI] [PubMed] [Google Scholar]
- Knoll N, Burkert S, Scholz U, et al. (2012) The dual-effects model of social control revisited: Relationship satisfaction as a moderator. Anxiety, Stress, & Coping 25: 291–307. [DOI] [PubMed] [Google Scholar]
- Kuehr L, Wiskemann J, Abel U, et al. (2014) Exercise in patients with non-small cell lung cancer. Medicine & Science in Sports & Exercise 46: 656–663. [DOI] [PubMed] [Google Scholar]
- Kuijer RG, Ybema JF, Buunk BP, et al. (2000) Active engagement, protective buffering, and overprotection: Three ways of giving support by intimate partners of patients with cancer. Journal of Social and Clinical Psychology 19: 256–275. [Google Scholar]
- Kwan ML, Cohn JC, Armer JM, et al. (2011) Exercise in patients with lymphedema: A systematic review of the contemporary literature. Journal of Cancer Survivorship 5: 320–336. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Rook KS. (1999) Social control in personal relationships: Impact on health behaviors and psychological distress. Health Psychology 18: 63–71. [DOI] [PubMed] [Google Scholar]
- Lowe SS. (2011) Physical activity and palliative cancer care. In: Courneya SK, Friedenreich MC. (eds) Physical Activity and Cancer. Berlin; Heidelberg: Springer, pp. 349–365. [DOI] [PubMed] [Google Scholar]
- Luszczynska A, Boehmer S, Knoll N, et al. (2007) Emotional support for men and women with cancer: Do patients receive what their partners provide? International Journal of Behavioral Medicine 14: 156–163. [DOI] [PubMed] [Google Scholar]
- Manne SL, Ostroff J, Sherman M, et al. (2003) Buffering effects of family and friend support on associations between partner unsupportive behaviors and coping among women with breast cancer. Journal of Social and Personal Relationships 20: 771–792. [Google Scholar]
- Manne SL, Siegel S, Kashy D, et al. (2014) Cancer-specific relationship awareness, relationship communication, and intimacy among couples coping with early-stage breast cancer. Journal of Social and Personal Relationships 31: 314–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcell AV, Howard TL, Plowden K, et al. (2010) Exploring women’s perceptions about their role in supporting partners’ and sons’ reproductive health care. American Journal of Men’s Health 4: 297–304. [DOI] [PubMed] [Google Scholar]
- Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. (2015) Supervised exercise reduces cancer-related fatigue: A systematic review. Journal of Physiotherapy 61: 3–9. [DOI] [PubMed] [Google Scholar]
- Miller E, Wortman C. (2002) Gender differences in mortality and morbidity following a major stressor: The case of conjugal bereavement. In: Weidner G, Kopp MS, Kristenson M. (eds) Heart Disease: Environment, Stress, and Gender. Amsterdam: IOS Press, pp. 251–266. [Google Scholar]
- Mishra SI, Scherer RW, Geigle PM, et al. (2012a) Exercise interventions on health-related quality of life for cancer survivors. The Cochrane Library 15: CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SI, Scherer RW, Snyder C, et al. (2012b) Exercise interventions on health-related quality of life for people with cancer during active treatment. The Cochrane Library 15: CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff LA, Karney BR. (2005) Gender differences in social support: A question of skill or responsiveness? Journal of Personality and Social Psychology 88: 79–90. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Frierson GM, Rabin C, et al. (2005) Home-based physical activity intervention for breast cancer patients. Journal of Clinical Oncology 23: 3577–3587. [DOI] [PubMed] [Google Scholar]
- Quick BL, Stephenson MT. (2007) Further evidence that psychological reactance can be modeled as a combination of anger and negative cognitions. Communication Research 34: 255–276. [Google Scholar]
- Rief H, Welzel T, Omlor G, et al. (2014) Pain response of resistance training of the paravertebral musculature under radiotherapy in patients with spinal bone metastases—a randomized trial. BioMed Central Cancer 14: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LQ, Courneya KS, Robbins KT, et al. (2008) Physical activity correlates and barriers in head and neck cancer patients. Supportive Care in Cancer 16: 19–27. [DOI] [PubMed] [Google Scholar]
- Rogers LQ, Markwell S, Hopkins-Price P, et al. (2011) Reduced barriers mediated physical activity maintenance among breast cancer survivors. Journal of Sport and Exercise Psychology 33: 235–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook KS, August KJ, Stephens MAP, et al. (2011) When does spousal social control provoke negative reactions in the context of chronic illness? The pivotal role of patients’ expectations. Journal of Social and Personal Relationships 28: 772–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF, Grossman RM, Pinski RB, et al. (1987) The development of scales to measure social support for diet and exercise behaviors. Preventive Medicine 16: 825–836. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, et al. (1985) Physical activity assessment methodology in the Five-City Project. American Journal of Epidemiology 121: 91–106. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Sarkin J, Campbell J, et al. (1997) Seven-day physical activity recall. Medicine & Science in Sports & Exercise 29: 89–103. [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, et al. (2010) American college of sports medicine roundtable on exercise guidelines for cancer survivors. Medicine & Science in Sports & Exercise 42: 1409–1426. [DOI] [PubMed] [Google Scholar]
- Seemann EA, Buboltz WC, Jenkins SM, et al. (2004) Ethnic and gender differences in psychological reactance: The importance of reactance in multicultural counselling. Counselling Psychology Quarterly 17: 167–176. [Google Scholar]
- Skoyen JA, Blank E, Corkery SA, et al. (2013) The interplay of partner influence and individual values predicts daily fluctuations in eating and physical activity. Journal of Social and Personal Relationships 30: 1000–1019. [Google Scholar]
- Speck RM, Courneya KS, Mâsse LC, et al. (2010) An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Journal of Cancer Survivorship 4: 87–100. [DOI] [PubMed] [Google Scholar]
- Speed-Andrews AE, Rhodes RE, Blanchard CM, et al. (2012) Medical, demographic and social cognitive correlates of physical activity in a population-based sample of colorectal cancer survivors. European Journal of Cancer Care 21: 187–196. [DOI] [PubMed] [Google Scholar]
- Stephens MAP, Fekete EM, Franks MM, et al. (2009) Spouses’ use of pressure and persuasion to promote osteoarthritis patients’ medical adherence after orthopedic surgery. Health Psychology 28: 48–55. [DOI] [PubMed] [Google Scholar]
- Stephens MAP, Rook KS, Franks MM, et al. (2010) Spouses use of social control to improve diabetic patients’ dietary adherence. Families, Systems, & Health 28: 199–208. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Lewis MA, Sterba KR. (2008) Reactions to health-related social control in young adults with type 1 diabetes. Journal of Behavioral Medicine 31: 93–103. [DOI] [PubMed] [Google Scholar]
- Traut-Mattausch E, Jonas E, Förg M, et al. (2008) How should politicians justify reforms to avoid psychological reactance, negative attitudes, and financial dishonesty? Zeitschrift für Psychologie/Journal of Psychology 216: 218–225. [Google Scholar]
- Trief PM, Sandberg J, Greenberg RP, et al. (2003) Describing support: A qualitative study of couples living with diabetes. Families, Systems, & Health 21: 57–67. [Google Scholar]
- Tucker JS, Anders SL. (2001) Social control of health behaviors in marriage. Journal of Applied Social Psychology 31: 467–485. [Google Scholar]
- Tucker JS, Orlando M, Elliott MN, et al. (2006) Affective and behavioral responses to health-related social control. Health Psychology 25: 715–722. [DOI] [PubMed] [Google Scholar]
- Uchino BN. (2006) Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine 29: 377–387. [DOI] [PubMed] [Google Scholar]
- Ungar N, Sieverding M, Stadnitski T. (2013) Increasing fruit and vegetable intake. “Five a day” versus “just one more.” Appetite 65: 200–204. [DOI] [PubMed] [Google Scholar]
- Ungar N, Sieverding M, Schweizer F, et al. (2015) Intervention-elicited reactance and its implications: Let me eat what I want. Zeitschrift für Psychologie 223: 247–256. [Google Scholar]
- Van Dyck D, Cardon G, Deforche B, et al. (2011) Environmental and psychosocial correlates of accelerometer-assessed and self-reported physical activity in Belgian adults. International Journal of Behavioral Medicine 18: 235–245. [DOI] [PubMed] [Google Scholar]
- Van Waart H, Stuiver MM, Van Harten WH, et al. (2015) Effect of low-intensity physical activity and moderate-to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. Journal of Clinical Oncology 33: 1918–1927. [DOI] [PubMed] [Google Scholar]
- Wesley KM, Zelikovsky N, Schwartz LA. (2013) Physical symptoms, perceived social support, and affect in adolescents with cancer. Journal of Psychosocial Oncology 31: 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller KM, Buboltz WC, Loveland JM. (2007) Psychological reactance: Examination across age, ethnicity, and gender. The American Journal of Psychology 15–24. [PubMed] [Google Scholar]
- Zhu SH, Nguyen QB, Cummins S, et al. (2006) Non-smokers seeking help for smokers: A preliminary study. Tobacco Control 15: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]