Abstract
The objective is to assess if longer life in Belgium is associated with more healthy years through the evaluation of trends (1997–2004) in health expectancy indicators at ages 65 and 80 covering different health domains: self-perceived health, chronic morbidity, disease clusters, and disability. Information was obtained from Belgian Health Interview Surveys. Health expectancies were calculated using the Sullivan method. Among males at age 65, the increase in years expected to live without chronic morbidity, without a disease cluster or without disability exceeded the increase of the life expectancy (LE). The rise in LE in good self-perceived health was equal to the gain in LE. Among women at age 65 and among men and women at age 80, none of the changes in the expected years of life in good health in any health domain were statistically significant. At age 65 among women, the increase in LE was smaller than the increase in years without chronic disease or without disability. The increase in years without disease clusters was less that the LE increase. At age 80 among men, the years without disability increased as the LE, with a shift toward years with moderate limitations. In any other health domains for men (except co-morbidity) and in all domains for women the years in good health either decreased or increased less than the LE. The recent rise in life expectancy in Belgium is, among the youngest old and especially among males, accompanied by an improved health status. At age 80 and particularly among women expansion of unhealthy years prevails.
Keywords: Life expectancy, Health expectancy, Disability free life expectancy, Belgium
Introduction
Reports on health trends in the oldest sector of populations in developed countries do not show a consistent pattern. The discussion on the consequences for society of both increasing life expectancy and the reduction in mortality of older people is linked to uncertainty about the future burden of morbidity. The burden of morbidity is driven by a complex interaction between on the one side the age distribution of the incidence rates and on the other side the prevalence, especially the severity of the prevalent morbid status and the outflow rate either through mortality or through cure. If the onset of health problems, morbidity or disability, is postponed and the postponement is greater than the increase in life expectancy the cumulative life time with morbidity will be less as is suggested by the compression of morbidity model (Fries 1980, 2003). The expansion of Morbidity (Gruenberg 1977) is the pessimistic opposite model assuming that the growing life expectancy is a result of a better survival of the sick rather than a decline of morbidity incidence inducing an increase in the years lived in poor health. A third scenario is the dynamic equilibrium, in which the increase in morbidity is counterbalanced by a decrease in the severity, as proposed by Manton (1982).
In light of the coexistence of opposite trends (Robine et al. 2003) it has been suggested that the different paradigms on the evolution of population health can be reconciled as part of a single, more general theory as they are different stages of a same epidemiologic transition, the long-term shift in mortality and disease patterns toward an era of delayed degenerative diseases (Olshansky and Ault 1986; Robine and Michel 2004).
Through simulation it has been shown that interventions with a similar improvement of the prevalence of morbidity (indicator of successful ageing), but with different improvements of the mortality rates, are associated with different gains in the total life expectancy and different changes in the years lived with or without morbidity (Nusselder and Peeters 2006). Therefore, to better understand the evolution of health and especially to be able to distinguish between compression, expansion and a dynamic equilibrium, composite measures combining morbidity and mortality information are necessary.
The objective of this paper is to assess trends in health expectancy indicators in Belgium between 1997 and 2004 covering different health domains: (1) self-perceived health, (2) chronic morbidity, (3) co-morbidity and (4) disability. The four health domains cover together largely the multiple facets of the disablement process (Verbrugge and Jette 1994) and different aspects of successful ageing. The health expectancy indicators—life expectancy in good self-perceived health, life expectancy without chronic morbidity, life expectancy without a disease cluster, with at least one disease cluster or with co-morbidity and life expectancy without disability, with moderate and with severe disability—are evaluated by gender at age 65 and at age 80 years. Evaluating the interaction of mortality with four different health domains provides a more holistic picture to understand population health dynamics and to capture the multidimensionality of health (Myers et al. 2003). Self-perceived health is a very large concept including many aspects of quality of life. The indicator overlaps partially, but not completely, with the other health indicators because it can include many health states not covered by other health indicators such as non-diagnosed sickness or depressive states. Self-assessed health on population level is a simple single item question, easy to use and to interpret. Poor self-perceived health is also a good predictor of mortality and hospitalisation (DeSalvo et al. 2006). The subjective evaluation of suffering from chronic conditions is not identical to a more objective assessment of the presence or absence of disease clusters in the past 12 months. In particular the latter indicator focuses on the presence of at least two disease clusters as a measure of severity compared to one or none. Both indicators are of major importance to estimate the mobilisation of the health care sector. They may or may not result in functional limitations. The disability question on restrictions in daily activities due to these chronic conditions captures this outcome in the most direct and relevant way regarding long term care. The inclusion of a severity level provides additional information, for example, even when there is a compression, there may be a change in the distribution of the severity of the disability toward the more severe levels. Information on changes in the distribution of the severity levels between unhealthy states may not only contribute to better understand the evolution in population health but is also essential for public health policy and health care planning. The focus of this paper on the older ages is indicated as they are the subgroup of the population with the most explicit decline in mortality rates and with a greater sensitivity to changes in morbidity.
Methods
Life expectancy in a particular health state, for example, life expectancy without disability or life expectancy with disability, is defined as the average number of years a person at a certain age is expected to live in the particular health state. To calculate these health expectancies, two types of data are needed. Data on mortality enable estimation of the total life expectancy. Data on prevalence of different health states are applied to the life table to estimate the person time lived in the different health states.
Data on mortality for the years 1997, 2001 and 2004 were obtained from the Belgian National Institute of Statistics. The information was provided in the form of 1 year-life tables starting at age 0 with a last closed year at age 105 years. The life tables were constructed by gender. The life table was transformed to an abridged table with 5 year age intervals and a last interval starting at age 100. The variance of life expectancy and the 95% confidence intervals (CI) were estimated (Chiang 1984).
Information by age and gender on health status was obtained from the 1997, 2001, and 2004 Health Interview Survey.1 The methodological approach of the survey did not change between the different surveys. In 2004 there was an oversampling of population 65–84 years and 85 years and older. The National Register was used as the sampling frame to select a sample of the Belgian population using a multistage sampling frame (Van Oyen et al. 1997). The number of subjects aged 65 years and over in the three surveys was 1,787, 2,153, and 3,594, respectively. The population residing in nursing homes is more difficult to reach. In the 2004 survey, specific efforts were made to reach this population. This could affect the prevalence estimates. Therefore the analyses were done including and excluding the nursing home population [n = 26 (1997), n = 31 (2001) and n = 345 (2004)]. Only the results of the analysis on the total population are presented as exclusion of the nursing home population did not alter the conclusions.
Health domains
Self-perceived health was defined by comparing those indicating their health as very good or good (self-perceived good health), to those who experienced their health as very bad, bad or fair (self-perceived bad health) when answering the standard question “How is your health in general?” (de Bruin et al. 1996).
The prevalence of chronic morbidity was assessed using the following question:
“Do you suffer from one or more longstanding diseases, chronic conditions or handicaps?”. Yes/no.
The reply to this question was also used together with the following question to define disability:
“Are you restricted in your daily activities due to this (these) illness(es), chronic condition(s) or handicaps?”. Continuously, every now and then, seldom or not.
Those answering ‘continuously’ were defined to be severely limited; those answering ‘every now and then’ were defined to be moderately limited. Subjects replying “not or seldom” or those who were without any longstanding morbidity were defined to be without limitations.
Interviewees were asked if they had a specific condition during the 12 months prior to the interview. The conditions were grouped in six clusters of major chronic conditions (Picavet and van den Bos 1997): heart disease (myocardial infarction and severe heart problem), respiratory disease (asthma, chronic bronchitis, chronic obstructive pulmonary disease, other longstanding lung disease), musculoskeletal disease (longstanding (longer than 3 months) back pain, disc problem, osteoarthritis knees, hips, hands, rheumatoid arthritis of hands or feet, other form of chronic inflammation of joints), cancer, neurological disease (epilepsy, dizziness with falling, stroke) and diabetes. Having two or more disease clusters was defined as co-morbidity compared to having one disease cluster and no disease cluster.
The prevalence data were used to estimate different health expectancy indicators (Table 1). All health expectancies were calculated using the Sullivan method (Jagger 1997; Sullivan 1971). The part of the variance due to the mortality data was ignored when estimating the variance of the health expectancies (Mathers 1991). To estimate the yearly change, the life and health expectancy were regressed on year using a weighted linear regression for each indicator separately. The regressions at age 65 and age 80 were done stratified by gender. Weights were defined as the inverse of the variance of the estimate of each indicator at each year (Mathers and Robine 1997). Furthermore, stratified by age, the likelihood ratio test was used to compare two nested models containing gender and year (model 1) versus gender, year and an interaction term “gender*year” (model 2) in order to identify a possible differential time trend in the life and health expectancies by gender. Given there were only three observations points over time, it is not appropriate to assume a more complex than simple linear relationship between life expectancy or health expectancy (dependent variable) and calendar time.
Table 1.
Health expectancy indicators
| Domain | Health status | Health expectancy indicator | Abbreviation |
|---|---|---|---|
| Self-perceived health | Good | Life expectancy (LE) in Good self-perceived Health | HLE |
| Bad | LE in bad self-perceived Health | BHLE | |
| Chronic morbidity | No | Chronic morbidity free LE | MFLE |
| Yes | LE With chronic morbidity | MLE | |
| Co-morbidity | No disease clustera | Disease cluster free LE | DisFLE |
| One disease cluster | LE with one disease cluster | DisLE | |
| 2–6 disease clusters | LE with Co-morbidity | ComLE | |
| Disability | No | Disability free LE | DFLE |
| Moderate | LE with moderate disability | MDLE | |
| Severe | LE with severe disability | SDLE |
aDisease cluster: heart disease (myocardial infarction and severe heart problem), respiratory disease (asthma, chronic bronchitis, chronic obstructive pulmonary disease, other longstanding lung disease), musculoskeletal disease (longstanding (longer than 3 months) back pain, disc problem, osteoarthritis knees, hips, hands, rheumatoid arthritis of hands or feet, other form of chronic inflammation of joints), cancer, neurological disease (epilepsy, dizziness with falling, stroke) and diabetes
The operationalisation of the relationship between morbidity, mortality and population health in terms of one of the three scenarios (compression, expansion and dynamic equilibrium) is complex, especially as initially the compression of morbidity hypothesis was defined under the assumption of fixed length of human life (Fries 1980). It is only under this condition that an increase in the life expectancy in a good health state automatically implies a decrease in the expected life years in ill health. This operationalisation has been the focus of many discussion in REVES (the International Network on Health Expectancy) (Robine et al. 1987; Robine 2002; Robine and Mathers 1993). Only evaluating the positive health expectancy indicator may give a too optimistic assessment of the changes in population health as e.g. an increase in life expectancy and in life expectancy in good health may be accompanied by a constant, an increasing or decreasing life expectancy in ill health. Therefore it is necessary to consider the ‘complete’ health expectancy including both ‘healthy’ and different levels of ‘unhealthy’ states (Mathers et al. 1994). In this paper the classification proposed by Nusselder (1998, 2003) is used. The classification distinguishes between absolute and relative compression or expansion is used. The relative measure or the change in the percentage of life time in a healthy state provides information on the distribution between the years in healthy and in unhealthy states in the gained life years as compared to the baseline situation. As life expectancy is increasing in Belgium, the classification used has been limited to this particular case (Table 2). There will be evidence for the dynamic equilibrium theory in case of expansion together with a shift from more to less severe unhealthy life years (Robine et al. 2003). The qualitative description of the direction of the change in distribution between different levels of severity of unhealthy states is therefore also provided.
Table 2.
Classification of compression and expansion of morbidity(Nusselder 1998)
| Life expecancy (LE) | Healthy LE | Unhealthy LE | % Healthy LE in LE | Classification | |
|---|---|---|---|---|---|
| Absolute | Relative | ||||
| ↑ | ↑ | ↓ | ↑ | Compression | Compression |
| ↑ | ↑ | = | ↑ | Equilibrium compression—expansion | Compression |
| ↑ | ↑ | ↑ | ↑ | Expansion | Compression |
| ↑ | ↑ | ↑ | = | Expansion | Equilibrium compression—expansion |
| ↑ | ↑ | ↑ | ↓ | Expansion | Expansion |
| ↑ | = | ↑ | ↓ | Expansion | Expansion |
| ↑ | ↓ | ↑ | ↓ | Expansion | Expansion |
Results
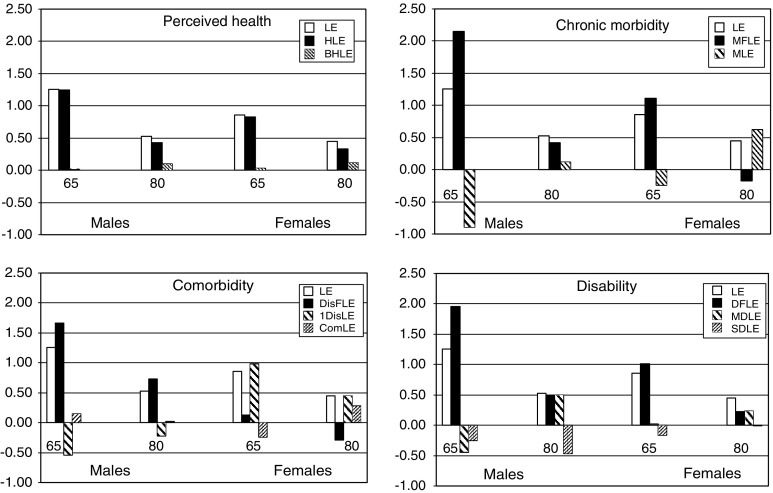
The results are presented stratified by gender at age 65 and at age 80. In Tables 3 and 4, for males and females, respectively, all estimates (with 95% confidence intervals) for the three survey years and for each of the health expectancy indicators are given together with an estimate of a yearly change (with P value). Figure 1 shows the absolute difference between the first and last year and Table 5 summarizes the results following the classification given in Table 2.
Table 3.
Total life expectancy (LE), life expectancy in good self-perceived health (HLE), life expectancy without chronic morbidity (MFLE), life expectancy without a disease clusterc (DisFLE), with at least one disease cluster (DisLE) or with co-morbidity (ComLE) and life expectancy without disability (DFLE), with moderate (MDLE) and with severe (SDLE) disability among males at age 65 and age 80, Belgium, 1997–2004
| At age | 1997 | 2001 | 2004 | Yearly changea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Year | % of LE | Year | % of LE | Year | % of LE | Coefficient | P-value | ||
| 65 | LE | 15.53 (15.51–15.55)b | 16.24 (16.22–16.26) | 16.79 (16.77–16.81) | 0.167 | 0.006 | |||
| HLE | 8.57 (7.98–9.16) | 55.16 | 8.68 (8.13–9.22) | 53.42 | 9.82 (9.34–10.29) | 58.46 | 0.179 | 0.022 | |
| MFLE | 6.83 (6.27–7.39) | 44.00 | 7.48 (6.96–8.00) | 46.07 | 8.98 (8.54–9.42) | 53.50 | 0.296 | 0.008 | |
| DisFLE | 4.89 (4.37–5.41) | 31.48 | 5.69 (5.18–6.20) | 35.02 | 6.55 (6.11–6.99) | 39.01 | 0.223 | 0.003 | |
| DisLE | 6.50 (5.94–7.06) | 41.31 | 6.11 (5.61–6.61) | 20.86 | 5.95 (5.52–6.39) | 35.31 | −0.070 | 0.241 | |
| ComLE | 4.14 (3.64–4.64) | 26.66 | 4.44 (3.97–4.91) | 27.35 | 4.29 (3.89–4.68) | 25.52 | 0.021 | 0.625 | |
| DFLE | 9.00 (8.44–9.56) | 57.98 | 9.69 (9.18–10.21) | 59.68 | 10.96 (10.54–11.38) | 65.28 | 0.289 | 0.011 | |
| MDLE | 2.69 (2.28–3.10) | 17.32 | 2.33 (1.96–2.70) | 14.33 | 2.25 (1.94–2.55) | 13.38 | −0.070 | 0.034 | |
| SDLE | 3.84 (3.35–4.33) | 24.70 | 4.22 (3.76–4.69) | 26.00 | 3.58 (3.22–3.94) | 21.34 | −0.041 | 0.457 | |
| 80 | LE | 6.76 (6.75–6.77) | 7.05 (7.04–7.06) | 7.29 (7.28–7.30) | 0.079 | 0.007 | |||
| HLE | 3.38 (2.73–4.04) | 50.04 | 2.68 (2.10–3.26) | 38.02 | 3.82 (3.41–4.22) | 52.35 | 0.043 | 0.596 | |
| MFLE | 3.00 (2.39–3.61) | 44.34 | 2.30 (1.80–2.80) | 32.64 | 3.41 (3.06–3.77) | 46.83 | 0.091 | 0.191 | |
| DisFLE | 1.76 (1.23–2.29) | 26.04 | 2.19 (1.68–2.71) | 31.12 | 2.49 (2.14–2.83) | 34.15 | 0.084 | 0.062 | |
| DisLE | 2.79 (2.19–3.40) | 41.31 | 1.47 (1.02–1.92) | 20.86 | 2.57 (2.22–2.92) | 35.31 | −0.004 | 0.954 | |
| ComLE | 2.21 (1.63–2.78) | 32.65 | 3.39 (2.83–3.94) | 48.02 | 2.23 (1.89–2.56) | 30.54 | 0.018 | 0.782 | |
| DFLE | 3.59 (2.97–4.20) | 53.05 | 3.24 (2.70–3.78) | 45.96 | 4.09 (3.73–4.44) | 56.05 | 0.097 | 0.116 | |
| MDLE | 0.59 (0.24–0.94) | 8.77 | 1.09 (0.71–1.48) | 15.53 | 1.09 (0.82–1.35) | 14.89 | 0.064 | 0.025 | |
| SDLE | 2.58 (1.99–3.18) | 38.18 | 2.71 (2.19–3.24) | 38.51 | 2.12 (1.80–2.44) | 29.06 | −0.071 | 0.050 | |
aWeighted least square regression
b95% Confidence interval
cDisease cluster: heart disease (myocardial infarction and severe heart problem), respiratory disease (asthma, chronic bronchitis, chronic obstructive pulmonary disease, other longstanding lung disease), musculoskeletal disease (longstanding (longer than 3 months) back pain, disc problem, osteoarthritis knees, hips, hands, rheumatoid arthritis of hands or feet, other form of chronic inflammation of joints), cancer, neurological disease (epilepsy, dizziness with falling, stroke) and diabetes
Table 4.
Total life expectancy (LE), life expectancy in good self-perceived health (HLE), life expectancy without chronic morbidity (MFLE), life expectancy without a disease clusterc (DisFLE), with at least one disease cluster (DisLE) or with co-morbidity (ComLE) and life expectancy without disability (DFLE), with moderate (MDLE) and with severe (SDLE) disability among females at age 65 and age 80, Belgium, 1997–2004
| At age | 1997 | 2001 | 2004 | Yearly changea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Year | % of LE | Year | % of LE | Year | % of LE | Coefficient | P-value | ||
| 65 | LE | 19.80 (19.78–19.82)b | 20.23 (20.21–20.25) | 20.66 (20.64–20.68) | 0.107 | 0.022 | |||
| HLE | 9.51 (8.84–10.18) | 48.03 | 9.61 (8.97–10.26) | 47.51 | 10.34 (9.82–10.85) | 50.04 | 0.105 | 0.300 | |
| MFLE | 9.78 (9.16–10.40) | 49.38 | 9.23 (8.64–9.82) | 45.63 | 10.88 (10.43–11.34) | 52.67 | 0.151 | 0.263 | |
| DisFLE | 6.37 (5.78–6.95) | 32.16 | 5.58 (5.05–6.11) | 27.58 | 6.50 (6.06–6.93) | 31.44 | 0.071 | 0.432 | |
| DisLE | 7.72 (7.11–8.33) | 39.00 | 9.08 (8.48–9.68) | 44.89 | 8.70 (8.24–9.17) | 42.12 | 0.097 | 0.131 | |
| ComLE | 5.71 (5.14–6.28) | 28.83 | 5.57 (5.02–6.11) | 27.53 | 5.46 (5.06–5.87) | 26.44 | −0.031 | 0.539 | |
| DFLE | 11.70 (11.09–12.30) | 59.08 | 11.07 (10.48–11.66) | 54.73 | 12.71 (12.26–13.15) | 61.50 | 0.129 | 0.353 | |
| MDLE | 2.60 (2.19–3.01) | 13.14 | 3.14 (2.71–3.57) | 15.53 | 2.62 (2.31–2.94) | 12.70 | 0.007 | 0.844 | |
| SDLE | 5.50 (4.95–6.06) | 27.78 | 6.02 (5.46–6.57) | 29.74 | 5.33 (4.94–5.72) | 25.81 | −0.016 | 0.857 | |
| 80 | LE | 8.76 (8.75–8.77) | 9.00 (8.99–9.01) | 9.21 (9.20–9.22) | 0.058 | 0.077 | |||
| HLE | 3.66 (3.04–4.28) | 41.80 | 3.94 (3.32–4.57) | 43.80 | 3.99 (3.61–4.37) | 43.32 | −0.006 | 0.936 | |
| MFLE | 4.27 (3.72–4.81) | 48.72 | 3.60 (3.06–4.14) | 40.03 | 4.09 (3.79–4.40) | 44.43 | −0.018 | 0.741 | |
| DisFLE | 2.61 (2.10–3.13) | 29.85 | 1.90 (1.44–2.35) | 21.09 | 2.33 (2.06–2.60) | 25.28 | −0.005 | 0.915 | |
| DisLE | 3.36 (2.81–3.91) | 38.39 | 4.06 (3.50–4.62) | 45.12 | 3.81 (3.50–4.12) | 41.41 | 0.032 | 0.382 | |
| ComLE | 2.78 (2.27–3.30) | 31.76 | 3.04 (2.51–3.57) | 33.79 | 3.07 (2.77–3.36) | 33.31 | 0.052 | 0.082 | |
| DFLE | 4.62 (4.09–5.16) | 52.78 | 4.42 (3.87–4.98) | 49.13 | 4.85 (4.54–5.16) | 52.62 | 0.027 | 0.576 | |
| MDLE | 0.65 (0.36–0.94) | 7.45 | 1.21 (0.83–1.59) | 13.47 | 0.89 (0.70–1.08) | 9.64 | 0.024 | 0.278 | |
| SDLE | 3.48 (2.95–4.02) | 39.78 | 3.37 (2.83–3.91) | 37.40 | 3.48 (3.18–3.77) | 37.73 | 0.014 | 0.728 | |
aWeighted least square regression
b95% Confidence interval
cDisease cluster: heart disease (myocardial infarction and severe heart problem), respiratory disease (asthma, chronic bronchitis, chronic obstructive pulmonary disease, other longstanding lung disease), musculoskeletal disease (longstanding (longer than 3 months) back pain, disc problem, osteoarthritis knees, hips, hands, rheumatoid arthritis of hands or feet, other form of chronic inflammation of joints), cancer, neurological disease (epilepsy, dizziness with falling, stroke) and diabetes
Fig. 1.
Change in total life expectancy (LE), health expectancy indicators based on self-perceived health (good: HLE, bad: BHLE), chronic morbidity (no: MFLE, yes: MLE), co-morbiditya (no: DisFLE, 1: DisLE, 2+: ComLE) and disability (no: DFLE, moderate: MDLE, severe: SDLE) at age 65 and age 85, Belgium, 1997–2004. aDisease cluster: heart disease (myocardial infarction and severe heart problem), respiratory disease (asthma, chronic bronchitis, chronic obstructive pulmonary disease, other longstanding lung disease), musculoskeletal disease (longstanding (longer than 3 months) back pain, disc problem, osteoarthritis knees, hips, hands, rheumatoid arthritis of hands or feet, other form of chronic inflammation of joints), cancer, neurological disease (epilepsy, dizziness with falling, stroke) and diabetes
Table 5.
Overview table of the absolute and relative evolution in population health in Belgium between 1997 and 2004 based on the complete health expectancy indicators in the domain of the self-perceived health, chronic morbidity, co-morbiditya and disability at age 65 years and at age 80 following the classification by Nusselder(Nusselder 1998)
| Gender | At age | Domain | Absolute Evolution | Relative Evolution | Shift between severity levels |
|---|---|---|---|---|---|
| Males | 65 | Self-perceived health | Equilibrium Compression-Expansion | Compression | |
| Chronic morbidity | Compression | Compression | |||
| Co-morbidity | Compression | Compression | Shift to severe level | ||
| Disability | Compression | Compression | Shift to severe level | ||
| 80 | Self-perceived health | Expansion | Compression | ||
| Chronic morbidity | Expansion | Compression | |||
| Co-morbidity | Compression | Compression | Shift to severe level | ||
| Disability | Equilibrium Compression-Expansion | Compression | Shift to moderate level | ||
| Females | 65 | Self-perceived health | Equilibrium Compression-Expansion | Compression | |
| Chronic morbidity | Compression | Compression | |||
| Co-morbidity | Expansion | Expansion | Shift to moderate level | ||
| Disability | Compression | Compression | Shift to moderate level | ||
| 80 | Self-perceived health | Expansion | Compression | ||
| Chronic morbidity | Expansion | Expansion | |||
| Co-morbidity | Expansion | Expansion | Shift to moderate level | ||
| Disability | Expansion | Expansion | Shift to moderate level |
aCo-morbidity: based on disease cluster: heart disease (myocardial infarction and severe heart problem), respiratory disease (asthma, chronic bronchitis, chronic obstructive pulmonary disease, other longstanding lung disease), musculoskeletal disease (longstanding (longer than 3 months) back pain, disc problem, osteoarthritis knees, hips, hands, rheumatoid arthritis of hands or feet, other form of chronic inflammation of joints), cancer, neurological disease (epilepsy, dizziness with falling, stroke) and diabetes
Males at age 65 years
Between 1997 and 2004, the total life expectancy (LE) for males at age 65 increased by 1.26 years (Table 3; Fig. 1). For all health domains, with the exception of self-perceived health, the increase in the health expectancy indicator was greater than the life expectancy gain, suggesting an absolute compression (Tables 2, 5). For self-perceived health there is an equilibrium between the absolute compression and absolute expansion as the life expectancy in good self-perceived health increased in the same way as the life expectancy and there is no increase in the expected years in bad self-perceived health. In 2004, the proportion of remaining life in good health for each of the 4 domains had all increased with at least 3% since 1997, indicating a relative compression (Tables 2, 5). For the co-morbidity and disability domain the data show a shift towards a more severe level of ill health. The life expectancy with one disease cluster decreased whereas the life expectancy with co-morbidity increased a little. The life expectancy with both moderate and severe disability decreased but the decrease was more pronounced for the moderate disability life expectancy. The yearly change during the period 1997–2004 was statistically significant for the increase in LE and all health expectancy indicators but the LE with one disease cluster, the LE with co-morbidity and LE with severe disability.
Males at age 80
The increase in the LE was 0.53 years (Table 3; Fig 1). There was an absolute and a relative compression for the co-morbidity domain only: (1) the increase in a health expectancy indicator was greater than the increase in LE and (2) the proportion of life without a chronic disease cluster increased substantially (Tables 2, 5). The LE without disability increased in the same way as the LE and the proportion of life without disability increased. For the other health domains there was an absolute expansion and relative compression. However, none of the yearly changes in the years of life in good health were statistically significant. The evolution of the distribution of the severity levels of ill-health within the years expected to live in ill health at age 80 indicates a shift towards a moderate level for the disability years (Table 5). There was a statistically significant increase and decrease, respectively in the years with moderate and severe disability. This is compatible with the dynamic equilibrium model. An opposite but non significant shift was observed between the years of life with one disease clusters and years with co-morbidity.
Females at age 65
The LE increased by 0.86 years (Table 4; Fig. 1). The increase in LE was smaller than the increase in the health expectancy indicator for the chronic morbidity and disability domain, indicating an absolute and relative compression (Tables 2, 5). In the domain of self-perceived health there was an equilibrium between an absolute compression and an absolute expansion as the increase in LE in good self-perceived health almost matched the rise in LE. The rise in the LE without a disease cluster was substantially lower suggesting an absolute and relative expansion. None of the yearly changes in any of the expected years in good health were statistically significant. For both LE with disease clusters and the LE expectancy with disability there was a tendency for a shift toward the moderate unhealthy stage.
Females at age 80
The LE increased with 0.41 years (Table 4; Fig. 1). For all health domains the increase in the health expectancy indicator was smaller than the life expectancy gain suggesting an absolute expansion. For all health domains, except the self-perceived health, the proportion of remaining life in good health dropped indicating a relative expansion. None of the yearly changes in the health expectancy indicators were statistically significant. For both LE with disease clusters and the LE expectancy with disability there was a tendency of a shift toward the moderate unhealthy stage.
Based on the weighted linear regression, there was no evidence that the trends in the health expectancy indicators were statistically significantly different by gender at any age.
Discussion
In this paper we focused on the evolution of population health to gain more insight on the question if longer life is associated, both absolutely and relatively, with an increase in years in good health or years in ill health. We evaluated the interaction of mortality with four different health domains, taking into account a level of severity for two of them, to capture the multidimensionality of health and to cover the multiple facets of the disablement process and different aspects of successful ageing (Myers et al. 2003; Verbrugge and Jette 1994). The relationship between the four measures is complex, for example, although poor self-perceived health is a good predictor of future health, data from the 2001 Belgian Census show that 2.3 and 10%, respectively of the subjects at age 55 with a very good and a good/very good self-perceived health declare to suffer from a chronic morbidity; at age 90, these percentages were 10 and 20%, respectively (Van Oyen et al. 2005). Similarly, it is evident from the different health models (Nagi 1991; Verbrugge and Jette 1994; WHO 2001) that the progression of the disablement process or the relationship between morbidity, function limitations and activity restrictions is not linear and is influenced by many intra-individual and extra-individual factors such as the physical environment and social role expectations. Disability is not an unitary concept and progresses in a non-linear way with increasing age (Nusselder et al. 2006).
As health is multidimensional, we did not expect that the observed trend in the different health domains should be uniform (Crimmins 2004) (Table 5). The evolution of population health is determined by different phenomena taking place with opposite effects. Declining mortality tends to increase the duration and the proportion of life in ill-health. This is because declining mortality among the healthy brings more people into ages where the probability of unhealthy outcomes is high and because the life of the unhealthy is extended. In such a situation substantial improvement in incidence and cure rates are needed to achieve an absolute compression. Simulation models have shown that in order to obtain compression the effect of reducing the incidence is larger than increasing recovering rates and that the improvement in the incidence and recovery rates have to be more substantial for a same change in mortality at older ages due to the higher concentration of ill health (Nusselder 1998). These processes may in part explain the observed difference in the evolution in the population health at age 65 and at age 80. Furthermore, the initially lower mortality rates in women and the fact that more women reach older ages can also explain in part the observed gender differences.
It is for the first time that health expectancy indicators in Belgium can be estimated for such a long period based on large national samples.
The sampling methodology, the fieldwork organisation and the participation rate did not change for the three studies. Over the study period, both the proportion of the Belgian population, 65 years and older in nursing homes (6.1% in 1997 and 6.6% in 2004) and receiving formal long-term care at home (6.6% in 1998 to 7.5% in 2004) increased slightly. In the 2004 survey; however, there was an oversampling of the 65–74 years and 85+ years population and the interviewers were specifically trained to reach selected subjects living in nursing homes. The data presented in the paper concerns the total study population, which could downgrade possible positive evolutions. However, exclusion of the nursing homes population did not affect the results to change the conclusion, probably because of a selection towards institutionalized subjects who were able to participate to the survey.
The period of observation (1997–2004) remains rather short compared to time series available in neighboring countries. This is, together with the fact that the evolution is only measured at three points in time, an important limitation. With only three observation points, the linear trend estimate of yearly change is not the most appropriate when there is a difference in the evolution in the 1997–2001 and the 2001–2004 periods. We should therefore, independently of the statistical significance, be careful in interpreting the observed trends. The lack of a consistent evolution of the different health domains over the two periods is explicit for males at age 80 years and for females at both age 65 and age 80 years and should caution inference about future evolution.
Data that enable international comparison are rare. The period considered and the health status definitions are often different and the estimates are not given at older ages. Recent publications suggest evidence for compression of disability at older ages during the last two decades with a decline in both mild and severe disability in the US (Manton et al. 2006). Although for several EU countries trends have been published (see, e.g., Bronnum-Hansen 2005; Doblhammer and Kytir 2001; Perenboom 2004; Perenboom et al. 2004), it is sufficient for the purpose of the discussion to focus on the European Community Household Panel (ECHP) which allows to estimate the disability free life expectancy (without severity level) between 1995 and 2001 for 12 EU member states (Robine et al. 2005).2 Similar to current observations described in this paper, the ECHP data for Belgium provides evidence for compression at age 65. When comparing the evolution in the 12 EU member states, there appear to be gender differences and three different trend patterns emerge: there are countries with evidence for compression (among males: Austria, Belgium, Finland, Greece, Germany, Italy, Spain; among females: Belgium, Italy, Sweden), there are countries with an equilibrium (among males: France; among females: Denmark, France, Spain) and there are countries with an expansion (among males: Denmark, Ireland, the Netherlands, Portugal, Sweden, UK; among females: Finland, Greece, Germany, Ireland, the Netherlands, Portugal, UK).
Although we used the published classification (Nusselder 1998) for the equilibrium when the health outcome is binary, evidence for the three health models can best be evaluated when a level of severity is taken into account. Under the assumption that survival with one or more disease clusters is gender independent, the observed compression in males and the observed dynamic equilibrium (absolute and relative expansion together with a shift to more moderate ill health) in females for the disease cluster domain could be explained by a delay in incidence in males without a delay in incidence in females. However, from a historical point of view women are ahead in the epidemiologic transition compared to men. The gains in life expectancy and changes in health among women are much more concentrated at the frontier of human life span, whereas men still have a large space for improvement both in life expectancy and in health.
Delay in incidence, more effective therapy and the greater availability of technical aids can be possible reasons for the observed evidence of the compression of the years with disability at age 65 and of the dynamic equilibrium theory in the disability domain at age 80. In particular, the drop (males, females at age 65) or status quo (females at age 80) in the years with severe disability corroborate the hypothesis (Manton 1982) and findings (Manton et al. 2006) that medical interventions and disease management are related to a joined increase in the life expectancy and the years with morbidity, but as a result of the medical interventions the progression of the chronic morbidity is reduced which induces a lower severity. Furthermore, through the delay in incidence, the effect of changing life styles such as the decline in smoking prevalence may, especially among males, be a contributing factor to the compression (Bronnum-Hansen and Juel 2001).
Conclusion
The recent rise in life expectancy in Belgium among the older adults is, in general and especially among the youngest old and among males, accompanied by an improved health status. The use of multiple health indicators for different age groups and gender among the older population demonstrates the differentiation in the ageing and disability process. Although there are some important limitations in our study (the short period with only three points of observations) there is evidence that increasing life expectancy does not necessarily result in expansion of morbidity. The gain in life expectancy under the age of 80 years is mainly translated into healthy years. At age 65 years and in particular among men the data support the compression of morbidity model. The gain in life expectancy after the age of 80 years is not in total a benefit towards more years in health. At age 80 and especially among women the expansion, both absolute and relative, is more prevailing. Future research agendas should include not only continuous monitoring through surveys but should also stimulate the use of available data to identify causes of transitions between health states. Population intervention studies should then be initiated on the determinants favoring compression.
Footnotes
References
- Bronnum-Hansen H. Health expectancy in Denmark 1987–2000. Eur J Public Health. 2005;15:20–25. doi: 10.1093/eurpub/cki106. [DOI] [PubMed] [Google Scholar]
- Bronnum-Hansen H, Juel K. Abstention from smoking extends life and compresses morbidity: a population based study of health expectancy among smokers and never smokers in Denmark. Tobacco Control. 2001;10:273–278. doi: 10.1136/tc.10.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CL. Statistical inference regarding life table functions. In: Chiang CL, editor. The life table and its applications. Malabar: Robert E Krieger Publishing Company; 1984. pp. 153–167. [Google Scholar]
- Crimmins EM. Trends in the health of the elderly. Annu Rev Public Health. 2004;25:79–88. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- de Bruin A, Picavet HS, Nosikov A. Health interview surveys: towards international harmonization of methods and instruments. Copenhagen: World Health Organisation; 1996. [PubMed] [Google Scholar]
- DeSalvo KB, Bloser N, Reynold K, He J, Muntner P. Mortality prediction with a single general self-rated health question A meta-analysis. J Gen Intern Med. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblhammer G, Kytir J. Compression or expansion of morbidity? Trends in healthy-life expectancy in the elderly Austrian population between 1978 and 1998. Soc Sci Med. 2001;52:385–391. doi: 10.1016/S0277-9536(00)00141-6. [DOI] [PubMed] [Google Scholar]
- Fries JF. Aging natural death and the compression of morbidity. New Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- Fries JF. Measuring and monitoring success in compression morbidity. Ann Intern Med. 2003;139:455–459. doi: 10.7326/0003-4819-139-5_part_2-200309021-00015. [DOI] [PubMed] [Google Scholar]
- Gruenberg E. The failure of success. Milbank Mem Fund Q Health Soc. 1977;45:3–24. doi: 10.2307/3349592. [DOI] [PubMed] [Google Scholar]
- Jagger C. Health expectancy calculation by the sullivan method: a practical guide. European Concerted Action on the Harmonization of Health Expectancy Calculations in Europe. Paris: Institut National d’ Etudes Démographiques; 1997. pp. 1–25. [Google Scholar]
- Manton KG. Changing concepts of morbidity and mortality in the elderly population. Milbank Mem Fund Q Health Soc. 1982;60:183–244. doi: 10.2307/3349767. [DOI] [PubMed] [Google Scholar]
- Manton KG, Gu X, Lamb V. Change in chronic disability from 1982 to 2004/5 as measured by long-term changes in function and health in the US elderly population. Proc Natl Acad Sci USA. 2006;103:18374–18379. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C. Health expectancies in Australia 1981 and 1988. Canberra: Australian Institute of Health, AGPS; 1991. [Google Scholar]
- Mathers CD, Robine JM. How good is Sullivan’s method for monitoring changes in population health expectancies. J Epidemiol Commun Health. 1997;51:80–86. doi: 10.1136/jech.51.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Robine JM, Wilkins R (1994) Health expectancy indicators: recommendations for terminology. In: Mathers CD, McCallum J, Robine JM (eds) Advances in health expectancies: proceedings of the 7th meeting of the international network on health expectancy (REVES). Australian Institute of Health and Welfare, AGPS, Canberra, pp 34–41
- Myers G, Lamb V, Agree E. Patterns of disability change associated with the epidemiologic transition. In: Robine jM, Jagger C, Mathers CD, Crimmins E, Suzman R., editors. Determining health expectancies. Chichester: Wiley; 2003. pp. 59–74. [Google Scholar]
- Nagi S. Disability concepts revisited: implications for prevention. In: Pope A, Tarlov A, editors. Disability in America: toward a national agenda for prevention. Washington: National Academy Press; 1991. pp. 307–327. [Google Scholar]
- Nusselder WJ (1998) Compression or expansion of morbidity? A life table approach. Erasmus University, Rotterdam, pp 1–253
- Nusselder WJ. Compression of morbidity. In: Robine JM, Jagger C, Mathers CD, Crimmins E, Suzman R, editors. Determining health expectancies. Chichester: Wiley; 2003. pp. 35–58. [Google Scholar]
- Nusselder WJ, Looman CWN, Mackenbach JP. The level and time course of disability: trajectories of disability in adults and young elderly. Disabil Rehabil. 2006;28:1015–1026. doi: 10.1080/09638280500493803. [DOI] [PubMed] [Google Scholar]
- Nusselder WJ, Peeters A. Successful aging: measuring the years lived with functional loss. J Epidemiol Community Health. 2006;60:448–455. doi: 10.1136/jech.2005.041558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Ault AB. The fourth stage of the epidemiologic transition: the age of delayed degenerative diseases. Milbank Q. 1986;64:355–391. doi: 10.2307/3350025. [DOI] [PubMed] [Google Scholar]
- Perenboom R. Health expectancies in the Netherlands. Amsterdam: University of Amsterdam; 2004. pp. 1–183. [Google Scholar]
- Perenboom R, van Herten L, Boshuizen H, van den Bos GAM. Trends in disability-free life expectancy. Disabil Rehabil. 2004;26:377–386. doi: 10.1080/0963828032000174098. [DOI] [PubMed] [Google Scholar]
- Picavet HS, van den Bos GA. The contribution of six chronic conditions to the total burden of mobility disability in the Dutch population. AJPH. 1997;87:1680–1682. doi: 10.2105/ajph.87.10.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine JM. A new health expectancy classification system. In: Murray CJL, Salomon JA, Mathers CD, Lopez AD, editors. Summary measures of population health. Concepts, ethics measurement and applications. Geneva: WHO; 2002. pp. 205–211. [Google Scholar]
- Robine JM, Brouard N, Colvez A. Les indicateurs d’espérance de vie sans incapacité (EVSI). Des indicateurs globaux de l’état de santé des populations. Rev Epidémiol Santé Publique. 1987;35:206–224. [PubMed] [Google Scholar]
- Robine JM, Jagger C, Van Oyen H, Cambois E, Romieu I, Clavel A et al (2005) Are we living longer healthier lives in the EU? Disability-free life expectancy (DFLE) in EU countries from 1991 to 2003 based on the European Household Panel (ECHP). EHEMU Technical Report 2; EHEMU, Montpellier, pp 1–29
- Robine JM, Mathers CD. Measuring the compression or expansion of morbidity through changes in health expectancy. In: Robine JM, Mathers CD, Bone MR, Romieu I, editors. Calculation of health expectancies; harmonization, consensus achieved and future perspectives. Montrouge: John Libbey Eurotext; 1993. pp. 269–286. [Google Scholar]
- Robine JM, Michel JP. Looking forward to a general theory on population aging. J Gerontol A Biol Sci Med Sci. 2004;59:M590–M597. doi: 10.1093/gerona/59.6.m590. [DOI] [PubMed] [Google Scholar]
- Robine JM, Romieu I, Michel JP. Trends in health expectancies. In: Robine JM, Jagger C, Mathers CD, Crimmins E, Suzman R, editors. Determining health expectancies. Chichester: Wiley; 2003. pp. 75–101. [Google Scholar]
- Sullivan DF. A single index of mortality and morbidity. HSMHA Health Reports. 1971;86:347–354. [PMC free article] [PubMed] [Google Scholar]
- Van Oyen H, Bossuyt N, Bellamammer L, Deboosere P, Demarest S, Lorant V, et al. Composite health measures in Belgium based on the 2001 Census. Arch Public Health. 2005;63:107–126. [Google Scholar]
- Van Oyen H, Tafforeau J, Hermans H, Quataert P, Schiettecatte E, Lebrun L, et al. The Belgian health interview survey. Arch Public Health. 1997;55:1–13. [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- WHO (2001) International classification of functioning disability and health: ICF WHO, Geneva