Abstract
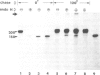
The biosynthesis and membrane topography of the neural cell adhesion molecule L1 have been studied in cerebellar cell cultures by metabolic labeling and immunoprecipitation. Pulse and pulse-chase experiments with [35S]methionine show that L1 is synthesized in its high mol. wt. form, the 200 kd component. The lower mol. wt. components with 40, 80 and 140 K apparent mol. wts. can be generated by proteolysis in intact cellular membranes. Peptide maps generated by protease treatment of L1 isolated from adult mouse brain show that the 80 and 140 kd components are related to the 200 kd component, but not to each other. The 200, 80 and 40 kd components can be biosynthetically phosphorylated. The 140 kd component is not phosphorylated and not released from the surface membrane during tryspinization. The phosphorylated amino acid is serine. In the presence of tunicamycin the 200 kd component is synthesized as a 150 kd protein. Pulse-chase experiments in the presence of tunicamycin indicate that the carbohydrate moieties are predominantly N-glycosidically linked and that the contribution of O-glycosylation is minimal. The carbohydrate moieties are of the complex type as shown by treatment with endoglycosidase H. Since monensin inhibits processing of the carbohydrate moieties, the 200 kd component appears to be transported to the surface membrane via the Golgi apparatus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Edelman G. M. Modulation of cell adhesion during induction, histogenesis, and perinatal development of the nervous system. Annu Rev Neurosci. 1984;7:339–377. doi: 10.1146/annurev.ne.07.030184.002011. [DOI] [PubMed] [Google Scholar]
- Faissner A., Kruse J., Goridis C., Bock E., Schachner M. The neural cell adhesion molecule L1 is distinct from the N-CAM related group of surface antigens BSP-2 and D2. EMBO J. 1984 Apr;3(4):733–737. doi: 10.1002/j.1460-2075.1984.tb01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G., Hirn M., Deagostini-Bazin H., Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM. The use of liposome-inserted radioiodinated N-CAM to study its transbilayer orientation. Eur J Biochem. 1984 Jul 2;142(1):65–73. doi: 10.1111/j.1432-1033.1984.tb08251.x. [DOI] [PubMed] [Google Scholar]
- Gennarini G., Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM. A monoclonal antibody recognizing a cytoplasmic domain and evidence for the presence of phosphoserine residues. Eur J Biochem. 1984 Jul 2;142(1):57–64. doi: 10.1111/j.1432-1033.1984.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Goridis C., Deagostini-Bazin H., Hirn M., Hirsch M. R., Rougon G., Sadoul R., Langley O. K., Gombos G., Finne J. Neural surface antigens during nervous system development. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):527–537. doi: 10.1101/sqb.1983.048.01.057. [DOI] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Chuong C. M., Edelman G. M. Polypeptide components and binding functions of neuron-glia cell adhesion molecules. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7989–7993. doi: 10.1073/pnas.81.24.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Edelman G. M. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984 Jan;81(1):267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen O. S., Bock E. Brain specific synaptosomal membrane proteins demonstrated by crossed immunoelectrophoresis. J Neurochem. 1974 Oct;23(4):879–880. doi: 10.1111/j.1471-4159.1974.tb04419.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen O. S., Delouvée A., Thiery J. P., Edelman G. M. The nervous system specific protein D2 is involved in adhesion among neurites from cultured rat ganglia. FEBS Lett. 1980 Feb 25;111(1):39–42. doi: 10.1016/0014-5793(80)80756-3. [DOI] [PubMed] [Google Scholar]
- Keilhauer G., Faissner A., Schachner M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature. 1985 Aug 22;316(6030):728–730. doi: 10.1038/316728a0. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Wienrich M., Schachner M. Antibody L1 ejected from a micropipette identifies neurons without altering electrical activity. Neurosci Lett. 1983 Oct 31;41(1-2):85–90. doi: 10.1016/0304-3940(83)90227-6. [DOI] [PubMed] [Google Scholar]
- Kruse J., Mailhammer R., Wernecke H., Faissner A., Sommer I., Goridis C., Schachner M. Neural cell adhesion molecules and myelin-associated glycoprotein share a common carbohydrate moiety recognized by monoclonal antibodies L2 and HNK-1. Nature. 1984 Sep 13;311(5982):153–155. doi: 10.1038/311153a0. [DOI] [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Burow E., Kinzel V. Substrate-effected release of surface-located protein kinase from intact cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4021–4025. doi: 10.1073/pnas.80.13.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Kinzel V. Protein kinase activity and substrates at the surface of intact HeLa cells. J Biol Chem. 1982 Jan 10;257(1):322–329. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennarz W. Role of intracellular membrane systems in glycosylation of proteins. Methods Enzymol. 1983;98:91–97. doi: 10.1016/0076-6879(83)98142-9. [DOI] [PubMed] [Google Scholar]
- Lindner J., Rathjen F. G., Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. 1983 Sep 29-Oct 5Nature. 305(5933):427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- Lyles J. M., Linnemann D., Bock E. Biosynthesis of the D2-cell adhesion molecule: post-translational modifications, intracellular transport, and developmental changes. J Cell Biol. 1984 Dec;99(6):2082–2091. doi: 10.1083/jcb.99.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles J. M., Norrild B., Bock E. Biosynthesis of the D2 cell adhesion molecule: pulse-chase studies in cultured fetal rat neuronal cells. J Cell Biol. 1984 Jun;98(6):2077–2081. doi: 10.1083/jcb.98.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes E. L., Waterfield M. D. Biosynthesis of the epidermal growth factor receptor in A431 cells. EMBO J. 1984 Mar;3(3):531–537. doi: 10.1002/j.1460-2075.1984.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70(A):210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Jockusch H., Faissner A., Schachner M. Synapse formation and synaptic activity in mammalian nerve-muscle co-culture are not inhibited by antibodies to neural cell adhesion molecule L1. Neurosci Lett. 1984 Feb 24;44(3):235–239. doi: 10.1016/0304-3940(84)90028-4. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Koide N., Ceccarini C., Atkinson P. H. Characterization of mannose-labeled glycopeptides from human diploid cells and their growth-dependent alterations. J Biol Chem. 1976 Aug 10;251(15):4673–4679. [PubMed] [Google Scholar]
- Orkand P. M., Lindner J., Schachner M. Specificity of histiotypic organization and synaptogenesis in reaggregating cell cultures of mouse cerebellum. Brain Res. 1984 Sep;318(1):119–134. doi: 10.1016/0165-3806(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Rathjen F. G., Rutishauser U. Comparison of two cell surface molecules involved in neural cell adhesion. EMBO J. 1984 Feb;3(2):461–465. doi: 10.1002/j.1460-2075.1984.tb01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Molecular and biological properties of a neural cell adhesion molecule. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):501–514. doi: 10.1101/sqb.1983.048.01.055. [DOI] [PubMed] [Google Scholar]
- Schachner M., Faissner A., Kruse J., Lindner J., Meier D. H., Rathjen F. G., Wernecke H. Cell-type specificity and developmental expression of neural cell-surface components involved in cell interactions and of structurally related molecules. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):557–568. doi: 10.1101/sqb.1983.048.01.060. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Schachner M. Developmental expression of cell type-specific markers in mouse cerebellar cells in vitro. J Neuroimmunol. 1981 Dec;1(4):471–487. doi: 10.1016/0165-5728(81)90024-2. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Schachner M. Expression of Thy-1, H-2, and NS-4 cell surface antigens and tetanus toxin receptors in early postnatal and adult mouse cerebellum. J Neuroimmunol. 1981 Dec;1(4):429–456. doi: 10.1016/0165-5728(81)90022-9. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin B. C., Hoffman S., Edelman G. M., Cunningham B. A. Sulfation and phosphorylation of the neural cell adhesion molecule, N-CAM. Science. 1984 Sep 28;225(4669):1476–1478. doi: 10.1126/science.6474186. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tempst P., Hunkapiller M. W., Hood L. E. Separation of peptides by reverse-phase high-performance liquid chromatography using propyl- and cyanopropylsilyl supports. Anal Biochem. 1984 Feb;137(1):188–195. doi: 10.1016/0003-2697(84)90369-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]