Abstract
Statistics Sweden has interviewed representative samples of the population annually since 1980. This study looks at ages 65–84 (n ≈ 3,000 per year) and presents prevalence rates for functional ability (walking and running ability, vision and hearing, and disability) for different age groups and for men and women. Prevalence rates of functional problems increase with age, for all indicators and for men and women. With the exception of hearing, women have poorer function than men. Different function indicators showed different trends over time. For example, vision (reading text) improved over the studied time period, while hearing (a conversation between two or more people) showed a clear worsening over the time period. Seen over the entire time period 1980–2005, mobility items (running, walking) and disability indicators showed improvement. However, figures suggested that most of this improvement occurred during the 1980s and early 1990s. Regression analyses of the estimated trends up until 1996 show for the most part significant improvement, but this positive development seems to cease after 1996 and in some cases there seems to be a significant upswing in problems. On other hand, for hearing, the negative trend of increased problems seems to have been broken after 1996. Results emphasize the necessity to follow population trends over long periods of time with multiple waves and multiple indicators.
Keywords: Health trends, Mobility, Functioning
Introduction
Tremendous gains were made during the twentieth century in regards to life expectancy. For the entire world, life expectancy at birth was 46.4 years in 1950; in 2005 this had increased to 66 years (UN 2007). Projections to 2020 estimate a life expectancy of 69.8 and for 2050 estimates lie at 75.4 years. Increased life expectancies, together with decreased fertility rates (from five children per woman in 1950 to 2 in 2005), have led to increases in both the number and proportion of elderly people in the world (UN 2007). This will inevitably lead to increases in needs for services, from specialized medical interventions to long-term care. The extent of the increases depends on the changes in health status we can expect in the elderly population. Will future cohorts of elderly people be healthier than today’s elderly cohorts? Projections made by the European Union’s Economic Policy Committee (Commission 2006) demonstrated that future spending on health care and long-term care is very sensitive to assumptions of health status.
Changes in mortality rates are relatively easy to measure and follow over long periods of time in most countries with census data. Morbidity, on other hand, is a more nebulous and diverse concept, difficult to measure at the population level. Few studies with morbidity measures stretch over more than a decade, particularly for the older sectors of the population (Lafortune et al. 2007). Epidemiology has traditionally focused on the prevalence of specific diseases or conditions in a population. Disease prevalence in the elderly population is important, but because of the higher rates of co-morbid conditions among elderly people and the interaction of disease with the aging process, indicators that reflect the cumulative effects of morbidity are more revealing when it comes to describing health in the elderly population (Manton 1990). While disease indicators reflect needs for medical care, indicators of disability and functioning are more helpful for capturing both living conditions and potential needs for long-term care.
Sweden has had a larger proportion of elderly people in the population for many years and still leads the world in regards to the proportion of very old people. Other countries are, however, fast approaching Swedish levels and will surpass Sweden in the not too distant future. According to projections by the United Nations (UN 2007), in 2030, 22.8% of Swedes will be over 65 years and 7.6% will be over 80. In Italy, for example, 27% will be over 65, and 8.6% over 80. According to the Organisation for Economic Co-operation and Development (OECD 2007), even ‘young’ countries such as Korea and the United States will surpass Sweden in the proportion of persons over the age of 85 by 2050.
Sweden was also ahead of many nations in developing a national health system. By the early 1970s, medical care was accessible to the entire population regardless of age or economic means (Parker 2000). However, most elderly people today lived the first part of their lives without a national health system, so it is debatable to what extent the system has been responsible for the aging of the population. In any case, it is clear that Sweden is somewhat of a forerunner in regard to its aging population. The health trends occurring in Sweden should therefore be of interest to other nations.
A study of Swedes aged 77 years and older found that a number of health indicators showed significant worsening between 1992 and 2002 (Parker et al. 2005). This study, the Swedish Panel Study of Living Conditions of the Oldest Old (SWEOLD) was based on an interview survey and included some objective tests in addition to self- and proxy reported health data. Self reports of, e.g., mobility, hearing, and musculoskeletal pain showed significant worsening between 1992 and 2002, adjusting for the differing age and sex distributions of the two time periods. Tests of lung function and physical ability also showed significant worsening. Interestingly, disability items (ADL and IADL) showed no significant change over time.
These findings seemed to conflict with previous findings from another national data base, Statistic Sweden’s Living Conditions Survey (ULF). The ULF study had an age ceiling of 84 with the exception of two survey periods, 1988/1989 and 2002/2003. In order to include the oldest age groups, previous Swedish studies compared only these two time points. Between 1988/1989 and 2002/2003, these studies found improvements in self-rated health, mobility, vision and ADL ability (Lagergren 2004; Larsson and Thorslund 2006; Malmberg and Sundström 2004; Persson et al. 2001). Two of the studies also examined reports of disease and pain and found increased prevalence rates during the same time period (Lagergren 2004; Larsson and Thorslund 2006).
Aim
An initial aim of this study was to examine the national study ULF in more detail, utilizing all the interview years starting in 1980. We hypothesized that health trends have not followed a purely linear trend over the past 25 years. We assumed, based on our SWEOLD findings that different patterns could be found for different indicators of health and function. Since the ULF included lower age groups and has greater statistical power compared to SWEOLD, we wanted to explore possible differences between age groups and between women and men.
Our research questions were:
What is the shape of trends in functioning and disability over the time period 1980–2005?
Do different functioning indicators show different trends over time?
Have all age groups followed the same trends?
Material and methods
Statistics Sweden (www.scb.se) conducts the annual Living Conditions Survey (ULF). The ULF surveys consist of interviews with a random sample of the Swedish population. Institutionalized persons are included and proxy interviews are carried out when necessary. From 1980 to 2001, the upper age limit has been 84 years with the exception of 1988–1989. Sample size for each survey year is about 3,000 persons1 over the age of 65. In 2002, the age ceiling was eliminated. Non-response in the samples aged over 64 years increased from 22 to 27% over the time period studied. Health items in ULF included a selection of function indicators.
Altogether there were four mobility items. The first question was “Can you run a short distance, say 100 m, if you are in a hurry?” Those persons who answered “yes” were assumed to have full mobility; those who answered “no” were asked the three following questions: “Can you take a short walk, say 5 min, at a brisk pace?”, “Can you step up onto a bus without difficulty?” and “Can you walk up and down stairs without difficulty?” All items had yes–no answers. We present results for running and walking.
Sensory function included hearing and vision. Hearing was measured with the question, “Can you hear without any difficulty what is said in a conversation between several people (with or without a hearing aid)?” The vision question was, “Can you read a newspaper without difficulty (with or without glasses)?” Both items had yes–no answers. Instructions to interviewers regarding proxy responses to the hearing and vision items varied over the years. To maintain comparability, we present only results from direct interviews for these two items.
Disability included items of personal activities of daily living (ADL) and instrumental activities (IADL). The questions were preceded by an introduction asking the respondent to consider the need for help with tasks, not the usual household division of tasks. An introductory question was read to the respondent, “Would you be able to manage the following activities without help from other persons?” followed by a list of activities.
The ADL items included personal hygiene, bathing or showering, getting up and going to bed, dressing and undressing, and eating. IADL items included housecleaning, shopping for food and preparing food. Alternative answers were “need help” or “manage by self”. Disability items were not included in 1992 and 1993.
Unfortunately, the ADL items included differed somewhat on different waves. Based on the 1988/1989 waves, when all items were included, calibrations were made for the ADL score by assuming that the relationships between frequencies of items were similar all years. For example, waves that did not include the bathing item were calibrated upwards corresponding to its relationship to other items on the 1988/1989 wave. To obtain better statistical power, the calibrations were calculated using all the age groups (65–84) with age standardization. The age standardization also considered the fact that relationships between ADL items differed somewhat by age group. Therefore, the ADL results are not broken down by age groups.
Prevalence rates for reported problems with functioning are shown in Figs. 1, 2, 3, 4, and 5 for the four age groups aged between 65 and 84. Statistics Sweden organizes the data in 2 year intervals, i.e., the time point 1990/1991 is the average for 1990 and 1991. Figures include both women and men.
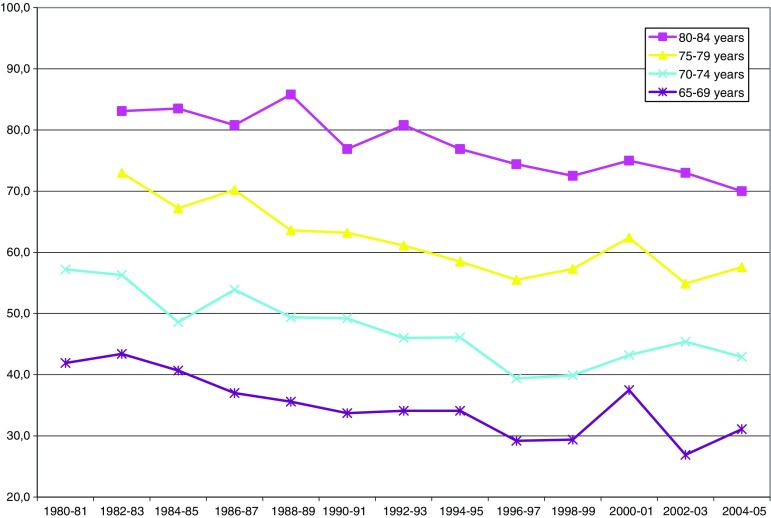
Fig. 1.
Reported inability to run 100 m (percent by age group)
Fig. 2.
Inability to take a short walk (percent by age group)
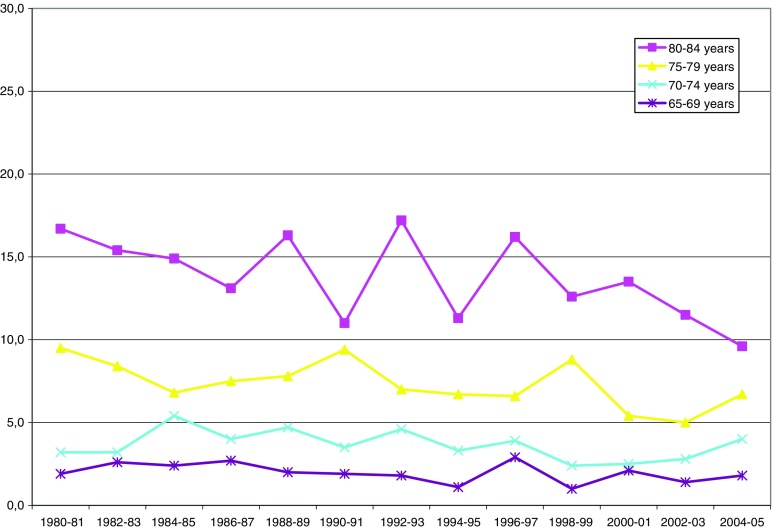
Fig. 3.
Inability to read a newspaper (with or without glasses, percent by age group)
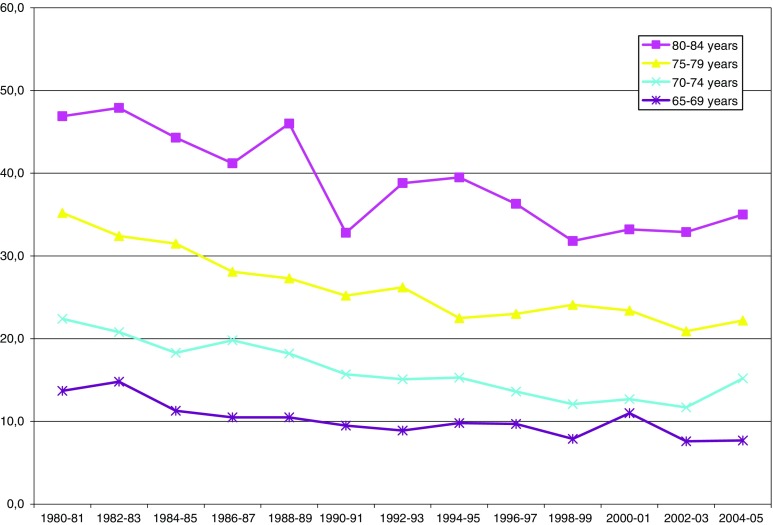
Fig. 4.
Inability to hear a conversation between several people (with or without hearing aid, percent by age group)
Fig. 5.
Inability to manage housecleaning without help (percent by age group)
Results from the regression analyses are shown in Tables 1, 2, 3, 4, 5, and 6 below the figures. The first column of each table shows the estimated trends for the entire period 1980 to 2005. The second column shows estimated trends for the time period up to and including the 1996/1997 wave of ULF. The third column shows the following time period, from 1996/1997 to 2005. The 1996/1997 wave was chosen because, according to the figures, the trends for several variables seemed to change at this point. A fourth column shows the difference between the first and second period. A positive value here shows that there is more deterioration (or less improvement) in the second period compared to the first. A negative value shows that there is less deterioration (or more improvement) in the second period compared to the first. The P values for the difference between trends are from t tests. T tests are made with STATA 9.0, regression and trend calculations with Excel 2003.
Table 1.
Estimated trends for reported inability to run 100 m
| 1980–2005a | 1980–1996/1997 | 1996/1997–2005 | Differenceb | ||
|---|---|---|---|---|---|
| Total | 65–69 | −1.07*** | −1.59*** | +0.13n.s. | +1.72n.s. |
| 70–74 | −1.25*** | −1.86*** | +1.25n.s. | +3.11*** | |
| 75–79 | −1.37*** | −2.31*** | +0.18n.s. | +2.49* | |
| 80–84 | −1.25*** | −1.22*. | −0.83n.s. | +0.39n.s. | |
| Men | 65–69 | −1.17*** | −1.72*** | −0.39n.s. | +1.33n.s. |
| 70–74 | −1.29*** | −1.88** | +0.63n.s. | +2.51** | |
| 75–79 | −1.58*** | −2.11** | −1.53n.s. | +0.58n.s. | |
| 80–84 | −1.30* | −1.62n.s. | −0.88n.s. | +0.74n.s. | |
| Women | 65–69 | −0.96** | −1.47** | +0.73n.s. | +2.20n.s. |
| 70–74 | −1.19** | −1.83** | +1.82* | +3.65*** | |
| 75–79 | −1.17** | −2.36*** | +1.38n.s. | +3.74*** | |
| 80–84 | −1.17** | −1.04*. | −0.58n.s. | +0.46n.s. | |
† P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001; n.s. non significant
aThe survey year 1980–1981 is missing for running for the age groups 75–79 and 80–84
bBetween time periods 1980–1996 and 1996–2005. A positive value reflects more deterioration (or less improvement) in the second period compared to the first. A negative value reflects less deterioration (or more improvement) in the second period compared to the first
Table 2.
Estimated trends for reported inability to take a short walk
| 1980–2005 | 1980–1996/1997 | 1996/1997–2005 | Difference | ||
|---|---|---|---|---|---|
| Total | 65–69 | −0.45*** | −0.61** | −0.43n.s. | +0.18n.s. |
| 70–74 | −0.79*** | −1.04*** | +0.28n.s. | +1.32** | |
| 75–79 | −1.05*** | −1.53*** | −0.48† | +1.05** | |
| 80–84 | −1.27*** | −1.45* | −0.15† | +1.30n.s. | |
| Men | 65–69 | −0.43* | −0.57† | −0.79n.s. | −0.22n.s. |
| 70–74 | −0.67** | −0.91** | +0.77n.s. | +1.68* | |
| 75–79 | −1.08*** | −1.63*** | −0.48n.s. | +1.15† | |
| 80–84 | −1.28** | −1.79* | +0.34n.s. | +2.13† | |
| Women | 65–69 | −0.46** | −0.63* | −0.11n.s. | +0.52n.s. |
| 70–74 | −0.89*** | −1.14** | −0.10n.s. | +1.04** | |
| 75–79 | −1.02*** | −1.44** | −0.50n.s. | +0.94n.s. | |
| 80–84 | −1.24*** | −1.30* | −0.21n.s. | +1.09n.s. | |
Table 3.
Estimated trends for reported vision problems (inability to read a newspaper with or without glasses)
| 1980–2005 | 1980–1996/1997 | 1996/1997–2005 | Difference | ||
|---|---|---|---|---|---|
| Total | 65–69 | −0.07n.s. | −0.04n.s. | −0.18n.s. | −0.14n.s. |
| 70–74 | −0.08n.s. | +0.02n.s. | +0.06n.s. | +0.04n.s. | |
| 75–79 | −0.22* | −0.24n.s. | −0.36n.s. | −0.12n.s. | |
| 80–84 | −0.38* | −0.20n.s. | −1.43* | −1.23* | |
| Men | 65–69 | −0.05n.s. | +0.04n.s. | −0.29n.s. | −0.33n.s. |
| 70–74 | +0.04n.s. | +0.27† | −0.10n.s. | −0.37n.s. | |
| 75–79 | +0.09n.s. | +0.09n.s. | +0.04n.s. | −0.05n.s. | |
| 80–84 | −0.46* | −0.21n.s. | −1.97** | −1.76** | |
| Women | 65–69 | −0.08n.s. | −0.11n.s. | −0.09n.s. | +0.02n.s. |
| 70–74 | −0.19* | −0.18n.s. | +0.16n.s. | +0.34n.s. | |
| 75–79 | −0.45** | −0.48† | −0.62n.s. | −0.14n.s. | |
| 80–84 | −0.32n.s. | −0.21n.s. | −0.97n.s. | −0.76n.s. | |
Table 5.
Estimated trends for reported inability to manage housecleaning without help
| 1980–2005 | 1980–1996/1997 | 1996/1997–2005 | Difference | ||
|---|---|---|---|---|---|
| Total | 65–69 | −0.20* | −0.11n.s. | −0.35n.s. | −0.24n.s. |
| 70–74 | −0.38** | −0.41n.s | −0.24n.s. | +0.17n.s. | |
| 75–79 | −0.77** | −0.82n.s. | −0.59n.s. | +0.23n.s. | |
| 80–84 | −0.83n.s. | −0.54n.s. | +0.03n.s. | +0.57n.s. | |
| Men | 65–69 | −0.27* | −0.25n.s. | −0.71n.s. | −0.46n.s. |
| 70–74 | −0.42** | −0.43n.s. | −0.31n.s. | +0.12n.s. | |
| 75–79 | −0.81** | −0.73n.s. | −0.77n.s. | −0.04n.s. | |
| 80–84 | −0.88n.s. | −0.02n.s. | −2.24† | −2.22n.s. | |
| Women | 65–69 | −0.12n.s. | +0.02n.s. | +0.01n.s. | −0.01n.s. |
| 70–74 | −0.34* | −0.37n.s | −0.16n.s. | +0.21n.s. | |
| 75–79 | −0.74** | −0.85† | −0.50n.s. | +0.35n.s. | |
| 80–84 | −0.76* | −0.86n.s. | +1.53† | +2.39*. | |
Table 6.
Estimated trends for reported need for help in at least one ADL (age adjusted)
| 1980–2005 | 1980–1996/1997 | 1996/1997–2004/2005 | Difference | |
|---|---|---|---|---|
| Total | −0.62*** | −1.03*** | +0.71* | +1.74*** |
| Men | −0.62*** | −1.02*** | +0.47* | +1.49*** |
| Women | −0.62** | −1.03*** | +0.89n.s. | +1.92*** |
Results
Mobility
The Figs. 1 and 2 show prevalence rates for limitations in running and taking a short walk. The downward slope of the curves indicates improvement. For the group aged 70–74, the proportion of persons reporting limitations in running decreased from 57% in 1980/1981 to 43% in 2004/2005. Among men in this age group, reported limitations in running decreased from 52 to 35%, and among women 61 to 50%.
According to Table 1, this downward trend in prevalence rates is significant for all age and sex groups analyzed. (Note that the tables show the estimated slopes of the changes in prevalence levels, not the levels themselves.) Looking at the two time periods, it is clear that the decline in running limitations occurred primarily in the first period, 1980–1996/1997. The trend then reverses direction, i.e., limitations increase, but significantly only for women aged 70–74. The difference between the two time periods (shown in the last column) is significant for those aged 70–79.
Prevalence levels for the inability to take a walk (Fig. 2) are much lower than for running, but show a similar pattern of improvement. Reported limitations for men aged 75–79 decreased from 32% in 1980 to 21% in 2005; figures for women were 37 and 23%. Similar patterns were seen for the ability to walk up and down stairs and step onto a bus (not shown). The improvement in walking was significant for the entire period, and for the first of the separated time periods (Table 2). Trend estimates during the second time period are not significant, that is, the improvement seems to have ceased or at least slowed. The difference between the two time periods is significant for the middle age groups (ages 70–79).
Note that, despite the large sample size, there are still blips and dips when looking across the entire time period. This was seen for all variables. The fluctuations of the graph lines may reflect some cohort differences, or some systematic methodological differences between surveys, as well as random variation in survey response.
Sensory function
Vision and hearing showed contradictory trends over the time period. Note that prevalence rates for both vision and hearing underestimate total population prevalence since proxy responses were omitted for these items.
Vision (Fig. 3, Table 3) improved significantly in the older age groups. Women reported more vision problems than men in all age groups. In the group aged 80–84 years, 17% had vision problems in 1980/1981 and 10% in 2004/2005. Change among the younger groups was negligible. There was no significant difference between the first and second time period (Table 3), with the exception of the oldest men. This group had an accelerated improvement during the second time period (1996/1997–2005).
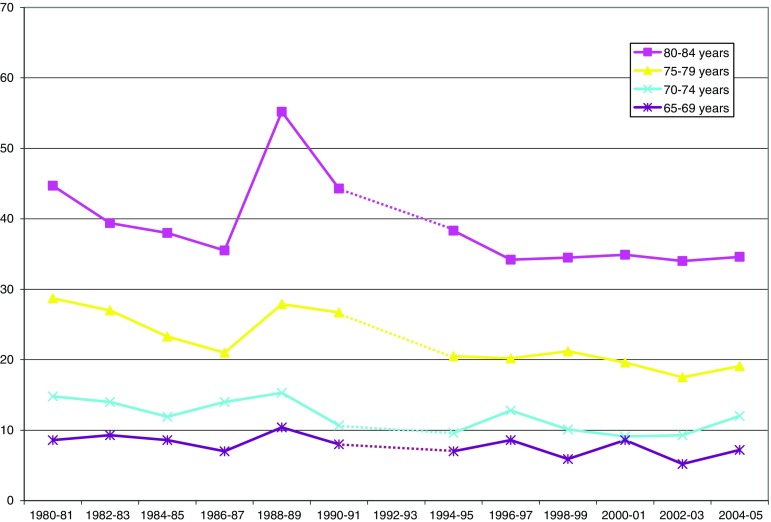
Hearing (Fig. 4, Table 4), on other hand, showed an increased prevalence of reported problems for all studied age groups. Hearing was also an exception to the gender ‘rule’ in that men had higher prevalence rates of problems compared to women. For example, in the age group 70–74, reported hearing problems among men increased from 22 to 37%, women from 17 to 19% from 1980 to 2005. When separating the two time periods, it is clear that here, too, there seems to be a significant difference and that the negative (worsening) trend from 1980 to 1996/1997 seems to taper off and in some groups becomes a trend of improvement.
Table 4.
Estimated trends for reported hearing problems (difficulty to hear a conversation between several people, with or without hearing aid)
| 1980–2005 | 1980–1996/1997 | 1996/1997–2005 | Difference | ||
|---|---|---|---|---|---|
| Total | 65–69 | +0.78** | +1.30** | +0.14n.s. | −1.16* |
| 70–74 | +0.89*** | +1.48** | −0.25n.s. | −1.73** | |
| 75–79 | +0.59* | +1.28** | −0.85* | −2.13*** | |
| 80–84 | +0.64* | +1.36** | −1.10n.s. | −2.46** | |
| Men | 65–69 | +0.96** | +1.78*** | −0.51n.s. | −2.29*** |
| 70–74 | +1.33*** | +2.03*** | −0.26n.s. | −2.29** | |
| 75–79 | +0.74* | +1.58** | −0.47n.s. | −2.05* | |
| 80–84 | +0.22n.s. | +1.03† | −2.13** | −3.16*** | |
| Women | 65–69 | +0.60** | +0.86* | +0.66n.s. | −0.20n.s. |
| 70–74 | +0.52* | +1.02* | −0.30n.s. | −1.32* | |
| 75–79 | +0.45n.s. | +0.99* | −1.08† | −2.07** | |
| 80–84 | +0.87** | +1.59** | −0.54n.s. | −2.13*. | |
Disability
The three IADL items (cleaning, shopping, preparing food) showed a similar pattern over time. Women had higher rates of problems with housecleaning and shopping for food, and men had higher rates for food preparation. As an example, the need for help with housecleaning is shown in Fig. 5 and Table 5. For the entire time period, all but the group aged 80–84 showed significant improvement. It seems that the improvement was spread over both studied time periods as there is no significant difference between trend estimates. One exception is the oldest group of women, where the decrease in problems during the first period was followed by an increase during the second period.
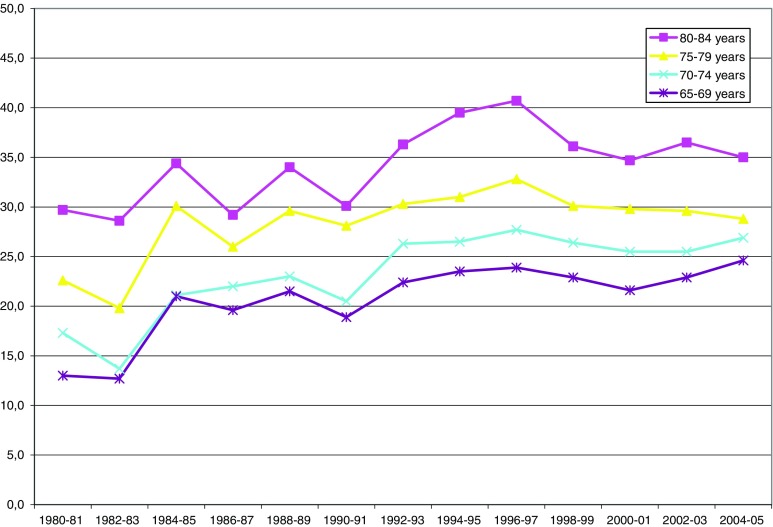
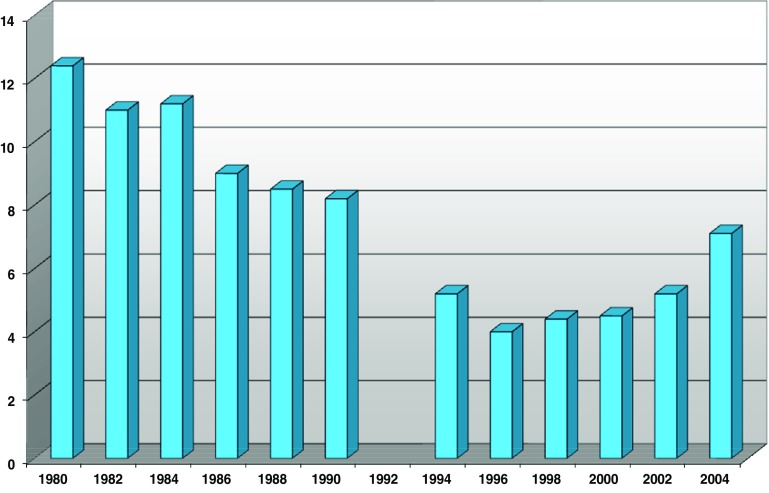
ADL is presented in Fig. 6 and Table 6 as a score of at least one problem with the personal ADL activities. As described above, the score was age standardized. Estimated trends for ADL scores improved significantly over the entire time period. However, this improvement occurred up until 1996/1997. After this wave, ADL limitations increased significantly. In the gender separate analyses; this increase was significant only for men, although the change in the estimated trend was significant for both men and women.
Fig. 6.
ADL: needing help with at least one limitation (65–84, age-adjusted)
Discussion
In summary, our aim was to describe the shape of health trends over the two and a half decades of survey data available and to describe differences between indicators.
We found improvement in some indicators (mobility, vision, ADL) and a clear worsening in hearing problems. Among the indicators that showed improvement, most of the improvement occurred during the 1980s and early 1990s. Any improvement after the mid-1990s was negligible, and some indicators, in particular ADL, showed signs of worsening.
The fact that different indicators of functioning reveal different patterns of change reflects the complex, multi-dimensional character of health (Parker and Thorslund 2007). Social and environmental factors, social policy, health behavior, and medical care developments can all have varying degrees of influence on population health trends. As these factors change over time, so changes their influence on health in the population.
Advances in technology may have contributed to improvement in some indicators during this period. An example is the progress made in cataract surgery that facilitates surgery even on old and frail persons. Another example is the increased use of assistive technology, in particular for walking. Improved aids, and improved access to aids, have certainly contributed to improvements in walking ability and IADL among Swedish older people. Other environmental changes, e.g., kneeling buses, microwave ovens, availability of semi-prepared food, have also facilitated management of IADLs.
The different trends seen in different age groups can reflect the fact that different birth cohorts experience changes in, e.g., social policy and medical care, at different times in their lives. Trends by cohort can reflect changes in health behavior, occupational exposure and other risk factors. The concept of ‘critical periods’ in the life course could explain some of these differences (Kuh et al. 2005). Additionally, medical advances have had different effects on different age groups. Advancements in eye surgery, for example, may be seen most clearly among the oldest age groups who had previously been denied surgery for medical reasons.
Gender differences in health trends can be explained by the assumption that social and environmental changes, as well as changes in medical care, have had different influences on the health of women and men. For example, improvements in factory working conditions have benefitted men more than women in current elderly cohorts since women worked more often in their homes. There have also been gender changes in health behavior, e.g., smoking. Changing gender roles over the past few decades may also have had different effects on women and men. Gender roles may influence health itself as well as how women and men respond to questions, in particular, how men respond to IADL items. We can expect improvement among men in regard to housecleaning and food preparation as gender roles become more flexible.
The figures from this study do not show a linear trend over the 25 year period studied. Rather, we can see a general pattern of improvement during the 1980s and into the 1990s, and a less positive pattern from the mid-1990s onward. The exception is hearing, which shows an opposite development. Robine and Michel (2004) describe how health trends in a population reflect the demographic trends together with the epidemiological stages and transitions. Wolf et al. (2007) take a closer look and describe trends in prevalence rates in the elderly population as a reflection of the complex interplay of incidence rates and prevalence rates of those persons entering old age. Their study of a community based population in the United States found downward trends for both onset and recovery of disability from 1982 to 1994. Clearly, different patterns can be expected as a result of the myriad of forces shaping health in the elderly population as cohort and period differences interact over time.
Explaining the upswing of poor functioning found in this study is a challenge beyond this article. One possible hypothesis is that survival rates from circulatory disease have increased, but that survivors may be left with limitations. This has been corroborated by epidemiological studies (Rosen and Haglund 2005) that show a 20% increase in prevalence of heart disease, hypertension and diabetes among men between the late 1980s and the late 1990s. This is a medical paradox, whereby successes in medicine lead to poorer health in the surviving population.
Our data, like most trend studies, can only describe trends in prevalence rates. Explanations for the patterns seen must be inferred given patterns in, e.g., morbidity, health behavior, and environmental factors. Numerous explanations, from both cohort and period effects, are possible. Certainly medical advancements at the end of life are responsible for increased survival late in life. But other factors, related to behavior, socioeconomic status, and social welfare, are also central. A study of mobility in the Swedish adult population (aged 45–71) found that the composition of the population regarding social class, as well as smoking and physical activities, could explain most of the improvement in mobility (Ahacic and Parker 2003). This study demonstrated how sensitive prevalence rates are to period and cohort effects.
Further challenges to the discernment of explanatory factors include the possibility of lag effects—an event or condition may not emerge as a health factor until years later—and the possibility of methodological explanations such as selective non-response or measurement error.
This study spanned two and a half decades with good consistency between surveys. A limitation with previous trend analyses in Sweden (e.g., Parker et al. 2005; Persson et al. 2001) and, indeed, many trend analyses in other countries (e.g., Lafortune et al. 2007) is that only two time points are compared. Due to the random fluctuations that can be expected in survey data, this limits the reliability of these findings. As seen in the case of ADL, measuring only two endpoints in time completely missed the upswing in limitations that seems to have occurred since the mid-1990s.
Limitations with this study include the fact that ADL items changed over the time period, leading to the need for calibrated ADL scores. There were other minor inconsistencies in the interview questionnaire over time which could have contributed to the fluctuations seen in the figures. In the regression analyses, the second time period had fewer interview waves and therefore there were fewer degrees of freedom in these regressions. This could explain the lack of significance for the second period for some of the indicators.
Unfortunately, the ULF study imposed an age ceiling for most survey years; those 85 and above were only interviewed on a few of the survey waves and are not included in this analysis. Response rates also decreased over the studied period, with increased non-response in later waves. The effect of this increase is uncertain; we don’t know how selective non-response has been. One study of ULF claimed that the effect of non-response on health was negligible (Johansson et al. 2006); another analyzed the health of non-responders using mortality and sickness registers and concluded that non-response leads to an underestimation of problems in the elderly population (Lagergren 2004). If this is true, then the results of this study may actually underestimate the extent of increases in poor function in later waves with greater non-response.
Conclusions
There are two main reasons to study trends in the health of elderly people over time. Trends provide hindsight to help us understand the effects of changes in, e.g., social policy and health care. This knowledge is useful in designing public health interventions and shaping public policy. Trends also help us to predict future trends and thus possible future needs for medical care and social services. This is useful information for planning the distribution of fiscal and other resources.
What will the future bring? Studies of past trends may increase our understanding of the factors that influence health and function in the elderly population. However, future trends cannot be discerned solely by studying past trends. We must also look at today’s younger generations to see what may be in store for future elderly cohorts. In Sweden, studies of persons aged 19–75 years have shown increases from 1981 to 2000 in prevalence rates of mean number of symptoms reported, self-reported health, musculoskeletal pain, and psychological distress (Fritzell et al. 2007). Will these cohorts carry these problems with them into old age? Looking at health behaviors, we also see that while smoking rates have decreased in general, rates among younger women have not been decreasing as much as other sectors of the population. Obesity, less prevalent than in many developed countries, is increasing and causing concern in Sweden. On a more positive side, education levels—correlated with better health and function—are higher in more recent cohorts of elderly people. Projections that included adjustments for increased educational level suggested that the benefits of education could counteract the expected increases in ill-health due to the aging of the population between 2000 and 2035 (Batljan 2007).
At first glance, the SWEOLD results (Parker et al. 2005) showing worsening of several function items between 1992 and 2002 seemed to conflict with previous studies based on ULF. Closer examination of ULF data showed that the results were not really so disparate. The improvement in functioning and disability found in previous studies (Lagergren 2004; Larsson and Thorslund 2006; Malmberg and Sundström 2004; Persson et al. 2001) occurred primarily between the 1980s and early 1990s. This positive development seems to have waned during the latter half of the 1990s and early 2000s. In particular, the upswing in prevalence rates for ADL limitations deserve attention, since they imply the need for long-term care, either home services, institutionalization, informal care or a combination of these. We intend to follow these developments to see if this increase in reported health problems and disability continues, or if it is a temporary anomaly. If it continues, and if Sweden is a forerunner in regard to aging populations, there is reason to view this development with some concern.
Acknowledgments
We are grateful for the financial support from the Swedish Council for Working Life and Social Research, grants 2003-0539 and 2002-0919.
Footnotes
N varies between survey waves and age/sex groups. For the smallest group, oldest men, n varies between 129 and 355 for different waves. For women aged 70–74, n varies from 342 to 438.
References
- Ahacic K, Parker MG. Mobility limitations 1974–1991: period changes explaining improvement in the population. Soc Sci Med. 2003;57:2411–2422. doi: 10.1016/S0277-9536(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Batljan I. Demographics and future needs for public long term care and services among the elderly in Sweden. Stockholm: Stockholm University; 2007. [Google Scholar]
- Commission E (2006) The impact of ageing on public expenditure: projections for the EU25 Member States on pensions, health care, long-term care, education and unemployment transfers (2004–2050) (No. 1/2006). Directorate-General for Economic and Financial Affairs, Brussels
- Fritzell J, Lennartsson C, Lundberg O. Health and inequalities in Sweden: long and short-term perspectives. In: Fritzell J, Lundberg O, editors. Health Inequalities and welfare resources, continuity and change in Sweden. Bristol: The Policy Press; 2007. pp. 19–41. [Google Scholar]
- Johansson S-E, Batljan I, Qvist J, Sundquist J. Förändringar i de äldres hälsotillstånd från 1988/89 till 2002/03–bortfallets betydelse (Changes in health status of elderly people from 1988/89 to 2002/03–the importance of non-response) In: Vogel J, Häll L, editors. Äldres levnadsförhållanden, Arbete, ekonomi, hälsa och sociala nätverk 1980–2003 (living conditions of the elderly, work, economy, health and social networks 1980–2003) Stockholm: Statistics Sweden; 2006. pp. 355–409. [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2005;57:778–783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafortune G, Balestat G, Members DSEG. Trends in severe disability among elderly people: Assessing the evidence in 12 OECD countries and the future implications (No. 26) Paris: Organisation for Economic Co-operation and Development; 2007. [Google Scholar]
- Lagergren M. Utvecklingen av de äldres hälsa och levnadslängd (Development of health and life expectancy among elderly people) Stockholm: Stiftelsen Stockholms läns Äldrecentrum; 2004. [Google Scholar]
- Larsson K, Thorslund M. Old people’s health in Sweden—the National Public Health Report 2005. Scand J Public Health Suppl. 2006;67(34):185–198. doi: 10.1080/14034950600677253. [DOI] [PubMed] [Google Scholar]
- Malmberg B, Sundström G (2004) Äldres levnadsförhållanden 1988–2002. Hälsa, funktionsförmåga och vård- och omsorgsmönster (Living conditions among elderly people 1988–2002. Health, functioning and care utilization patterns). Socialstyrelsen (National Board of Health and Welfare), Stockholm
- Manton KG. Mortality and morbidity. In: Binstock RH, George LK, editors. Handbook of aging and the social sciences. San Diego: Academic Press; 1990. pp. 64–90. [Google Scholar]
- OECD (2007) Population pyramids in 2000 and 2050. Retrieved September 2007, from http://www.oecd.org/dataoecd/52/31/38123085.xls
- Parker MG. Sweden and the United States: is the challenge of an aging society leading to a convergence of policy? J Aging Soc Policy. 2000;12(1):73–90. doi: 10.1300/J031v12n01_06. [DOI] [PubMed] [Google Scholar]
- Parker MG, Thorslund M. Health trends in the elderly population: Getting better and getting worse. Gerontologist. 2007;47(2):150–158. doi: 10.1093/geront/47.2.150. [DOI] [PubMed] [Google Scholar]
- Parker MG, Ahacic K, Thorslund M. Health changes among Swedish oldest old: prevalence rates from 1992 and 2002 show increasing health problems. J Gerontol A Biol Sci Med Sci. 2005;60(10):1351–1355. doi: 10.1093/gerona/60.10.1351. [DOI] [PubMed] [Google Scholar]
- Persson G, Bostrom G, Allebeck P, Andersson L, Berg S, Johansson L, et al. Elderly people’s health–65 and after. Health in Sweden: The National Public Health Report 2001, Chapter 5. Scand J Public Health Suppl. 2001;58:117–131. [PubMed] [Google Scholar]
- Robine JM, Michel JP. Looking forward to a general theory on population aging. J Gerontol A Biol Sci Med Sci. 2004;59(6):M590–M597. doi: 10.1093/gerona/59.6.m590. [DOI] [PubMed] [Google Scholar]
- Rosen M, Haglund B. From healthy survivors to sick survivors–implications for the twenty-first century. Scand J Public Health. 2005;33(2):151–155. doi: 10.1080/14034940510032121. [DOI] [PubMed] [Google Scholar]
- UN (2007) World population prospects: the 2006 revision population database. Retrieved September 2007, from http://esa.un.org/unpp
- Wolf DA, Mendes de Leon CF, Glass TA. Trends in rates of onset of and recovery from disability at older ages: 1982–1994. J Gerontol Soc Sci. 2007;62(1):S3–S10. doi: 10.1093/geronb/62.1.s3. [DOI] [PubMed] [Google Scholar]