Abstract
Charge-balanced direct current (CBDC) nerve block can be used to block nerve conduction in peripheral nerves. Previous work demonstrated that the CBDC waveform could be used to achieve a 10% duty cycle of block to non-block repeatedly for at least two hours. We demonstrate that the duty cycle of this approach can be significantly increased by utilizing multiple electrode contacts and cycling the CBDC waveform between each contact in a “carousel” configuration. Using this approach, we demonstrated in an acute rat sciatic nerve preparation, that a 30% duty cycle complete block can be achieved with two contacts; and a 100% duty cycle block (>95% complete block) can be achieved with four contacts. This latter configuration utilized a four second block plateau, with three seconds between successive plateaus at each contact and a recharge phase amplitude that was 34% of the block amplitude. Further optimization of the carousel approach can be achieved to improve block effectiveness and minimize total electrode length. This approach may have significant clinical use in cases where a partial or complete block of peripheral nerve activity is required. In one example case, we achieved continuous block for 22 minutes without degradation of nerve conduction. Future study will be required to further optimize this technique and to demonstrate safety for chronic human use.
Index Terms: Biomedical electrodes, biomedical electronics, direct current, electrical stimulation, electrodes, high capacitance electrodes, implantable biomedical devices, nerve block, neural engineering, neural prosthesis, platinum black
I. Introduction
MANY different neurological diseases can benefit from electrical nerve block. The possible applications represent a wide variety of systems in the body including pain relief, spasticity, and autonomic nervous system regulation. Although each application will have unique needs, the basic requirements of rapid block, rapid reversal, safety, and stability/repeatability are common to all applications. Electrical nerve block is well suited to satisfy all of these criteria.
Kilohertz high frequency alternating current (KHFAC) block has been previously shown to provide a very safe solution that meets all the goals of electrical nerve block [1–4]. However, at the initiation of KHFAC a period of transient firing occurs which is referred to as the “onset response” [5, 6]. This onset response could produce undesirable responses which prevent the therapy from being effective. Although different waveform modifications have been implemented, such as amplitude ramping, the KHFAC onset has been reduced, but not completely eliminated [7–9].
Direct current (DC) nerve block has also been demonstrated in acute experiments to create a complete block. Historically, the use of DC block was for the purpose of blocking larger axons so that small axons could be studied independently [10, 11]. The earliest study in this area was by Pflüger [12] (as described by Bhadra [13]) to selectively block larger fibers. Further studies by Kuffler [11] in the 1940’s demonstrated in the frog sciatic that direct current block was reversible and repeatable for the study of fiber size physiology. Later studies continued in the periphery on the sciatic [14–16], saphenous [17] and sural nerves [18–22], on the dorsal roots [23] as well as in the autonomic system on the vagus [24–27] and phrenic [28] nerves. This property of DC block was also exploited to demonstrate micturition in the paralyzed bladder by preferentially blocking the larger fibers controlling the sphincter to allow micturition to occur [22, 29]. Activation at the start of DC block will occur if there is a step change to block amplitude, but activation can be eliminated by ramping the DC level slowly from zero [14, 18, 30, 31]. Excitation can also occur when the DC block is turned off, but this can also be eliminated using a ramp at the end of DC block [14, 16, 18, 30, 31]. A more complete examination of the use of ramps and their mechanisms was explored by Bhadra et al [13] with simulation using the Hodgkin Huxley squid model.
Despite the demonstrated usefulness of DC block in acute preparations, it is unsuitable for repeated acute or chronic applications because the delivery of DC to the nerve results in a degradation in conduction due to the creation of toxic reactants produced at the electrode interface [10, 17, 32, 33]. Electrodes that prevent these reactants from forming [30] or isolate these reactants from the nerve [30, 34–37] can extend the amount of time that DC can be provided before a degradation of conduction occurs.
Platinum black is one electrode material that has successfully been used to prevent toxic reactions at the electrode interface [30, 34–37]. Platinum black has a high effective surface area relative to the geometric surface area, resulting in a significant reduction in the effective charge density when current is delivered through the electrode. Platinum black can be applied using several techniques including electroplating [38] and sputtering [39], but electrodeposition produces a surface with a higher charge capacity [40, 41]. We demonstrated previously that this method can be used to produce electrodes with charge capacities in excess of 10 mC, allowing for DC block to be applied for ten seconds or more before irreversible reactions begin based on in vitro electrochemical measurements and in vivo experiments on the sciatic nerve in rats [30].
DC block can be repeatedly delivered without causing irreversible reactions by combining high charge capacity materials such as platinum black with a charge balanced waveform that restores the electrode potential to its original resting value and prevents the buildup of charge at the electrode interface. This waveform has a cathodic blocking phase, lasting many seconds, and an anodic recharge phase, which can last 1–2 minutes. We referred to this waveform as a “charge balanced direct current waveform” (CBDC) because the effect on the nerve (conduction block) is due to the initial DC phase, yet the total net charge delivered to the tissue is zero. Acute testing of this waveform on motor fibers was performed using platinum black electrodes on the sciatic nerve in six rats [30]. This waveform was demonstrated to provide as much as ten seconds of nerve block every 113 seconds for 100 cycles without a degradation in nerve conduction using a platinum black electrode [30]. This waveform was also used in combination with a KHFAC electrode to block the onset produced by the KHFAC [31]. These acute experiments were performed on the sciatic nerve using platinum black electrodes in four rats.
One major drawback of the CBDC waveform is that it can only block for a few seconds before the charge phase must be delivered, and no block effect occurs during the recharge phase. We refer to this as the duty cycle of block, expressed as the percent of time during which the nerve is blocked compared to the time during which the nerve is not blocked (while the electrode is in the recharge phase). For applications where CBDC block is used in combination with KHFAC to mitigate the onset, this limitation is typically not an issue, as the required duty cycle is well below 10%. However, CBDC block has desirable features by itself, including the generation of nerve block without producing an onset and the possibility of using simpler electrode designs not requiring nerve cuffs. We hypothesized that it would be possible to increase the duty cycle of CBDC block by using multiple electrode contacts placed longitudinally along a nerve and cycling the block between each contact. We refer to this approach as “CBDC carousel block”, in which block is delivered through successive contacts while the other contacts are in their recharge phases. With sufficient contacts, it should be possible to generate a 100% duty cycle block in the nerve by cycling back to the first contact once its recharge phase is complete. In this study, we tested the feasibility of two and four contact CBDC electrodes to increase the duty cycle of block, and we evaluated the effect of waveform parameters on the effectiveness of block in an in vivo motor fiber rat model.
II. Material and Methods
Several different experiments were performed to test key parameters of CBDC carousel block. The objective of these experiments was first to provide proof of concept of the carousel technique, and secondly to characterize the design parameters so that an optimal configuration could be determined. Experiments verified that complete block could be achieved using a two contact configuration. The timing interval of the waveform was then tested to determine a range that could feasibly provide 100% duty cycle block. Since the recharge level has an impact on the number of channels needed, several levels were evaluated to make sure that block could be maintained during the recharge. Finally a four contact solution was tested using a 100% duty cycle. The list of experiments is shown in Table 1.
A. Electrode Fabrication
Several different electrode configurations were generated for testing, using the J-cuff electrode design described in Foldes et al [42]. Electrodes were fabricated using platinum foil for the contact surface attached to either a platinum or stainless steel wire. The foil was then encapsulated in silicone sheeting and a window was cut in the silicone sheeting to expose the platinum surface. Window sizes were 3×2 mm in area. These electrodes were fabricated in either a monopolar, bipolar, or tripolar conformation.
The electrodes were electrochemically cleaned using a square wave of +3 mA for 10 sec, −3 mA for 10 sec, for 25 cycles in 0.1 M H2SO4. An electrodeposition process was used to create a platinum black surface as described in Feltham [43]. The platinum black was deposited from a chloroplatinic acid solution (5g H2PtCl6 in 500ml H2O, with NaCl (2.9 g) and lead acetate (0.3 g)) and applying a galvanic square wave (−56 mA/cm2 ‘on’ current for 5 sec, followed by 5 sec at open circuit = 10 sec/cycle) to platinize the surface. The total charge capacity of the electrode was controlled by regulating the number of deposition cycles.
The maximum charge that can be delivered through each electrode contact was determined by measuring the electrochemical water window and calculating the charge capacity, as described previously [21]. The electrochemical water window is defined as the voltage range where neither oxidation nor reduction of water or protons occurs [44]. To analyze the water window, a cyclic voltammogram [44] for each of the electrodes was generated using a Solartron Inc., Model 1280B potentiostat using a BASi RE5B reference electrode (measurements are accurate to within 10 mV) with sweep rate 10 mV/s, voltage range of −0.225 to +1.20 V, and sampled at 10 Hz. For platinum and platinum black, the amount of charge that could be safely delivered by these electrodes (referred to as the ‘Q value’) was estimated by calculating the pseudocapacitive charge associated with hydrogen adsorption by integrating between −0.25 to +0.15 V as described in Vrabec et al [30].
B. Waveform Design and Parameters
Successfully production of 100% duty cycle depolarization block through multiple contacts on an electrode surrounding the nerve requires knowledge of the interaction of the various physical and electrical parameters of the system. We based our 100% duty cycle block on the CBDC waveform as described by Vrabec et al. [30]. This waveform has a zero net charge and is capable of producing depolarization block in nerves for periods of a few seconds. It consists of a linear ramp to a plateau amplitude (cathodic current), a plateau of a few seconds (during which the nerve is blocked), a linear ramp back to zero, and a rectangular phase with an amplitude (anodic current) and duration sufficient to charge-balance the first phase (Figure 1).
Fig. 1.
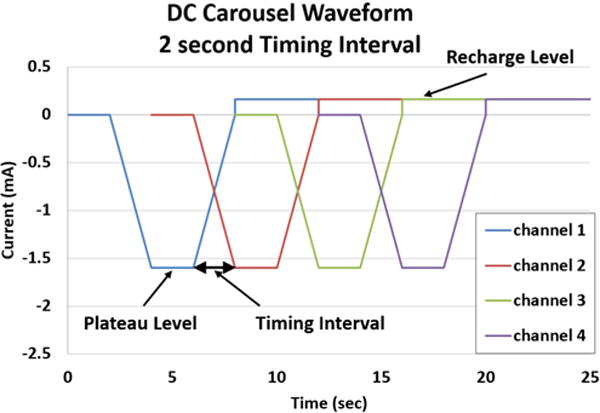
DC Carousel Waveform: Four channels each produce a CBDC waveform separated in time by 2 seconds.
When multiple contacts are used, the amplitude of the current required for the blocking plateau of the waveform depends on the individual block threshold determined for that contact. The maximum usable duration of the blocking plateau depends on the Q value limit for the electrode, as described previously [30]. The Q value is expressed in as total charge in mC, so the total charge in the blocking phase, which depends on the current of the plateau and the time of the plateau, must be lower than the Q value. In addition, the ramp to plateau and the ramp to recharge must be long enough to prevent DC onset, but as short as possible to maximize the amount of time spent at the DC block level. In order to precisely and efficiently describe this waveform, we develop a shorthand notation convention for this waveform that will be utilized in this paper. The specific notation for the blocking waveform is as follows: Time duration of the Ramp to DC block plateau/Plateau duration/duration of the Ramp to start of Recharge. Using this notation, a waveform with a ramp to DC time of two seconds, a plateau time of four seconds, and a ramp to recharge time of two seconds, the specification would be “2/4/2”.
When multiple contacts are utilized in a carousel configuration with a goal of producing a 100% duty cycle block, there are multiple parameters that can be optimized, as outlined in Table 2. One critical parameter is the timing between the CBDC waves delivered on each contact. An example of the waveform timing parameters for a four contact electrode is shown in Figure 1. The waveform is generated on each contact offset in time from the previous contact by a specified time duration (“Timing Interval” in Figure 1). Figure 1 shows the CBDC waveform with a 2 second timing interval between contacts. When the waveform timing interval is zero, a current amplitude sufficient to block nerve conduction is always being applied on at least one of the contacts, and thus continuous block should be achieved across the entire four-contact electrode. However, increasing the waveform timing interval between contacts could result in a longer total block time for the same total charge output, since it may be possible that ramping the current on two nearby contacts will maintain a block even if the current amplitude is slightly below the block threshold. In order to provide the most efficient continuous block, the timing offset between contacts must be maximized, but not extended beyond the point where block is lost between the plateau phases of sequential contacts.
A second critical parameter in optimizing the efficiency of continuous multi-contact CBDC block is the amplitude of the recharge phase of the waveform. Vrabec et al. [30], used a recharge level set to 10% of the blocking phase plateau level. This results in a recharge phase duration that is more than ten times the duration of the blocking phase, which for a single channel, results in a low duty cycle of 10%. The recharge phase amplitude and duration significantly impacts the number of contacts that are needed. If a higher recharge amplitude can be utilized, the duration of the recharge phase can be decreased while still providing complete recharge of the applied blocking phase. A shorter recharge duration means that the entire CBDC waveform is shorter, allowing a single contact to begin the next blocking phase more rapidly. The net effect of a higher recharge phase amplitude is that fewer contacts are needed for 100% duty cycle block as shown in table 2. The number of contacts required directly affects the total length of the electrode assembly, and thus it is desirable to minimize the total number of contacts required for 100% duty cycle block.
The duration of the plateau was set to maximize the amount of time at blocking levels. This was related to the number of channels and the level of recharge that could be used while still achieving a 100% duty cycle block. Depending on the block threshold, the length of time may be limited by the amount of charge that can be delivered and remain below the Q value (charge capacity) of the electrode. Since this is a complex relationship, for these experiments, the plateau duration was selected to be a particular value, typically fixed at four seconds.
The number of parameters involved in the implementation of the CBDC carousel requires a methodical approach to achieve optimization. Table 3 summarizes the CBDC parameters and the goals for each.
C. Experimental Setup and Waveform
Acute experiments were performed on Sprague-Dawley rats to test the viability of the CBDC carousel. All experiments were approved by our institutional animal usage committee. The surgical exposure is described previously in detail [1, 45, 46]. The animal was placed under anesthesia (Isoflurane) and then the sciatic nerve and the gastrocnemius muscle were exposed. The Achilles tendon was detached and fixed to a force transducer (Entran, Fairfield, NJ; resolution 0.005 N). Gastrocnemius tendon tension was measured on the force transducer at a data sampling rate of 1000 Hz. This allows for a test of the activity of the motor fibers of the sciatic nerve.
Bipolar stimulating electrodes were placed proximal and distal to the blocking electrodes to measure nerve block and conduction reduction. As shown in Figure 2, up to four platinum black contacts are used for the CBDC carousel with a single needle placed in the tissue as a common return for all contacts. The DC carousel waveform was generated by a Labview application and was output through a National Instruments DAQ (NI USB-6229 BNC). Four output channels were used, one for each contact. The timing of the four outputs were coordinated by the Labview application. Each voltage output controlled a voltage-controlled current source (built in house), which was then output to each of the CBDC carousel contacts. The ground of all four current sources were connected together and then connected to a needle placed subcutaneously in the animals’ back, which served as a common return electrode for all four monopolar DC contacts.
Fig. 2.
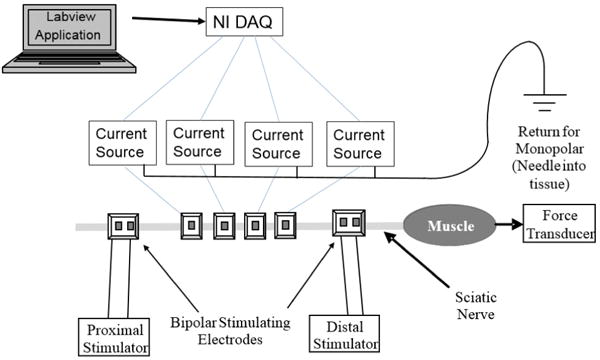
Experimental setup for multi contact block: Four current sources with a common ground are controlled by Labview through a National Instruments DAQ. Monopolar block electrodes are flanked by stimulating electrodes which are used to evaluate nerve block and nerve health. The muscle response is measured using a force transducer.
D. Complete Block Using DC Carousel
Complete block using multiple contacts was tested in two animals. For these experiments, no attempt was made to provide a block with 100% duty cycle. The recharge amplitude (percentage of block plateau amplitude) was maintained below an amplitude that would produce unwanted neural activity. Two contacts were used in the first animal and up to four contacts were used in the second animal.
The first step in these two experiments was to determine the CBDC block threshold for each electrode contact using the following steps. The proximal stimulation was adjusted to provide maximal muscle twitches at 1Hz. A 2/4/2 CBDC waveform was applied with a plateau at 1 mA and the force output was measured. The plateau level was then increased or decreased until the block threshold (complete elimination of any muscle twitch due to proximal stimulation) was found using a binary search pattern with a block threshold resolution of 0.1 mA. The total charge was calculated in each test to ensure it did not exceed 90% of the Q value. The block threshold determined in this manner was used as the starting amplitude on each channel for the DC carousel.
In the first animal, two platinum black monopolar blocking electrodes (2 contacts) were spaced approximately 1 mm apart on the nerve. The 2/4/2 waveforms had a timing interval of 2 seconds and a 15% recharge phase (Rat 1) which is a 25% duty cycle of block. In the second animal two platinum black electrodes with two contacts each (4 contacts total) were placed approximately 1 mm apart on the nerve. The spacing between the contacts on each electrode was 2 mm. The 2/12/2 waveforms had a timing interval of 2 seconds with a 10% recharge phase (Rat 2) which is a 36% duty cycle of block.
E. Effect of Timing Interval Between Block Plateaus
The effect of the timing interval between block plateaus was tested by using different timing intervals and measuring the force output to determine the effectiveness of the block. Two monopolar platinum black blocking electrodes were spaced approximately 1mm apart on the nerve. A proximal stimulating electrode was used to create a force output at either 1 Hz or 2 Hz. The waveform consisted of a 2/4/2 waveform on each channel with the plateau set at the block threshold level. The recharge amplitude was set to 10% of the block threshold. Timing intervals of 0 sec, 1 sec, 2 secs, 3 secs and 4 secs were tested and the force output was measured.
F. Effect of Different Recharge Levels
Although complete block can be obtained while using a recharge amplitude of 10%, a 10% recharge results in a long recharge phase duration in order to completely reverse the charge. Since typically no block occurs during the recharge phase, it is difficult to achieve 100% duty cycle block without reducing the length of the recharge. In order to do this, the recharge amplitude must be higher than 10%. Two contacts of a tripolar electrode were used for blocking. A 2/4/2 waveform with plateaus of 0.5 mA were used on each channel. Recharge levels of 10%, 20%, 30%, and 40% were compared. In each case the timing interval between channels was fixed at 0 seconds. For 10%, 20% and 30%, the distal contact was activated first, and then the middle contact. In order to verify if the block was dependent on the order of the channels, for 30% and 40% the order of the channel activation was reversed. A 2Hz proximal stimulation was used to generate twitches and the effectiveness of the block was evaluated. Once block effectiveness was determined for a 0 second timing interval, timing intervals of 1 sec, 2 sec, and 3 sec were also tested with a 30% recharge (Rat 1).
G. 100% Duty Cycle Block Using Four Contacts
In one animal a 100% duty cycle block configuration was attempted using four contacts, based on the parameters determined from the previous sets of experiments. For a 2/4/2 waveform with a timing interval of 2 sec, a four contact configuration would require a recharge of 37.5 % as described in Table 2. From the recharge results, it was determined that this value was likely to be too large. The waveform was modified to use a 2/4/2 waveform with 3 seconds between channels and 34% recharge, allowing the possibility of a 100% duty cycle block with only four contacts. During the recharge phase, a 2 second ramp was added on the rising edge and a 1 second ramp on the falling edges. This adjustment prevents neural activation during these phases, and also allows a 34% recharge to be used instead of the 37.5% described in Table 2. In this configuration, the first channel has completed its recharge phase immediately after the fourth contact has completed block. This allows the first channel to restart the cycle and produce a continuous block.
The four contact configuration consisted of 2 bipolar electrodes (Rat 4) with 2 mm between contacts and the electrodes spaced approximately 1 mm apart on the nerve. DC block threshold was first determined as described previously for each channel and these amplitudes were used as the starting value for the four contact configuration. A 1 Hz proximal stimulation (PS) was applied, and the amount of block was measured. The amplitude levels were further adjusted to provide the optimal block. In addition to the 1Hz proximal stimulation, a 50Hz tetanic proximal stimulation was also tested to verify that block could be maintained during tetanic stimulation.
For the four contact carousel testing, the amount of block was determined by calculating the area under the force curve during the blocked phase of the trial. This was compared to the estimated force output that would have been generated if the block had not been in effect. To estimate the force that would have been generated for the 1 Hz stimulation, the area under the force curve was determined for five PS twitches. This value was extrapolated over the duration of block by multiplying by the total duration of block divided by the duration of the five PS twitches. For the tetanic pulses, the maximum force of the tetanic stimulation before block was multiplied by the duration of block. The blocking area was then divided by the estimated force area to determine the percentage of force that was not blocked.
The four contact carousel testing was repeated for a total of 45 cycles in 5–10 cycle segments. At the start of each segment, proximal stimulation at 1 Hz was initiated to measure the muscle force and then the carousel was started to demonstrate block of the proximal signal. The block was recorded for several cycles to demonstrate that the block was still effective, and then both the recording and proximal stimulation was halted until the end of the segment. At the end of the segment, both recording and proximal stimulation were restarted to measure the result of the termination of the carousel block. After each segment had completed, a trial was performed to compare the force from the proximal stimulation (PS) electrode as compared to the distal stimulation (DS) electrode. The ratio of the force from the proximal electrode to the distal electrode was calculated to determine if the nerve conduction was affected by the block. A ratio of one indicates that the force is the same from either electrode and demonstrates that the nerve is conducting normally. A ratio below 0.9 was specified as the level at which conduction reduction had occurred. This ratio is referred to as the PS/DS ratio. A total of 30 cycles of force data to demonstrate block were measured over the 45 cycles of carousel block. The PS/DS ratio to check nerve integrity was measured a total of six times.
III. Results
A. Complete Block Using CBDC Carousel
Complete conduction block using the CBDC carousel is shown in Figure 3. For animal 1 (2 contacts), the complete CBDC block was on for 12 seconds with a duty cycle of 25%. The Q values of the electrodes were measured as 28.8 mC and 29.0 mC, and the block thresholds for each channel were 1.0 mA and 1.2 mA. Neural activation occurred when the second channel transitioned to the recharge phase (occurs at 24 seconds in Figure 3). For animal 2 (4 contacts), the complete DC block was continuous for 56 seconds with a duty cycle of 36%. The Q values for the four contacts were determined to be 23.5 mC, 27.6 mC, 24.3 mC and 21.2 mC and the block thresholds were 1.2 mA, 1.4 mA, 1.0 mA, and 2.2 mA. In this example, no activation was observed during the transition phases in the CBDC waveform on any of the four contacts.
Fig. 3.
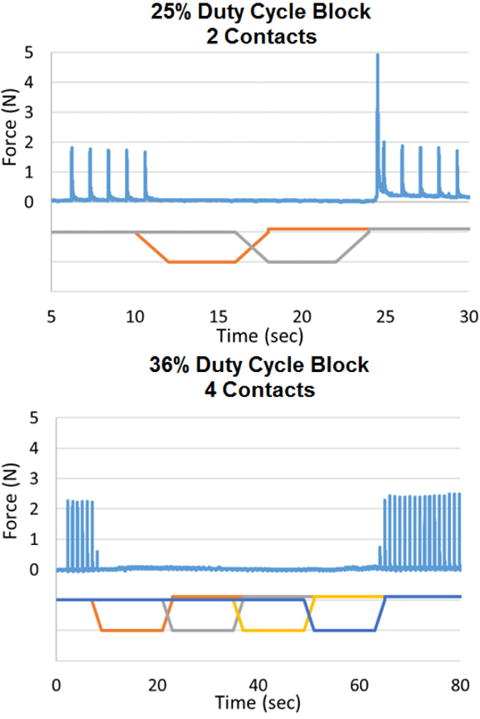
Complete block using DC carousel block: In the 2 contact configuration, the nerve is blocked for 12 seconds, and in the 4 contact configuration, the nerve is blocked for 56 seconds.
B. Effect of Waveform Timing Interval
The effect of waveform timing interval is shown in Figure 4 A–E. The Q values of the electrodes used in this test were 24.3 mC and 21.4 mC, and the block threshold was 0.6mA for each contact. The results show that for intervals of 0, 1, or 2 seconds, complete block occurs throughout the entire waveform, even during the transition periods. For a timing interval of 3 seconds we observed that motor activity at an attenuated level could pass through the nerve unblocked during the transition period between the plateaus. When the timing interval was increased to 4 seconds, there was a distinct period of time during which the nerve was not blocked at all during the transition period.
Fig. 4.
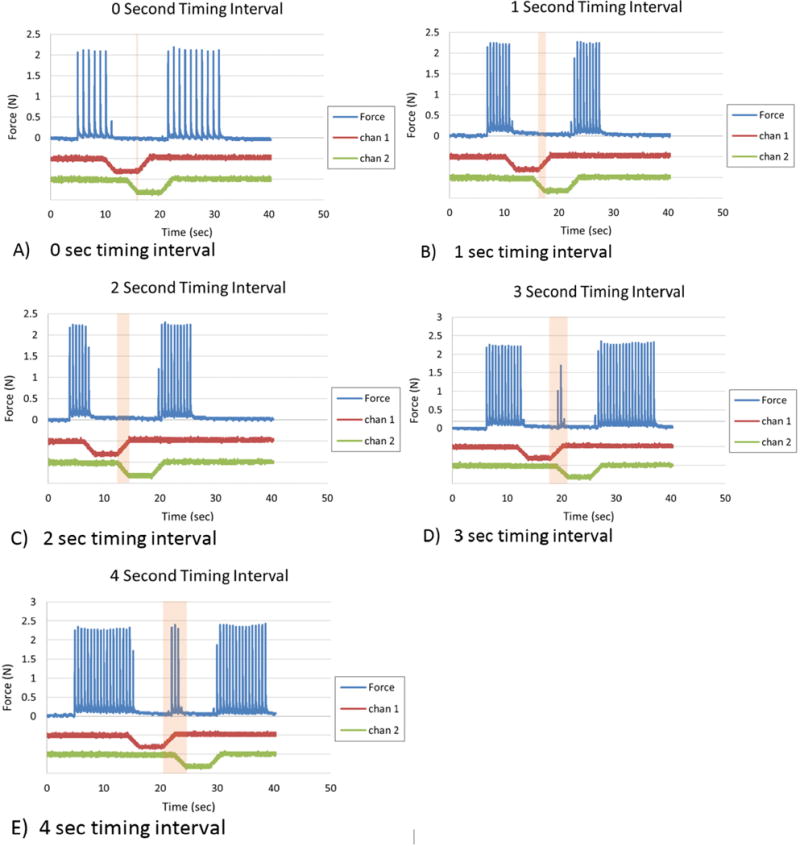
The effect of timing interval on block effectiveness: A timing interval of 0–2 seconds results in complete block between the plateau phases. Increasing the timing interval above 3 seconds causes an incomplete block between the plateaus. The timing intervals of the waveforms are increased by 1 second in trials A-E. The top line in each graph shows the force output due to a 2Hz stimulation. The middle line in each graph is the first CBDC channel and the bottom line is the second CBDC channel.
C. Effect of Different Recharge Levels
Figure 5 shows the effect of different levels of recharge on the neural response. For this test, the Q value of all three contacts together and then divided by three for an estimated Q value per channel of 13.3 mC. The nerve block threshold was measured to be 0.5 mA for each contact. The results show that for 10% recharge, block occurred throughout the plateau and ramps on channel 1. When channel 1 began recharge and channel 2 was in the block phase (shaded in pink), the force was blocked completely even though channel 1 was in recharge. For 20% and 30% the force was blocked completely, but neural activation was observed when the recharge on channel 2 was activated. If additional channels are added it should be possible to suppress this activity by adding additional contacts to the carousel configuration. The order of the contact activation was reversed for 30% recharge, and this resulted in an incomplete block during the plateau phase for the second activated contact. It was possible to suppress this activity by increasing the plateau level of the second activated electrode. The results of the carousel with a 40% recharge indicated that the blocking effect during the plateau for the second activated contact was almost completely eliminated. This indicated that the use of a 40% recharge would not be feasible for use in the carousel configuration. Based on these results, the 30% recharge appeared to provide the best balance between complete, continuous block and the shortest recharge phase duration.
Fig. 5.
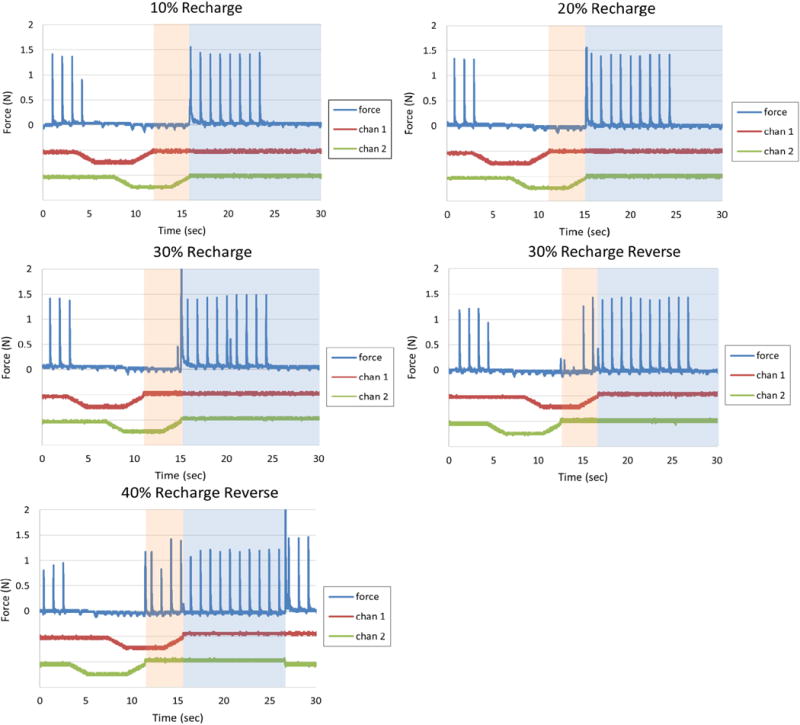
The effect of recharge on blocking: At 10% and 20% recharge it is possible to completely block the second channel during the recharge phase of the first channel. At 30% recharge, some partial twitches appear, but can be blocked with higher plateau amplitudes. If the channel order is reversed, twitches at 30% are no longer blocked. At 40%, full twitches appear making it difficult to produce an amplitude that will block completely.
In order to create a 100% duty cycle block using the carousel approach, not only does the recharge amplitude need to be optimized, the timing interval between the waveforms needs to be optimized. Three different intervals were tested with the 30% recharge level and the results are shown in Figure 6. For timing intervals of 1 second and 2 seconds, complete block occurs throughout the plateaus for both contacts. For a timing interval of 3 seconds, a small force twitch was observed in between the plateaus of each contact.
Fig. 6.
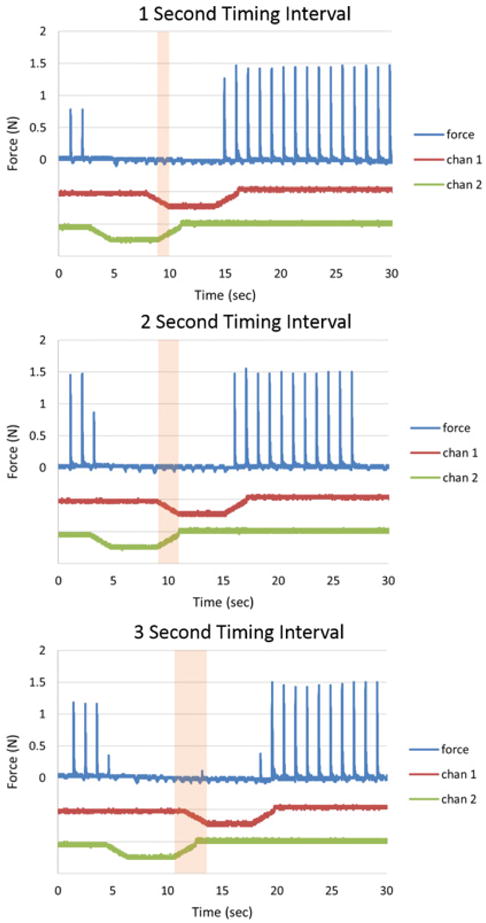
Effect of timing interval on 30% recharge waveforms: For timing intervals of 1 and 2 seconds, complete block occurs during the plateau phases. For a 3 second timing interval, a small twitch occurs between the plateaus.
D. 100% Duty Cycle Block Using Four Contacts
The results for the test of the 100% duty cycle block in Rat 4 are shown in Figure 7. The waveform used in this test had a timing interval of 3 seconds and a recharge level of 34%. This configuration produced a nearly complete block throughout the waveform delivery to each channel. The Q values were determined to be 23.2 mC, 32.95 mC, 33.9 mC, and 29.0 mC for each contact. The plateau values were set to 2.0 mA, 2.4 mA, 1.6 mA and 1.1 mA (block threshold level). With this configuration and parameter set, a few action potentials are able to pass through unblocked during the transition periods between the complete block at each contact. The block of the 1 Hz stimulation was evaluated using the force area calculation and determined to be a 97.5% block. Tetanic block shows a similar trend. The tetanic force block was determined to be a 99.4% block.
Fig. 7.
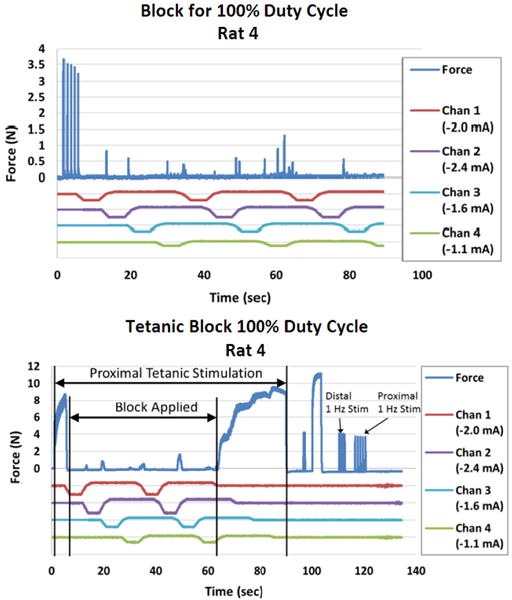
Continuous block using four contacts for a 100% duty cycle: A 4 contact carousel block can produce nearly complete block of with a 100% duty cycle as demonstrated using a 1 Hz input signal and a tetanic input signal.
Figure 8 shows continued CBDC carousel block in Rat 4 for 45 continuous cycles adding up to a total of 22 minutes of nerve block. Nerve conduction block was measured in 30 cycles and the results are shown in figure 9. The mean percentage of nerve block was 96.7% with a 2.8% standard deviation. The PS/DS ratio was measured at six points during the 22 minute trial and the results are shown in figure 9. The mean PS/DS ratio was 0.94 with a 0.05 standard deviation. At the end of this period of block, the ratio of proximal stimulation to distal stimulation was still at 0.88, indicating a minimal effect of the CBDC block on axon survival.
Fig. 8.
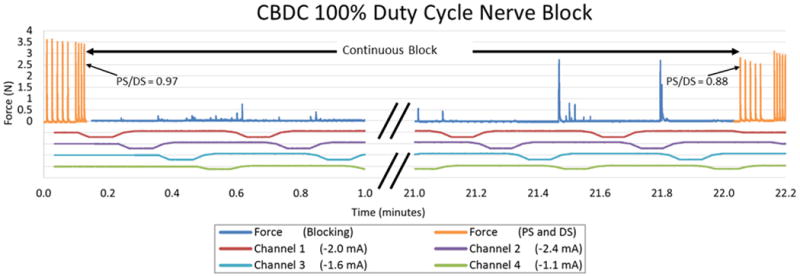
100% duty cycle block using four contacts: Block was applied continuously between the PS/DS measurements for up to 22 minutes. Force output is shown in blue, and the result of the proximal and distal stimulation without blocking are shown in orange.
Fig. 9.
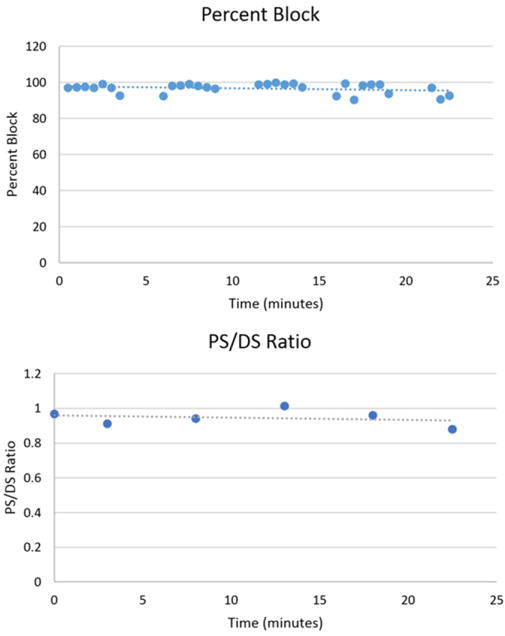
Block percentages and conduction integrity for a 100% duty cycle: Block percentages as measured at the start and end of each block segment are plotted vs time. Block is maintained above 90% for all cycles. The ratio of proximal to distal stimulation force as measured between segments is plotted vs time. Ratios do not drop below 0.88. Both trends show a negligible decline.
IV. Discussion
The delivery of cathodic current to a peripheral nerve can produce a conduction block that is highly repeatable and rapidly reversible. Cathodic block can be achieved using a charge-balanced direct current waveform. In our previous work [30], we demonstrated that it was possible to use CBDC to block nerve conduction at a duty cycle of approximately 10 seconds of block to 100 seconds of no block (10% duty cycle). In this paper, we demonstrate that it is ∼possible to significantly increase this duty cycle by delivering the CBDC waveform sequentially through multiple independent electrode contacts arranged longitudinally along a peripheral nerve, referred to as “carousel block”. Specifically, we demonstrated that a 25% duty cycle complete block of motor fibers can be achieved with two contacts; and a 100% duty cycle block (>95% complete block) can be achieved with four contacts. It is even possible to maintain this latter block for periods of up to 22 minutes with minimal residual effect on the nerve.
The waveform parameters and electrode configuration have a direct impact on the outcome of the carousel block. The current delivered through each contact can either enhance or suppress the block under the adjacent contact as the field extents longitudinally along the nerve. If the electrode contacts are physically separated longitudinally, both the enhancement and suppression effects will be lessened. In our experiments, we kept the distance between the contacts fixed at 1–2 mm in order to minimize the total length of the electrode assembly. Further study is needed to determine the optimal distance between contacts. In addition, it may be possible to design electrodes with minimal longitudinal spread of current along the nerve. Specifically, the use of bipolar configurations might allow each contact to act in a more isolated manner on the nerve. Our experiments exclusively utilized a monopolar CBDC block configuration, with a common return placed remotely in the tissue.
When a subthreshold block level is applied on adjacent contacts, such as occurs during the ramp up from the plateau phase of the CBDC waveform, the block can be enhanced by both contacts and complete block can occur even during these transition periods. Our results indicate that the block plateaus of adjacent channels can be separated by up to two seconds and still produce complete block in between the block plateaus. Using four contacts, we demonstrated that even a separation of three seconds blocks almost all neural activity, resulting in a 90% or greater effective block.
The amplitude of the current delivered during the recharge phase has a significant impact on the design of the CBDC carousel block. When the recharge (anodic) current is delivered through one contact, that current can spread to the contact delivering block (cathodic) and partially or fully suppress the block effect. In addition, the total number of contacts required to produce a continuous carousel block is determined primarily by the duration of the recharge phase. A higher amplitude during the recharge phase allows a shorter recharge duration, minimizing the number of contacts required. For a four contact configuration, the recharge amplitude needs to be at least 34% of the block plateau amplitude to achieve a continuous block. We tested adjacent contacts at recharge values of 10%, 20%, 30% and 40%. The 10% and 20% recharge values did not significantly impact the block of an adjacent contact. At 40%, the recharge prevented the blocking electrode from producing any measurable block, demonstrating that this level is unlikely to be usable for carousel block. At 30%, we found that the block could be maintained at the adjacent contact in some cases, but that the order in which the contacts were cycled impacted this result. Thus, we found a tradeoff occurring around this level of recharge. Based on our results, a recharge amplitude of ∼27% would likely eliminate interaction between contacts, but this would have required a five contact electrode. In our experiments, we chose a recharge amplitude of 34% because this could be implemented with only four contacts, even though the results indicated that this level might not allow 100% block. This configuration proved to be highly successful, producing a continuous block effect that was greater than 95% in both cases tested.
The results we obtained indicating that the interaction between contacts could depend on the order in which the contacts were blocked (Figure 5) indicates an opportunity for optimization. The interaction between contacts is dependent on the exact shape of the electrical field around each contact. Thus, it may be possible to optimize the block effect in a carousel configuration by altering the order in which contacts are blocked. In our study, we always blocked each contact in consecutive longitudinal order, and maintained the same timing interval between each contact. Future studies could explore these parameters, and the effect is likely to depend on the exact electrode geometry and tissue properties within and around the electrode. Therefore, carousel block might be optimized by customizing the parameters with each application. It is also possible that these parameters could vary over time, possibly requiring re-tuning at regular intervals.
The physical size of a CBDC carousel electrode is an important parameter to be minimized. For our four contact configuration, with 2 mm contact width, 2 mm spacing between contacts, and 1 mm insulation on each end, the resulting electrode would have a total length of 16 mm. Currently, cuff electrodes used in clinical applications vary in length from 10 mm for sensory applications [47], 20 mm on the optic nerve [48] and as high as ∼75mm on the femoral nerve [49, 50]. The total length of the electrode could be reduced by reducing the contact width. A smaller geometrical size of each contact would reduce the total charge that can be delivered during the blocking phase, which would translate into a shorter block plateau. It may still be possible to maintain continuous block, as long as block can be maintained during the timing interval between plateaus, noting that the ramp times are fixed and do not scale with the block plateau duration.
In this study only a small number of animals were evaluated. Additional acute studies would be needed to account for underlying nerve anatomy, electrode-nerve contact, or nerve-muscle dissection across experiments. Also, for the parameters tested, only single trials were performed to evaluate the potential effect of these parameters. Now that these exploratory tests have been performed, more rigorous acute tests can be designed, which include multiple trials using appropriate randomization techniques.
We previously demonstrated that a single contact, delivering a 10% duty cycle block, could be repeatedly delivered to peripheral nerves without any measurable decrement in conduction. Similar experiments will need to be repeating using the carousel configuration. Although this study evaluated recharge and spacing, there are several other parameters which may affect safety and effectiveness such as, channel order, spacing of contacts and contact geometry. These parameters would need to be evaluated as well before a configuration could be developed to demonstrate acute safety.
The chronic safety of this approach will also need to be tested in future studies. Previous studies have shown that for Platinum Black electrodes, the Q value degrades during acute experiments [30, 31]. A degradation of the Q value could result in the application of charge beyond the safe levels of the electrode. This surface instability will need to be addressed before a clinical solution is feasible. In addition, previous studies have shown a possible pathway to damage when the applied plateau level is well above the block threshold for the electrode [30]. This damage mechanism needs to be studied in more detail to determine the acceptable parameters to deliver block without reducing the conduction of the nerve. Chronic studies would also allow for many more cycles of nerve block to be performed. These studies would need to include single electrode studies to verify the chronic feasibility of the CBDC waveform as well as CBDC carousel studies to determine the effect of prolonged block on the nerve.
The optimum CBDC carousel configuration will depend on the requirements of each clinical application. The in vivo testing performed in this study focused on the motor activity of the sciatic nerve which comprises <10% of the sciatic nerve fibers [51]. For some clinical applications, such as in the control of autonomic nervous system activity, a continuous block may not be required and may, in fact, be undesirable. For those applications, a 10%–30% duty cycle of block may be entirely sufficient, requiring only one or two contacts. For applications such as the suppression of pain, a multi-contact continuous carousel block may be highly desirable, but the total block effectiveness may not need to approach 100%. Significant pain reduction might be obtained at considerably lower levels of block effectiveness, potentially allowing block to be achieved with only three or four contacts. For clinical applications where there is a significant length of peripheral nerve over which to apply the blocking effect, it may be desirable to apply the carousel block through two or more physical electrodes, each of which may have one or two contacts, thus reducing the total length of the nerve cuff required at any one point along the nerve.
In this study, we focused on the use of nerve cuff electrodes to deliver the CBDC block. However, it is likely that CBDC block can be achieved using other electrode configurations that may be easier to implement clinically. For example, electrodes inserted alongside the nerve could also produce effective block. Electrodes designed for minimally-invasive insertion could have multiple contacts through which the carousel approach could be delivered. The results of our study can help to establish the initial design requirements for such electrodes.
V. Conclusions
In this paper, we demonstrated that it is possible to produce a 100% duty cycle nerve conduction block using a multi-contact configuration. A CBDC waveform was delivered sequentially through four electrode contacts, producing a continuous motor block of 95% or greater effectiveness which could be maintained for up to 22 minutes. We determined that the parameters related to the timing between block plateaus on each contact and the amplitude of the recharge phase can be adjusted to optimize the electrode configuration. This approach may have significant clinical use in cases where a partial or complete block of peripheral nerve activity is required. Future study will be required to further optimize this technique and to demonstrate safety for chronic human use.
TABLE I.
Summary of Experiments (Rev = Reverse contact firing order)
| Animal | Contacts (N) | Timing Interval (s) | Recharge (%) | Waveform (s/s/s) | Cycles (N) | Animals (N) | |
|---|---|---|---|---|---|---|---|
| I Parameter Assessment | |||||||
| A. Timing Interval | Rat 3 | 2 | 0 | 10 | 2/4/2 | 1 | 1 |
| 2 | 1 | 10 | 2/4/2 | 1 | |||
| 2 | 2 | 10 | 2/4/2 | 1 | |||
| 2 | 3 | 10 | 2/4/2 | 1 | |||
| 2 | 4 | 10 | 2/4/2 | 1 | |||
| B. Recharge Level | Rat 1 | 2 | 0 | 10 | 2/4/2 | 1 | 1 |
| 2 | 0 | 20 | 2/4/2 | 1 | |||
| 2 | 0 | 30 | 2/4/2 | 1 | |||
| 2 | 0 | 30 Rev | 2/4/2 | 1 | |||
| 2 | 0 | 40 Rev | 2/4/2 | 1 | |||
| 2 | 1 | 30 | 2/4/2 | 1 | |||
| 2 | 2 | 30 | 2/4/2 | 1 | |||
| 2 | 3 | 30 | 2/4/2 | 1 | |||
| 2 | 3 | 30 | 2/4/2 | 1 | |||
| 2 | 4 | 30 | 2/4/2 | 1 | |||
| II Carousel Block | |||||||
| A. Complete Block | Rat 1 | 2 | 2 | 15 | 2/4/2 | 1 | 2 |
| Rat 2 | 4 | 2 | 10 | 2/12/2 | 1 | ||
| B. 100% Duty Cycle Block Using Four Contacts | Rat 4 | 4 | 3 | 34 | 2/4/2 | 45 | 1 |
TABLE II.
Required recharge times and amplitude percentages for different numbers of contacts and waveform timing intervals to produce a 100% duty cycle (Plateau timing fixed at 2/4/2 for all waveforms).
| Contacts (n) | Timing Interval (s) | Recharge Time (s) | Recharge plateau amplitude (%) |
|---|---|---|---|
| 3 | 0 | 4 | n/a |
| 4 | 0 | 8 | 75 |
| 5 | 0 | 12 | 50 |
| 6 | 0 | 16 | 37.5 |
| 16 | 0 | 60 | 10 |
| 3 | 2 | 10 | 60 |
| 4 | 2 | 16 | 37.5 |
| 5 | 2 | 22 | 27.3 |
| 6 | 2 | 28 | 21.4 |
| 11 | 2 | 60 | 10 |
| 3 | 3 | 13 | 46 |
| 4 | 3 | 20 | 30 |
| 5 | 3 | 27 | 22.2 |
| 6 | 3 | 34 | 17.6 |
| 10 | 3 | 62 | 9.7 |
| 4 | 3 | 2/16/1* | 34 |
This refers to a 2 second ramp to recharge, 16 second plateau, and 1 second ramp to zero
TABLE III.
CBDC Parameters and Goals
| Carousel Parameter | Goal |
|---|---|
| Plateau Level | Provide Block |
| CBDC Ramps |
|
| Timing Interval | Close enough to maintain complete conduction block between plateaus |
| Recharge Level |
|
| Number of Contacts |
|
| Duration of Plateau |
|
| Q Value of Each Contact | Large enough to accommodate the charge delivered during the blocking phase. |
Acknowledgments
Research supported by the Case Coulter Translational Partnership Program National Institutes of Health, National Institute of Neurological Disorders and Stroke 1R01NS074149
Biographies

Tina Vrabec, Ph.D BS (1990), MS (1995) Electrical Engineering from Case Western Reserve University, Cleveland, PhD (2016) Biomedical Engineering from Case Western Reserve University. She worked 7 years designing firmware for Bailey Controls and Rockwell automation then spent 10 years designing medical implant software and firmware at the Cleveland FES Center. She is currently an assistance research professor at Case Western Reserve University. Her research interests include the use of direct current by itself and in combination with kilohertz frequency alternating current (KHFAC) nerve block to provide therapies in both the peripheral and autonomic nervous systems.

Niloy Bhadra Ph.D, MBBS (1982), MS Ortho-pedics (1985), FRCS Edinburgh (1989), MS Biomedical Engineering (1999), and PhD Biomedical Engineering (2005) from Case Western Reserve University, Cleveland, Ohio. He is currently Research Assistant Professor in Biomedical Engineering in Case Western Reserve University, Cleveland. His research interests include the implementation of neuroprosthetic systems in individuals with paralysis and electrical nerve conduction block.

Gustaf Van Acker III, MD, Ph.D BS Biochemistry and Molecular Biophysics, in Molecular and Cellular Biology (2004) at the University of Arizona, (Magna Cum Laude and Honors). Ph.D Molecular and Integrative Physiology in Neuroelectrophysiology (2011), MD (2013) University of Kansas Medical Center. His research interests focus on increasing functional recovery following neurological injury and disease, such as SCI and stroke. Active research areas include investigation of non-invasive methods for nerve conduction block to block muscle spasticity in individuals with central nervous system damage or disease, and potentially to abolish pain in the periphery in those with acute or chronic pain.

Narendra Bhadra, Ph.D MBBS (1978), MS Orthopedics (1983), University of Calcutta, India, PhD (2001) in bioengineering from Case Western Reserve University, Cleveland, OH. He was an Assistant Professor at the National Institute for Orthopedics, India and has worked as Staff Scientist at Axon Engineering Inc. Cleveland. Dr. Bhadra is currently Principal Researcher at the Neural Engineering Center, Department of Biomedical Engineering, Case Western Reserve University, and Biomedical Engineer at the Louis Stokes Cleveland Veterans Administration Medical Center. His principal research interests are in design of neural stimulation electrodes for functional electrical stimulation and clinical applications of electrical nerve conduction block.

Kevin Kilgore, Ph.D. received the BS degree in Biomedical Engineering from the University of Iowa, Iowa City, in 1983, and the MS and Ph.D. degrees in Biomedical Engineering from Case Western Reserve University, Cleveland, in 1987 and 1991. He is currently Professor, Department of Orthopaedics at MetroHealth Medical Center and School of Medicine, Case Western Reserve University. He is also a Biomedical Engineer at the Louis Stokes Cleve-land Veterans Affairs Medical Center. His research interests are in the clinical applications of functional electrical stimulation and in the application of electrical currents to control unwanted neural activity.
Contributor Information
Tina Vrabec, Department of Biomedical Engineering, Case Western Reserve University 10900 Euclid Ave., Cleveland, Ohio 44106 (phone: 440-749-7628).
Niloy Bhadra, Department of Biomedical Engineering, Case Western Reserve University 10900 Euclid Ave., Cleveland, Ohio 44106.
Gustaf Van Acker, Department of Biomedical Engineering, Case Western Reserve University 10900 Euclid Ave., Cleveland, Ohio 44106.
Narendra Bhadra, Department of Biomedical Engineering, Case Western Reserve University 10900 Euclid Ave., Cleveland, Ohio 44106.
Kevin Kilgore, Department of Biomedical Engineering, Case Western Reserve University 10900 Euclid Ave., Cleveland, Ohio 44106; Metrohealth Medical Center 2500 Metrohealth Dr Cleveland, OH 44109; Louis Stokes Cleveland Department of Veterans Affairs Medical Center, Cleveland, Ohio, 44106.
References
- 1.Bhadra N, Kilgore KL. High-frequency nerve conduction block. Conf Proc IEEE Eng Med Biol Soc. 2004;7:4729–32. doi: 10.1109/IEMBS.2004.1404309. [DOI] [PubMed] [Google Scholar]
- 2.Boger A, Bhadra N, Gustafson KJ. Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourol Urodyn. 2008;27:435–9. doi: 10.1002/nau.20538. [DOI] [PubMed] [Google Scholar]
- 3.Franke M, Bhadra N, Bhadra N, Kilgore K, Gustafson K. Chronic bladder control post SCI via Electric KHFAC Pudendal Nerve Block. presented at the IEEE EMBS Conference Neural Engineering; San Diego, CA. 2013. [Google Scholar]
- 4.Kilgore KL, Bhadra N. Reversible Nerve Conduction Block Using Kilohertz Frequency Alternating Current. Neuromodulation. 2014 Apr;17:242–255. doi: 10.1111/ner.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve. 2005 Dec;32:782–90. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- 6.Ackermann DM, Jr, Bhadra N, Foldes EL, Wang XF, Kilgore KL. Effect of nerve cuff electrode geometry on onset response firing in high-frequency nerve conduction block. IEEE Trans Neural Syst Rehabil Eng. 2010 Dec;18:658–65. doi: 10.1109/TNSRE.2010.2071882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miles JD, Kilgore KL, Bhadra N, Lahowetz EA. Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. J Neural Eng. 2007 Dec;4:390–8. doi: 10.1088/1741-2560/4/4/005. [DOI] [PubMed] [Google Scholar]
- 8.Ackermann D, Foldes EL, Bhadra N, Kilgore KL. Electrode design for high frequency block: effect of bipolar separation on block thresholds and the onset response. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:654–7. doi: 10.1109/IEMBS.2009.5332738. [DOI] [PubMed] [Google Scholar]
- 9.Gerges M, Foldes EL, Ackermann DM, Bhadra N, Bhadra N, Kilgore KL. Frequency- and amplitude-transitioned waveforms mitigate the onset response in high-frequency nerve block. J Neural Eng. 2010 Dec;7:066003. doi: 10.1088/1741-2560/7/6/066003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitman JG, Kidd C. The use of direct current to cause selective block of large fibres in peripheral nerves. 1975 doi: 10.1093/bja/47.11.1123-b. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1218139. [DOI] [PubMed]
- 11.Kuffler SW, Gerard RW. The Small-Nerve Motor System to Skeletal Muscle. Journal of Neurophysiology. 1947;10:383–394. doi: 10.1152/jn.1947.10.6.383. [DOI] [PubMed] [Google Scholar]
- 12.Pflüger EFW. Untersuchungen uber die Physiologie des Electrotonus. Berlin: Hirschwald; 1858. [Google Scholar]
- 13.Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng. 2004 Sep;12:313–24. doi: 10.1109/TNSRE.2004.834205. [DOI] [PubMed] [Google Scholar]
- 14.Petruska JC, Hubscher CH, Johnson RD. Anodally focused polarization of peripheral nerve allows discrimination of myelinated and unmyelinated fiber input to brainstem nuclei. Exp Brain Res. 1998 Aug;121:379–90. doi: 10.1007/s002210050472. [DOI] [PubMed] [Google Scholar]
- 15.Cangiano A, Lutzemberger L. The action of selectively activated group II muscle afferent fibers on extensor motoneurons. Brain Res. 1972 Jun 22;41:475–8. doi: 10.1016/0006-8993(72)90519-7. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney JD, Mortimer JT. An asymmetric two electrode cuff for generation of unidirectionally propagated action potentials. IEEE Trans Biomed Eng. 1986 Jun;33:541–9. doi: 10.1109/TBME.1986.325818. [DOI] [PubMed] [Google Scholar]
- 17.Manfredi M. Differential block of conduction of larger fibers in peripheral nerve by direct current. Arch Ital Biol. 1970;108:52–71. [PubMed] [Google Scholar]
- 18.Zimmermann M. Selective activation of C-fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301:329–33. doi: 10.1007/BF00362643. [DOI] [PubMed] [Google Scholar]
- 19.Sassen M, Zimmermann M. Differential blocking of myelinated nerve fibres by transient depolarization. Pflugers Arch. 1973;341:179–95. doi: 10.1007/BF00592788. [DOI] [PubMed] [Google Scholar]
- 20.Casey KL, Blick M. Observations on anodal polarization of cutaneous nerve. Brain Res. 1969 Mar;13:155–67. doi: 10.1016/0006-8993(69)90150-4. [DOI] [PubMed] [Google Scholar]
- 21.Accornero N, Bini G, Lenzi GL, Manfredi M. Selective Activation of peripheral nerve fibre groups of different diameter by triangular shaped stimulus pulses. J Physiol. 1977 Dec;273:539–60. doi: 10.1113/jphysiol.1977.sp012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rijkhoff NJ, Hendrikx LB, van Kerrebroeck PE, Debruyne FM, Wijkstra H. Selective detrusor activation by electrical stimulation of the human sacral nerve roots. Artif Organs. 1997 Mar;21:223–6. doi: 10.1111/j.1525-1594.1997.tb04654.x. [DOI] [PubMed] [Google Scholar]
- 23.Mccloske Di, Mitchell JH. Reflex Cardiovascular and Respiratory Responses Originating in Exercising Muscle. Journal of Physiology-London. 1972;224:173–&. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mccloske Di, Mitchell JH. Reflex Cardiovascular and Respiratory Responses Originating in Exercising Muscle. Journal of Physiology-London. 1972;224:173–&. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopp FA, Zuperku EJ, Coon RL, Kampine JP. Effect of anodal blockade of myelinated fibers on vagal C-fiber afferents. Am J Physiol. 1980;239:R454–62. doi: 10.1152/ajpregu.1980.239.5.R454. [DOI] [PubMed] [Google Scholar]
- 26.Hopp FA, Seagard JL. Respiratory responses to selective blockade of carotid sinus baroreceptors in the dog. Am J Physiol. 1998 Jul;275:R10–8. doi: 10.1152/ajpregu.1998.275.1.R10. [DOI] [PubMed] [Google Scholar]
- 27.Coleridge HM, Coleridge JC, Dangel A, Kidd C, Luck JC, Sleight P. Impulses in slowly conducting vagal fibers from afferent endings in the veins, atria, and arteries of dogs and cats. Circ Res. 1973 Jul;33:87–97. doi: 10.1161/01.res.33.1.87. [DOI] [PubMed] [Google Scholar]
- 28.Sant’Ambrogio G, Decandia M, Provini L. Diaphragmatic contribution to respiration in the rabbit. J Appl Physiol. 1966 May;21:843–7. doi: 10.1152/jappl.1966.21.3.843. [DOI] [PubMed] [Google Scholar]
- 29.Bhadra N, Grunewald V, Creasey G, Mortimer JT. Selective suppression of sphincter activation during sacral anterior nerve root stimulation. Neurourol Urodyn. 2002;21:55–64. doi: 10.1002/nau.2068. [DOI] [PubMed] [Google Scholar]
- 30.Vrabec T, Bhadra N, Wainright J, Bhadra N, Franke M, Kilgore K. Characterization of high capacitance electrodes for the application of direct current electrical nerve block. Medical & Biological Engineering & Computing. 2015:1–13. doi: 10.1007/s11517-015-1385-5. 2015/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franke M, Vrabec T, Wainright J, Bhadra N, Bhadra N, Kilgore K. Combined KHFAC + DC nerve block without onset or reduced nerve conductivity after block. J Neural Eng. 2014 Oct;11:056012. doi: 10.1088/1741-2560/11/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005 Feb 15;141:171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Guz A, Trenchard DW. The role of non-myelinated vagal afferent fibres from the lungs in the genesis of tachypnoea in the rabbit. J Physiol. 1971 Mar;213:345–71. doi: 10.1113/jphysiol.1971.sp009386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negi S, Bhandari R, Rieth L, Solzbacher F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed Mater. 2010 Feb;5:15007. doi: 10.1088/1748-6041/5/1/015007. [DOI] [PubMed] [Google Scholar]
- 35.Rui YF, Liu JQ, Yang B, Li KY, Yang CS. Parylene-based implantable platinum-black coated wire microelectrode for orbicularis oculi muscle electrical stimulation. Biomed Microdevices. 2012 Apr;14:367–73. doi: 10.1007/s10544-011-9613-8. [DOI] [PubMed] [Google Scholar]
- 36.Shah KG, Tolosa VM, Tooker AC, Felix SH, Pannu SS. Improved chronic neural stimulation using high surface area platinum electrodes. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:1546–9. doi: 10.1109/EMBC.2013.6609808. [DOI] [PubMed] [Google Scholar]
- 37.Mailley S, Hyland M, Mailley P, McLaughlin JA, McAdams ET. Thin film platinum cuff electrodes for neurostimulation: in vitro approach of safe neurostimulation parameters. Bioelectrochemistry. 2004 Jun;63:359–364. doi: 10.1016/j.bioelechem.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 38.Mills A. Porous Platinum Morphologies: Platinised, Sponge and Black. Platinum Metals Review. 2007 Jan;51:52–52. [Google Scholar]
- 39.Maya L, Brown GM, Thundat T. Porous platinum electrodes derived from the reduction of sputtered platinum dioxide films. Journal of Applied Electrochemistry. 1999 Jul;29:883–888. [Google Scholar]
- 40.Taylor EJ, Anderson EB, Vilambi NRK. Preparation of High-Platinum-Utilization Gas-Diffusion Electrodes for Proton-Exchange-Membrane Fuel-Cells. Journal of the Electrochemical Society. 1992 May;139:L45–L46. [Google Scholar]
- 41.Vrabec T, Wainright J, Bhadra N, Bhadra N, Kilgore K. Use of High Surface Area Electrodes for Safe Delivery of Direct Current for Nerve Conduction Block. Bioengineering Based on Electrochemistry. 2013;50:31–37. doi: 10.1149/05028.0031ecst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foldes EL, Ackermann DM, Bhadra N, Kilgore KL, Bhadra N. Design, fabrication and evaluation of a conforming circumpolar peripheral nerve cuff electrode for acute experimental use. J Neurosci Methods. 2011 Mar 15;196:31–7. doi: 10.1016/j.jneumeth.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feltham AM, Spiro M. Platinized Platinum Electrodes. Chemical Reviews. 1970;71:177–193. [Google Scholar]
- 44.Hayyan M, Mjalli FS, Hashim MA, AlNashef IM, Mei TX. Investigating the electrochemical windows of ionic liquids. Journal of Industrial and Engineering Chemistry. 19:106–112. 1/25/2013. [Google Scholar]
- 45.Ackermann DM, Jr, Bhadra N, Foldes EL, Kilgore KL. Conduction block of whole nerve without onset firing using combined high frequency and direct current. Med Biol Eng Comput. 2011 Feb;49:241–51. doi: 10.1007/s11517-010-0679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilgore KL, Bhadra N. High frequency mammalian nerve conduction block: simulations and experiments. Conf Proc IEEE Eng Med Biol Soc. 2006;1:4971–4. doi: 10.1109/IEMBS.2006.259254. [DOI] [PubMed] [Google Scholar]
- 47.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler DJ. Stability and selectivity of a chronic, multi-contact cuff electrode for sensory stimulation in human amputees. J Neural Eng. 2015 Apr;12:026002. doi: 10.1088/1741-2560/12/2/026002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, et al. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Research. 1998 Nov 30;813:181–186. doi: 10.1016/s0006-8993(98)00977-9. [DOI] [PubMed] [Google Scholar]
- 49.Fisher LE, Tyler DJ, Anderson JS, Triolo RJ. Chronic stability and selectivity of four-contact spiral nerve-cuff electrodes in stimulating the human femoral nerve. Journal of Neural Engineering. 2009 Aug;6:046010. doi: 10.1088/1741-2560/6/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher LE, Miller ME, Bailey SN, Davis JA, Anderson JS, Rhode L, et al. Standing After Spinal Cord Injury With Four-Contact Nerve-Cuff Electrodes for Quadriceps Stimulation. Ieee Transactions on Neural Systems and Rehabilitation Engineering. 2008 Oct;16:473–478. doi: 10.1109/TNSRE.2008.2003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmalbruch H. Fiber Composition of the Rat Sciatic-Nerve. Anatomical Record. 1986 May;215:71–81. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]


