Abstract
Perry syndrome (PS) is a rare hereditary neurodegenerative disease characterized by autosomal dominant parkinsonism, psychiatric symptoms, weight loss, central hypoventilation, and distinct TDP-43 pathology. The mutated causative gene for PS is DCTN1, which encodes the dynactin subunit p150Glued. Dynactin is a motor protein involved in axonal transport; the p150Glued subunit has a critical role in the overall function. Since the discovery of DCTN1 in PS, it has been increasingly recognized that DCTN1 mutations can exhibit more diverse phenotypes than previously thought. Progressive supranuclear palsy- and/or frontotemporal dementia-like phenotypes have been associated with the PS phenotypes. In addition, DCTN1 mutations were identified in a family with motor-neuron disease before the discovery in PS. In this review, we analyze the clinical and genetic aspects of DCTN1-related neurodegeneration and discuss its pathogenesis. We also describe three families with PS, Canadian, Polish, and Brazilian. DCTN1 mutation was newly identified in two of them, the Canadian and Polish families. The Canadian family was first described in late 1970’s but was never genetically tested. We recently had the opportunity to evaluate this family and to test the gene status of an affected family member. The Polish family is newly identified and is the first PS family in Poland. Although still rare, DCTN1-related neurodegeneration needs to be considered in a differential diagnosis of parkinsonian disorders, frontotemporal dementia, and motor-neuron diseases, especially if there is family history.
Keywords: Atypical parkinsonism, DCTN1, Dynactin, FTD, Genetics, TDP-43, Perry syndrome, PSP, Review
1. Introduction
Axonal transport is mediated by a number of microtubule motor proteins and is fundamental to neurons [1]. Deficits in axonal transport contribute to several neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease (PD), Huntington’s disease (HD), hereditary neuropathies, and motor-neuron diseases [2]. A direct link between disrupted axonal transport and neurodegeneration has been emphasized by the identification of mutations in the microtubule motors of patients with neurodegenerative diseases [3]. Dynactin is a multi-subunit protein complex that binds and activates dynein, which engages retrograde axonal transport [4]. The dynein-dynactin motor complex conveys cargo in a retrograde fashion along the microtubules. The dynactin subunit p150Glued, encoded by the DCTN1 gene, has an essential role in binding microtubules and molecular motors, including dynein [4].
Perry syndrome (PS) is a rare neurodegenerative disorder characterized by autosomal dominant inheritance, psychiatric symptoms, parkinsonism, weight loss, and central hypoventilation. DCTN1 is a causative gene for PS [5], but interestingly, a DCTN1 mutation had previously been identified in a family with motor-neuron disease [6]. In addition, rare DCTN1 variants have been found in patients with other neurodegenerative phenotypes, such as amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and progressive supranuclear palsy (PSP)-like syndrome. In this review, we provide current knowledge of PS and discuss the pathogenesis of DCTN1-related neurodegeneration.
2. Ethical standards
The blood specimens from Canadian, Polish, and Brazilian families described in this manuscript were collected under the Institutional Review Board (IRB) Committee approved protocols of the participating institutions. All research subjects signed informed consent. The brain autopsy of the Colombian family index case was performed at the Mayo Clinic Florida after permission obtained from the legal next-of-kin. The Mayo Clinic IRB Committee exempts the autopsy studies from human subject research regulations.
3. Perry syndrome
3.1. From the first clinical description to the discovery of the gene
In 1975, a new neuropsychiatric disorder was described in a Canadian (British Columbia) family by Perry et al [7]. The affected members spanned three generations, and their disorder was passed down via autosomal dominant inheritance [7, 8]. The proband presented with depression, a loss of interest, and withdrawal from society when he was 50 years old. As the disease progressed, he developed fatigue, sleep disturbances, weight loss, and parkinsonian symptoms, such as hypomimia, reduced arm swing, resting tremor, dysarthria, dysphagia, and hypoventilation. None of the drugs typically used for treating neuropsychiatric disorders, including levodopa, benefitted his condition. He died from unexpected respiratory failure at the age of 56 years. His brain showed depigmentation and severe neuronal loss in the substantia nigra; however, no distinct Lewy bodies were seen.
Perry followed this family for over 15 years and identified at least ten affected members with this unique disease. All of these patients essentially developed the same clinical presentation: psychiatric symptoms such as depression and apathy appeared as an early feature, followed by parkinsonism, weight loss, and central hypoventilation (respiratory failure), which could be prominent at the end stage of the disease and the cause of death. Some patients suffered from sleep disturbances, such as insomnia which could have been related to the hypoventilation. Levodopa and antipsychotic therapies usually were not effective in these patients. The mean age of onset was 50 years (n = 10, range: 45–54 years), and the disease duration from onset to death was 5 years (range: 3–6 years).
By 2002, six other families had been reported from Canada (Ontario), the US (West Virginia), France, the UK, Japan (Fukuoka 1), and Turkey [9–15]. These families showed very similar phenotypes and an autosomal dominant pattern of inheritance. Exceptionally, the UK family did not exhibit weight loss or hypoventilation, but other clinical and pathological findings were compatible to that of the original family [13]. Although none of the families were mutually related or had a common ancestor, we hypothesized that a single gene might cause PS based on the uniform and unique phenotype of this disease.
In 2001, an ad hoc international consortium to study PS was established by ZKW with the aim of finding a genetic cause for PS. We collected DNA samples from all but one of the 7 families and from 2 additional families (Japan [Fukuoka 4] and US [Hawaii]) [16, 17]. Unfortunately, we could not obtain any DNA samples from the second Canadian (Ontario) kindred at that time since this family was lost for follow up. Using genome-wide linkage analysis, we identified that a locus on chromosome 2p12–14 was linked to the disease, and finally we discovered DCTN1 as a causative gene for PS in 2009 [5]. Five different mutations of the DCTN1 gene were identified in all 8 families with disease segregation: p.Gly71Arg, p.Gly71Glu, p.Gly71Ala, p.Thr72Pro, and p.Gln74Pro. No common founder was predicted by haplotype analysis. After discovery of the gene, four other PS families with DCTN1 mutations were reported from Korea (p.Gly67Asp) [18], the US (Georgia) (p.Gly71Arg) [19], New Zealand (p.Tyr78Cys) [19], and Colombia (p.Gly71Arg) [19, 20], indicating that PS with DCTN1 mutation could be found worldwide.
3.2 Typical findings of PS
PS is a fatal disease, and the cardinal features of PS are autosomal dominant parkinsonism, psychiatric symptoms, weight loss, and hypoventilation [21]. Typically, parkinsonism and psychiatric symptoms develop in the initial stage of the disease accompanied with or followed by weight loss. Hypoventilation usually appears in the later stage [21]. Levodopa is usually ineffective or has only partial effect for the parkinsonism, and if so in the initial stage of the disease. However, occasionally a substantial beneficial effect can be observed [10, 14, 15]; notably, it is worth trying a high dose of carbidopa/levodopa (>2,000 mg of levodopa per day) if the usual dose does not sufficiently benefit the patient. Psychiatric symptoms mainly manifest as apathy or depression. The weight loss is usually progressive, even if the sufficient nutrition is provided and the extent of the weight loss has been reported to range from 15 kg in 6 years to 10 kg in 2 months. PS-associated central hypoventilation is life-threatening, with the majority of patients dying due to respiratory failure. Polysomnography is useful for detecting central hypoventilation with hypoxia [14], and noninvasive positive-pressure ventilation may be helpful. A successful case treated with a bilateral diaphragmatic pacemaker implantation has been reported [20].
Additional findings that are more similar to PD have been observed in the Japanese families with PS. Impulse control disorders and punding have been reported in two cases from the Japanese (Fukuoka 1) family [22]. These findings might be related to dopaminergic therapy like those seen previously in PD patients. Indeed, one of these patients also had motor complications such as wearing-off and dyskinesia. In another Japanese (Fukuoka 4) kindred, the family members presented with dysautonomia (e.g. orthostatic hypotension, increased urinary frequency, anhidorosis) [17]. A patient from this family showed decreased cardiac metaiodobezylguanidine uptake, which is frequently observed in Lewy body diseases [23], indicating that sympathetic denervation could occur in PS.
No specific findings on brain imaging of PS patients are known. However, striatal dopaminergic and cortical/subcortical serotonergic dysfunctions were indicated by positron emission tomography [18, 24]. Hypoperfusion in the frontal cortex and basal ganglia has also been detected using 123I-single photon emission computed tomography [17], and transcranial sonography revealed hyperechogenicity of the substantia nigra, which is compatible to findings in PD [25].
3.3. DCTN1 mutation in the second Canadian family and in a newly identified Polish family
Very recently, we had the opportunity to evaluate the second Canadian (Ontario) family and found a DCTN1 mutation, p.Gly71Glu, in an affected family member (Figure 1A). Patient IV-2 was one of the daughters of the proband’s identical twin brother, who had been reported as a patient III-7 in 1979 by Purdy et al [9]. Patient IV-2 was admitted to the hospital when she was 57 years old because she had a decreased level of consciousness and had experienced falls. She was found to have hypercapneic respiratory failure and was intubated. A neurological assessment revealed that she had hypometric saccades, facial masking, mild rigidity in her upper and lower extremities bilaterally, hyperreflexia, and positive Babinski signs bilaterally. In the past two years, she became anxious, depressed, and socially withdrawn, which forced her to retire from her job. She also lost a significant amount of weight and fell multiple times during this period. Her symptoms were quite similar to her father who had typical PS. Additionally, her cousin, a daughter of the proband (IV-1), passed away with suspected PS, but we could not gain any detailed information about her.
Figure 1. Pedigrees of a Canadian (Ontario) Family (DCTN1, p.Gly71Glu) and a Polish Family (DCTN1, p.Gly71Glu) with Perry Syndrome.


(A) A Canadian (Ontario) family. This pedigree is generated by combining the original one [9] with the newly obtained information (dashed rectangle). An arrow and an arrowhead indicate the original proband [9] and the new mutation carrier, respectively. The numbers at the left upper side of the symbols indicate pedigree position. (B) A Polish family. An arrow indicates the proband. The numbers at the right lower side of the symbols indicate the age of death. For (A) and (B), the square indicates a male, the circle indicates a female, the diamond indicates a blinded sex. The numbers inside the symbols indicate the number of siblings.
We identified the first Polish family with PS (Figure 1B). A 45-year-old man was initially referred for apathy and bradykinesia. He had been suffering from difficulties in learning, planning, and concentration for last 3 years. He was diagnosed with depression and catatonia by a local psychiatrist who treated him with neuroleptics. Due to the lack of improvement, he was referred to neurology department. Neurological exam revealed mild cognitive impairment, hypomimia, slow speech with dysarthria, severe bradykinesia, more prominent on the left side, and right Babinski sign. He was not able to walk independently. He had difficulty falling asleep. Initially, his parkinsonism was dramatically improved by benserazide/levodopa (1,125 mg of levodopa per day) and ropinirole (8 mg per day). He became ambulatory again. Over the subsequent 3 years, the responsiveness to dopaminergic medications had decreased and his cognitive impairment and parkinsonism gradually progressed. Rest tremor appeared in his left arm. Wearing off and impulse control disorders including increased sexual activity, trichotillomania, compulsive behaviors, and pathological shopping developed. At 3 years after his first visit, he noticed breathing problems and exhibited tachypnea at night. At age of 51 years, he developed acute respiratory failure requiring intubation and supportive ventilation. After recovery, he had two more episodes of acute respiratory failure. At the present time his respiratory problems are managed through nocturnal assisted ventilator support delivered through tracheostomy. He also had dysphagia, oromandibular dyskinesia, restless legs syndrome, and orthostatic hypotension. The “round the houses” sign was observed in his vertical saccades. He experienced an episode of tonic-clonic seizure. Obvious weight loss was not observed. Brain MRI demonstrated only mild cerebellar atrophy. The dopamine transporter imaging showed severely reduced radiotracer uptake in striatum bilaterally. Genetic tests were negative for MAPT, GRN, and C9orf72, but positive for DCTN1, p.Gly71Glu mutation. The pedigree indicates an autosomal dominant inheritance (Figure 1B). Three other family members were diagnosed with atypical parkinsonism and died at relatively young age.
Both families presented with typical PS but weight loss was not obvious in the Polish family. Weight loss is one of the cardinal signs of PS, however, not always reported in previous cases [10, 13, 16]. Interestingly, some symptoms reminiscent of PSP were observed in the patient IV-2 of Canadian family (e.g. hypometric saccades, frequent falls) and in the proband of Polish family (e.g. the “round the houses” sign [26]).
4. Pathology of Perry syndrome
Pathologically, PS is characterized by neuronal loss and gliosis in the substantia nigra (Figure 2). Lewy bodies and neurofibrillary tangles are not present in brains with PS [7–10, 13, 14], however very sparse Lewy bodies had been reported in two Canadian families, a family from the US (West Virginia) and from the UK [7–10]. These observations were made prior to the discovery of α-synuclein and no Lewy bodies have been reported in PS using more specific methodologies. Neurons with focal clearing of cytoplasm or eosinophilic intranuclear inclusions have been reported, but the significance of these findings are unknown [7, 9]. A variable degree of neuronal loss and gliosis is also evident in the caudate nucleus, globus pallidus, subthalamic nucleus, hypothalamus, locus ceruleus, periaqueductal gray matter, ventrolateral medulla, dorsal raphe nucleus, and pontine reticular formation [27]. From an immunohistochemistry analysis of a single autopsied case from a Japanese (Fukuoka 1) family, neuronal loss in the pre-Bötzinger complex of the ventrolateral medulla and serotonergic neuronal loss in the medullary raphe and ventrolateral medulla were found [28]. These latter findings may be responsible for the respiratory failure seen in PS patients.
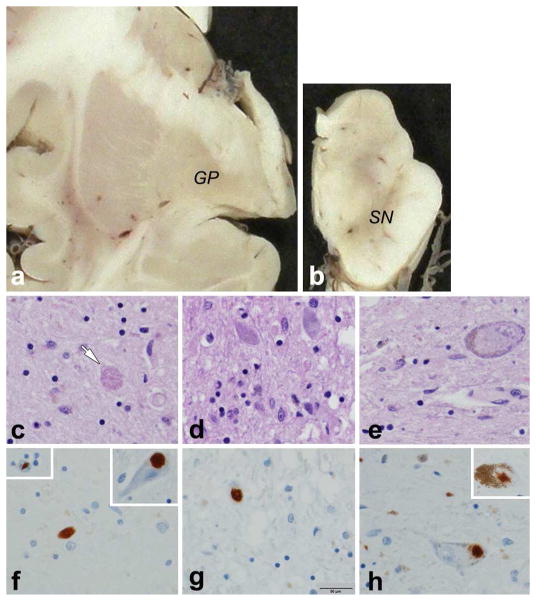
Figure 2. Neuropathology of Perry Syndrome from the Proband of the Colombian Family (DCTN1, p.Gly71Arg).
This case has been reported previously as the proband of the Colombian family [19, 20]. She died at age of 60 years due to pneumonia. Her cognitive function was preserved until her condition was terminal. a. mild atrophy and brown discoloration of the globus pallidus (GP); b. marked loss of neuromelanin pigment in the substantia nigra (SN); C. axonal spheroids (arrow) and gliosis in the globus pallidus; d. neuronal loss and gliosis in subthalamic nucleus; e. neuronal loss and gliosis in substantia nigra, and cytoplasmic clearing in a nigral neuron; f. TDP-43 positive dystrophic neurites in globus pallidus (upper left inset: neuronal intranuclear inclusion; upper right inset: TDP-43 neuronal cytoplasmic inclusion); g. TDP-43 neuronal cytoplasmic inclusion in subthalamic nucleus; h. TDP-43 neuronal cytoplasmic inclusion in substantia nigra (upper right inset: TDP-43 neuronal cytoplasmic inclusion in locus ceruleus). (bar in g = 50 μm applies to panels c - h)
Shortly before identifying the causative gene, PS was discovered to be a transactive response DNA-binding protein of 43 kDa (TDP-43) proteinopathy [27]. Based on our neuropathological analysis of eight patients with PS from Canadian (British Columbia), American (West Virginia), French, and Japanese (Fukuoka 1) families, abnormal TDP-43-positive neuronal cytoplasmic inclusions, dystrophic neurites, glial cytoplasmic inclusions, and axonal spheroids were identified mostly in the substantia nigra and the globus pallidus [5, 16, 27]. The morphology of the TDP-43 pathology was comparable to those seen in other TDP-43 proteinopathies, such as frontotemporal lobar degeneration with TDP-43 (FTLD-TDP) and ALS; however, the distribution was distinct from these diseases (Figure 2). TDP-43 pathology in PS was present mainly in the extrapyramidal system. The neocortex, hippocampus, and upper and lower motor neurons, which are typically affected in FTLD and ALS, were spared. In addition, abnormal phosphorylated and 25-kDa truncated forms of TDP-43 were detected in the insoluble fraction of the substantial nigra and globus pallidus from PS [27]. Therefore, PS is a unique atypical parkinsonian syndrome that is characterized by TDP-43 proteinopathy.
5. Atypical phenotypes of Perry syndrome
The clinical uniformity of PS led to the identification of affected families and the discovery of a causative gene. Indeed, diagnostic criteria based on the cardinal clinical and pathological features had been proposed before the gene was discovered [21]; however, the clinical picture of PS has been expanding since the gene’s discovery. Newsway et al described a UK PS family with atypical features [29]. The proband displayed disinhibited behavior and PSP-like phenotypes such as early axial rigidity, frequent falls, marked slowing of downward saccades, and progressive midbrain atrophy on MRI scans. This patient also had the cardinal PS features but showed a continuously good response to levodopa. He was initially diagnosed as having FTD with parkinsonism, but PS was considered when he later developed central hypoventilation. This patient was observed to harbor the pathogenic p.Gly71Arg substitution in DCTN1 [29]. Another UK patient initially presented with anxiety and depression followed by hypomimia, nuchal ridigity, slow horizontal saccades, eyelid apraxia, and the applause sign when he was in his mid-40s. This patient had frontal-lobe dysfunction and some episodes of disinhibited behavior. He seemed to be a sporadic case and was suspected to have PSP at that time. He later developed weight loss and hypoventilation and was reported to have PS with a DCTN1 p.Gly67Asp mutation [30].
Additional families showing some degree of frontal lobe dysfunction and supranuclear gaze palsy were also reported from Korea and Japan [18, 31], with the Japanese case displaying frontotemporal atrophy on MRI scan [31]. Caroppo et al then identified a French family carrying DCTN1 p.Gly71Glu by analyzing 19 families with PSP-like phenotypes [32]. This pathogenic mutation segregated with disease in four affected members of a family and interestingly showed intrafamilial variability in the clinical phenotypic presentation: PD, PS, PSP, and the behavioral variant of FTD were observed. In addition, a DCTN1 mutation, p.Lys56Arg, was identified in two Asian patients who were clinically diagnosed as PSP by a comprehensive screening in patients with parkinsonism [33].
Recently, a Portuguese family was reported at the Nineteenth International Congress of Parkinson's Disease and Movement Disorders [34]. The PS in this family was passed down via autosomal-dominant inheritance, and affected members showed frontal lobe dysfunction, staring eyes, eyelid apraxia, oculogyric crisis, frequent falls, insomnia, apathy, and parkinsonism. Weight loss and hypoventilation were not observed in the proband or affected siblings, which was similar to the UK family [13], but at least one other affected relative had severe weight loss and respiratory failure. A DCTN1 p.Phe52Leu mutation was reported in the proband.
To summarize these reports, the atypical findings mainly consisted of PSP-like phenotypes, FTD-like phenotypes, and other features (Table 1). These symptoms are atypical when compared with those of typical PS, but they may be not uncommon. Indeed, some of these atypical findings were described in the early reported PS families including the second Canadian and Polish families reported here (Table 2). Clinical heterogeneity within the mutation carriers of the same family has been reported. Although there is no evidence yet, some genetic modifiers or environmental factors may have a role in the phenotypic variability of PS. It is well-established that both caffeine intake [35] and exercise [36] can modify the disease presentation in parkinsonism and for TDP-43 proteinopathy ATXN2 [37] and TMEM106B [38] are recognized as genetic modifiers of penetrance. To date, no pathological analyses have been conducted on atypical PS cases and as such this remains a clinical phenomenon. In addition, for those rare mutations that lack clear patterns of co-segregation with disease in families, caution must be used when conveying pathogenicity.
Table 1.
Typical and atypical clinical features of Perry syndrome
| Typical features |
| Autosomal dominant inheritance |
| Psychiatric symptoms (apathy, depression, and social withdrawal) |
| Parkinsonism |
| Weight loss |
| Central hypoventilation |
| Limited response to L-dopa |
| Fatigue |
| Sleep disturbancea |
| Rapid progression |
| Atypical features |
| PSP-like |
| Supranuclear gaze palsy |
| Eyelid apraxia |
| Frequent falls |
| FTD-like |
| Cognitive impairment |
| Frontal lobe dysfunction |
| Personality and behavioral changes |
| Others |
| Oculogyric crisis |
| Pyramidal signs |
| Dysautonomia |
| Good response to L-dopa |
FTD, frontotemporal dementia; PSP, progressive supranuclear palsy
Including difficulty in falling asleep, insomnia, nightmare, sleep walking, and excessive sleepiness
Table 2.
Demographic and clinical findings of Perry syndrome families with DCTN1 mutations
| Country | Mutation | Number of affected member (M) |
Age at onset (y) |
Age at death (y) |
Disease duration (y) |
Psychiatric symptomsa |
Parkinsonisma | Weight lossa |
Central hypoventilationa |
PSP-likea | FTD-likea | Positive response to L-dopab |
References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typical PS families | Korea | G67D | 3 (0) | 53 | NA | NA | 1 | 3 | 1 | 3 | 0 | 0 | 1 | Chung et al (2014)[18] |
| US | G71A | 6 (2) | 47 | NA | NA | 0 | 2 | 0 | 2 | 0 | 0 | 1 | Wider et al (2010)[16] | |
| Japan | G71A | 14 (10) | 41 | 49 | 6 | 6 | 7 | 5 | 3 | 0 | 0 | 5 | Tsuboi et al (2002)[14], Mishima et al (2015)[22] | |
| UK | G71A | 8 (4) | 39 | 47 | 4 | 5 | 6 | 0 | 0 | 1 | 0 | 1 | Bhatia et al (1993)[13] | |
| Canada | G71R | 13 (9) | 50 | 53 | 5 | 12 | 9 | 8 | 10 | 0 | 0 | 1 | Perry et al (1975, 1990)[7, 8], Felicio et al (2014)[24], Wider et al (2009)[27] | |
| Turkey | G71R | 5 (2) | 48 | 52 | 4 | 4 | 3 | 2 | 4 | 0 | 2 | 2 | Elibol et al (2002)[15], Saka et al (2010)[25] | |
| US | G71R | 5 (1) | 49 | 58 | 9 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | Tacik et al (2014)[19] | |
| Colombia | G71R | 10 (2) | 51 | NA | NA | 1 | 2 | 1 | 8 | 0 | 0 | 1 | Pretelt et al (2014)[20], Tacik et al (2014)[19] | |
| Canada | G71E | 7 (3) | 49 | 47 | 3 | 6 | 5 | 3 | 4 | 1 | 0 | 0 | Purdy et al (1979)[9] | |
| France | G71E | 8 (3) | 49 | 55 | 7 | 6 | 3 | 0 | 6 | 0 | 0 | 0 | Lechevalier et alf (1992, 2005)[11, 12] | |
| Poland | G71E | 4 (3) | 42 | 53 | NA | 1 | 4 | 0 | 1 | 1 | 0 | 1 | ||
| US | T72P | 7 (4) | 48 | 51 | 3 | 5 | 7 | 2 | 3 | 0 | 1 | 5 | Roy et al (1988)[10], Wider et al (2009)[27] | |
| Japan | Q74P | 4 (1) | 56 | 59 | 4 | 1 | 3 | 3 | 3 | 0 | 0 | 3 | Wider et al (2010)[16], Oshima et al (2010)[17] | |
| New Zealand | Y78C | 5 (4) | 54 | 57 | 4 | 3 | 1 | 1 | 4 | 0 | 0 | 0 | Tacik et al (2014)[19] | |
|
| ||||||||||||||
| Atypical PS families | Japan | F52L | 11 (4) | 57 | 65 | 7 | 3 | 6 | 3 | 4 | 1 | 2 | 3 | Araki et al (2014)[31] |
| Portugal | F52L | 12 (2) | 50 | NA | NA | 3 | 3 | 1 | 1 | 3 | 2 | 1 | Barreto et al (2015)[34] | |
| Canadac | K56Rd | 1 (1) | 61 | NA | NA | 0 | 1 | 0 | 0 | 1 | 0 | 0 | Gustavsson et al (2016)[33] | |
| Taiwan | K56Rd | 1 (1) | 57 | NA | NA | 0 | 1 | 0 | 0 | 1 | 0 | 0 | Gustavsson et al (2016)[33] | |
| UK | G67D | 1 (1) | mid-40s | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Aji et al (2013)[30] | |
| UK | G71R | 2 (2) | 46 | 56 | NA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Newsway et al (2010)[29] | |
| Korea | G71R | 3 (2) | 43 | 55 | NA | 1 | 3 | 1 | 1 | 1 | 0 | 1 | Chung et al (2014)[18] | |
| France | G71E | 8 (NA) | 47 | 52 | 6 | 6 | 8 | 1 | 3 | 2 | 4 | 2 | Caroppo et al (2014)[32] | |
| Korea | Y78C | 3e (2) | 51 | 52 | NA | 0 | 2 | 0 | 0 | 2 | 1 | 2 | Chung et al (2014)[18] | |
FTD, frontotemporal dementia; M, male; NA, not available; PS, Perry syndrome; PSP, progressive supranuclear palsy
Number of symptom-positive member in each family,
Number of L-dopa responsive member in each family. The effect varies from limited to significant.
Chinese origin
These two patients were clinically diagnosed as progressive supranuclear palsy (no autopsy confirmation) without other signs of PS. This variant is to be yet proven pathogenic.
None of them had clinical signs of PS other than parkinsonism.
Includes previously unpublished data provided by Drs. Françoise Chapon and Bernard Lechevalier.
6. Demographic summary of PS with DCTN1 mutation
Twenty-three families exhibiting typical and atypical PS symptoms, including three sporadic atypical PS cases (two of them were clinically diagnosed as PSP [33]), have been reported from various countries (Figure 3, Table 2). DCTN1 mutation has been confirmed in at least one affected member of each family. If we analyzed all available data from clinically affected family members regardless of whether their gene statuses were confirmed or not, the age at onset was 49 ± 6 years (mean ± SD, range: 35–70 years). The age of death was 53 ± 7 years (mean ± SD, range 39–70 years), and the disease duration was 5 ± 3 years (mean ± SD, range 2–14 years). The cumulative incidence was calculated using the onset age of the symptomatic carriers (Figure 4). Half of these patients developed the disease by age of 49 years, and the incidence reached to 90% by age of 58 years. The most common mutation in DCTN1 is p.Gly71Arg, which was identified in families with both the typical and atypical presentation of PS.
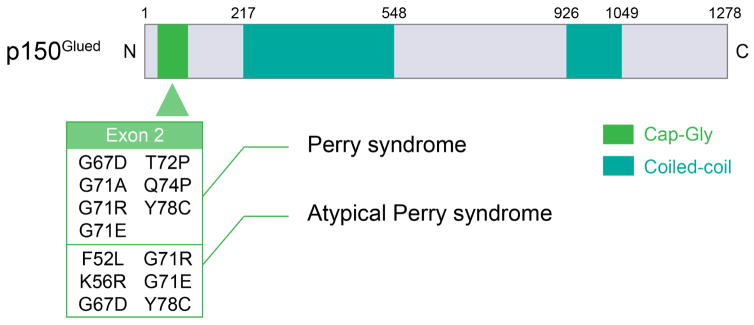
Figure 3. DCTN1 mutations in Typical and Atypical Perry syndrome.
(A) Worldwide distribution of families with typical and atypical Perry syndrome carrying DCTN1 mutations. The numbers in the parentheses indicate the number of the families. (B) Schema of p150Glued and DCTN1 mutations. All mutations associated with typical and atypical Perry syndrome are located on exon 2.
Figure 4. Cumulative Incidence of Perry Syndrome.
The cumulative incidence is 50% at the age of 49 years and 90% at the age of 58 years.
7.DCTN1 mutations/polymorphisms associated with other phenotypes
Before the discovery of DCTN1’s causative role in PS, a DCTN1 p.Gly59Ser mutation had been identified in a family with distal spinal and bulbar muscular atrophy, also known as distal hereditary motor neuropathy 7B (HMN7B) [6]. The disorder in this family also had an autosomal dominant inheritance pattern; however, the clinical phenotype was quite different from that of the PS families. In a majority of the affected cases, stridor and shortness of breath caused by vocal-fold paresis initially appeared around 30 years of age (mean age of onset was 34 years, range 23–39 years) [39]. The disease slowly progressed, and the symptoms included facial and distal dominant limb weakness without any sensory disturbances. In one autopsied case, neuronal loss was seen in the hypoglossal nucleus and the ventral horn of the spinal cord, but no pathology in the substantia nigra was described. Immunostaining of the dynactin p50 subunit and the intermediate chain subunit of dynein showed neuronal cytoplasmic inclusions in the hypoglossal nucleus. All affected family members possessed the same mutation. This is currently the only confirmed family with HMN7B and a pathogenic DCTN1 mutation. Recently, a DCTN1 p.Glu340Gly substitution has been found in a Korean patient with pure motor neuropathy. However, pathogenicity of this variant remains unclear because there was no obvious family history and segregation was not established [40].
Although not proven to be pathogenic, several variants of DCTN1 have been identified in both sporadic and familial cases with ALS [41–46]. One familial case who had DCTN1 p.Arg1101Lys variant was a member of a family with ALS and FTD. His sibling who had affected FTD had the same DCTN1 variant [42]. In Guam, a family with ALS/parkinsonism-dementia complex (PDC) was identified to harbor a DCTN1 p.Thr54Ile substitution. In this family, one ALS case and her sibling with PDC carried the p.Thr54Ile substitution; interestingly they also had a heterozygous PINK1 variant [45]. Notably, in addition to the DCTN1 variants, some of the patients carried one or two variants in other ALS-associated genes [46]. Using whole exome sequencing, a DCTN1 variant was identified in three other cases; they were diagnosed as having hereditary sensory and autonomic neuropathy type 1 [47], hereditary motor and sensory neuropathy [48], and a complex neurological disease with a slowly progressive, chronic axonal-distal motor neuropathy and extrapyramidal syndrome [49], respectively. The last case also had variants in the KIF5A and NEFH genes, which have been associated with similar phenotypes as described above including neuropathy, extrapyramidal signs, and ALS. The phenotypes of these non-PS cases mainly consisted of motor-neuron signs unlike those seen in PS, but there was a variation from peripheral neuropathy to FTD.
A genetic screening analysis of DCTN1 in 286 samples from patients with neurodegenerative diseases (156 familial PD, 100 pathologically-confirmed TDP-43 proteinopathies [79 FTLD and 21 Alzheimer’s disease], and 27 clinically diagnosed FTD and ALS patients [8 FTD-ALS, 5 FTD and 14 ALS]) identified 36 novel variants and 19 known polymorphisms [50], including p.Thr1249Ile, which was also identified in an ALS patient and an primary lateral sclerosis patient [41, 51]. However, none of these variants demonstrated complete segregation within families. Additional genotyping analyses for these variants using samples from other independent disease series (440 PD, 374 FTD, and 372 ALS samples) and 435 control samples concluded that DCTN1 variants were not a common genetic risk for PD, FTD, or ALS [50]. Moreover, Stockmann et al identified 24 exonic variants among more than 2,700 mixed samples comprised of 1,708 sporadic ALS, 218 striatal diseases (multiple system atrophy, PSP, and HD), and 778 healthy controls. However, none of them had confirmed segregation in affected family members, and some of them were found in both the disease and control groups [52]. Therefore, with the exception of the p.Gly59Ser mutation seen in the HMN7B family, the pathogenicity of these DCTN1 variants identified in non-PS phenotypes remains to be established.
8. DCTN1-unrelated Perry syndrome
A Japanese family with PS phenotype but without a DCTN1 mutation was reported in 2012 [53]. The proband developed apathy, tremor, rigidity, bradykinesia, weight loss, hypoventilation, and pyramidal signs in her mid-40’s. Levodopa was not sufficiently beneficial, and the disease progressed rapidly. She died within two years of onset due to sudden apnea. A sibling had hallucination and spasticity, and her mother showed rapid-progressive parkinsonism. The clinical picture of the proband fit with that of PS; however, pathological and biochemical analyses revealed that she had a tauopathy comparable to PSP without any TDP-43 pathology. No mutations were identified in DCTN1, but the proband and her affected sibling had an intronic 10 + 14 splice site mutation of MAPT [53]. The affected regions of the proband’s brain were similar to that of brains from patients with PS, eg, the pre-Bötzinger complex of the ventrolateral medulla were impacted, indicating that a TDP-43 proteinopathy caused by a DCTN1 mutation and a tauopathy due to a MAPT mutation can produce the cardinal clinical features of PS.
We have identified a Brazilian family clinically diagnosed as having PS. A proband was 48-year-old man who developed weight loss followed by parkinsonism that was characterized by hypomimia, resting hand tremor (left>right), cogwheel rigidity (left>right), bradykinesia, walking difficulties, bradyphrenia, depression, and apathy. He lost 80 kg of his body weight over a period of 12 months despite having a normal appetite. He was treated with benserazide/levodopa (800 mg of levodopa per day) combined with entacapone and mirtazapine but without improvement. Five years after disease onset, he was totally bedridden. He developed dementia, insomnia, and cachexia. A brain MRI scan showed diffuse cerebral atrophy. His medical history was only notable for epilepsy, which began when he was 20 years old, however, it was well controlled with carbamazepine and clonazepam. His father died at young age of unknown neurological disease indicating possible autosomal dominant inheritance. Molecular genetic testing was performed but revealed no pathogenic variants in exon 2 of DCTN1 or exons 1, 7, and 9–13 of MAPT.
9. The relevance of DCTN1 mutations to neurodegeneration
All nine mutations identified in the 14 typical and nine atypical PS families are clustered in exon 2 of DCTN1 among 32 coding exons. However, while the HMN7B-associated mutation p.Gly59Ser was also located in exon 2, the variants in other phenotypes were found in variable positions in DCTN1 (Figure 3B, Table 3). Exon 2 encodes the cytoskeleton-associated protein, glycine-rich (CAP-Gly) domain of p150Glued. The CAP-Gly domain is required for enriching dynactin in neurite tips associated with the end-binding proteins EB1 and EB3, which are microtubule-binding proteins [54]. Dynactin accumulation enhances recruitment and sustained engagement of dynein and promotes the retrograde flux from the distal axon [55–57]. The CAP-Gly domain has a 67GKNDG71 motif, which is evolutionarily well conserved and plays a pivotal role in microtubule binding [4, 58, 59]. All of the mutations found in typical/atypical PS and HMN7B were located within or close to the 67GKNDG71 motif. The p.Gly59Ser mutant p150Glued showed reduced binding affinity for microtubules and disrupted the folding of the CAP-Gly domain, inducing aggregation of the p150Glued in the cell bodies in vitro [6, 60]. The aggregates were immunopositive for the dynein intermediate chain and were seen in the affected neurons in a HMN7B patient with a p.Gly59Ser mutation [39]. The motor-neuron disease phenotype also developed in both p150Glued G59S knock-in and transgenic mice [61–63]. Intracellular inclusions seen in these mice consisted of mutant p150Glued [62]. PS-related mutant p150Glued proteins (p.Phe52Leu, p.Gly71Arg, p.Gln74Pro, and p.Tyr78Cys) showed reduced microtubule binding, and some (p.Phe52Leu, p.Gly71Arg, and p.Gln74Pro) formed cytoplasmic inclusions in the transfected cells [5, 19, 31]. The dynactin subunit p50- and p62-immunopositive neuronal cytoplasmic inclusions, which were occasionally co-localized with TDP-43, were seen in the substantia nigra in PS [5]. However, functional analyses of the ALS-associated mutant p150Glued (p.Met571Thr, p.Arg785Trp, p.Arg1101Lys, and p.Thr1249Ile) did not show any deficits. Microtubule binding abilities were preserved in all these mutations, and no aggregations were observed in the mutant-overexpressed culture cells [52, 64].
Table 3.
DCTN1 variants in other phenotypes
| Exon | Varianta | Phenotypes | References |
|---|---|---|---|
| 2 | T54I | ALS, PDC | Steel et al (2015)[45] |
| 2 | G59R | ALS | Liu et al (2014)[44] |
| 2 | G59S | HMN7B | Puls et al (2003, 2005)[6, 39] |
| 3 | S111C | ALS | Cady et al (2015)[46] |
| 8 | I196V | ALS | Cady et al (2015)[46] |
| 10 | E340G | Inherited peripheral neuropathy | Nam et al (2016)[40] |
| 12 | D424H | Hereditary sensory and autonomic neuropathy | Klein et al (2014)[47] |
| 16 | M571T | ALS | Münch et al (2004)[41] |
| 17 | Y670F | Hereditary motor and sensory neuropathy | Schabhüttl et al (2014)[48] |
| 21 | R785W | ALS | Münch et al (2004)[41] |
| 23 | I935M | ALS | Cady et al (2015)[46] |
| 25 | R997W | ALS | Takahashi et al (2008)[43] |
| 26 | R1049Q | ALS | Cady et al (2015)[46] |
| 28 | S1080F | ALS | Cady et al (2015)[46] |
| 28 | R1101K | ALS, FTD | Münch et al (2005)[42] |
| 32 | T1249I | ALS, PLS | Münch et al (2004)[41], Cady et al (2015)[46], Mitsumoto et al (2015)[51] |
| 32 | H1270Q | ALS | Cady et al (2015)[46] |
| 32 | R1275C | Distal motor neuropathy with ataxia and an Extrapyramidal syndrome of tremor and focal dystonia | Daud et al (2015)[49] |
ALS, amyotrophic lateral sclerosis; FTD, frontotemporal dementia; HMN7B, hereditary motor neuropathy 7B; PDC, parkinsonism-dementia complex; PLS, primary lateral sclerosis
Not yet proven to be pathogenic except G59S.
Given the distinctly different phenotypes of PS and HMN7B, there may be a certain mechanism by which specific cell types, dopaminergic neurons or motor neurons, are damaged preferentially in each disease. Structural analyses revealed that HMN7B-related p.Gly59Ser was located in the center of the CAP-Gly domain, while PS-related mutations were surface-exposed, suggesting that these mutations could lead to different phenotypes depending on their locations [55, 65]. Although a precise mechanism discriminating between both is unknown, mutation-dependent phenotypic differences were observed in vitro and in vivo. HMN7B mutant was more prone to aggregation than PS mutants, and HMN7B mutant had decreased interactions with dynein, suggesting HMN7B mutation affected the confirmation and stability of the mutant protein [55]. When the HMN7B mutant was overexpressed in primary dorsal root ganglion neurons, the insufficient association with dynein resulted in disruption of transport of cargo throughout the axon. In contrast, PS mutants can associate with dynein similar to the wild-type and did not alter the axonal transport itself. However, a reduction in distal dynactin enrichment was caused by impaired binding of mutant p150Glued to EBs, leading to a decrease the retrograde flux of PS mutants [55]. In a transgenic fly model, aggregates of mutant p150Glued in the cytoplasm of the motor neurons and the accumulation of dynein heavy chains at the synaptic termini were observed only when HMN7B mutant was overexpressed, but this was not the case for PS mutants [56].
The mechanism by which PS-related DCTN1 mutations induce TDP-43 proteinopathies remains unclear. Given that dynactin/dynein play a role in retrograde transport of autophagosomes [66], one hypothesis is that a disruption of the retrograde transport of lysosomes and autophagosomes may lead to accumulated and aggregate pathological proteins in the soma [3]. Xia et al. showed that a loss of TDP-43 decreased the expression of DCTN1, leading to the inhibition of autophagosome-lysosome fusion in TDP-43-depleted cells. As a result, the accumulation of autophagic cargo induced neurotoxicity [67]. The loss of function of the DCTN1 protein might result in such a vicious cycle in affected cells and contribute to TDP-43 accumulation. Similarly, dynein mutations also impair autophagosome-lysosome fusion and cause the aggregation of pathological proteins [68]. These observations indicated that functional loss of molecular motors associated with axonal transport and/or intracellular trafficking might induce protein aggregation in the cytoplasm.
10. Conclusion
DCTN1 mutations have been identified in several neurodegenerative diseases, indicating that the deficits in axonal transport play a crucial role in the development of neurodegeneration. Although not always confirmed, the pathogenicity of the PS/HMN7B-related mutations is well established. Interestingly, closely located mutations in the CAP-Gly domain of p150Glued can cause different cell-type specific neurodegeneration, leading to clinically distinct disorders. PS can mimic PSP and FTD, and HMN7B partially resembles ALS; therefore the diagnosis of DCTN1-related neurodegeneration should be in differential diagnosis for patients presenting with neurodegenerative phenotypes, especially in familial cases. In addition, other genes like MAPT might be associated with PS phenotype. Further studies are needed to find a therapy for DCTN1-related neurodegeneration based on the phenotype-specific mechanisms.
Highlights.
Twenty-three families with Perry syndrome due to DCTN1 mutation have been reported
Patients with DCTN1 mutations can exhibit diverse phenotypes
Atypical findings of Perry syndrome consisted of PSP-like and FTD-like phenotypes
DCTN1 mutation was found in the Canadian family which was first described in 1979
We identified the first Polish family with Perry syndrome
Acknowledgments
Study Funding: None.
We would like to thank Ms. Audrey J. Strongosky, BA, for her assistance with the genealogical research, Ms. Kelly Viola, MPS, ELS, and Mr. Jean G. Davit for their assistance with the technical preparation of this manuscript, Drs. Françoise Chapon and Bernard Lechevalier for the providing the updated information on the French family with PS. We also would like to thank Drs. Felipe Pretelt, MD, and Mercedes Olaya, MD, for assisting us with obtaining the autopsy material from the Colombian family.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- DCTN1
dynactin 1
- FTD
frontotemporal dementia
- HMN7B
distal hereditary motor neuropathy 7B
- MAPT
microtubule-associated protein tau
- PD
Parkinson disease
- PS
Perry syndrome
- PSP
progressive supranuclear palsy
- TDP-43
transactive response DNA-binding protein of 43 kDa
Footnotes
Author Contributions Takuya Konno: drafting the manuscript, design and conceptualization of the review, acquisition, analysis, and interpretation of the literature, and final approval of the manuscript. Owen A. Ross: reviewing the manuscript, design and conceptualization of the review, interpretation of the literature, and final approval of the manuscript. Hélio A.G. Teive: reviewing the manuscript, acquisition of the clinical information from the Brazilian family, and final approval of the manuscript. Jarosław Sławek: reviewing the manuscript, acquisition of the clinical information from the Polish family, and final approval of the manuscript. Dennis W. Dickson: reviewing the manuscript, creating a pathological figure, design and conceptualization of the review, interpretation of the literature, and final approval of the manuscript. Zbigniew K. Wszolek: reviewing the manuscript, design and conceptualization of the review, interpretation of the literature, and final approval of the manuscript.
Disclosure: T. Konno received research support from JSPS Overseas Research Fellowships and is partially supported by a gift from Carl Edward Bolch, Jr. and Susan Bass Bolch. O. Ross is supported by NINDS R01 NS078086 and the Mayo Clinic Morris K. Udall Parkinson’s Disease Research Center of Excellence (NINDS P50 #NS072187). He also receives support from Mayo Clinic Center for Regenerative Medicine, Mayo Clinic Center for Individualized Medicine, Mayo Clinic Neuroscience Focused Research Team (Cecilia and Dan Carmichael Family Foundation, and the James C. and Sarah K. Kennedy Fund for Neurodegenerative Disease Research at Mayo Clinic in Florida). H. Teive has nothing to disclose. J. Sławek has nothing to disclose. D. Dickson is supported by the NIH/NINDS P50 NS072187, P50 AG16574. Z. Wszolek is supported by the NIH/NINDS P50 NS072187, NIH/NIA (primary) and NIH/NINDS (secondary) 1U01AG045390-01A1, Mayo Clinic Center for Regenerative Medicine, Mayo Clinic Center for Individualized Medicine, Mayo Clinic Neuroscience Focused Research Team (Cecilia and Dan Carmichael Family Foundation, and the James C. and Sarah K. Kennedy Fund for Neurodegenerative Disease Research at Mayo Clinic in Florida), and The Sol Goldman Charitable Trust.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84(2):292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millecamps S, Julien JP. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14(3):161–76. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- 3.Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL. Retrograde axonal transport: pathways to cell death? Trends Neurosci. 2010;33(7):335–44. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–79. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 5.Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, Calne S, Calne DB, Lechevalier B, Chapon F, Tsuboi Y, Yamada T, Gutmann L, Elibol B, Bhatia KP, Wider C, Vilarino-Guell C, Ross OA, Brown LA, Castanedes-Casey M, Dickson DW, Wszolek ZK. DCTN1 mutations in Perry syndrome. Nat Genet. 2009;41(2):163–5. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, Brown RH, Jr, Ludlow CL, Fischbeck KH. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33(4):455–6. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 7.Perry TL, Bratty PJ, Hansen S, Kennedy J, Urquhart N, Dolman CL. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch Neurol. 1975;32(2):108–13. doi: 10.1001/archneur.1975.00490440058009. [DOI] [PubMed] [Google Scholar]
- 8.Perry TL, Wright JM, Berry K, Hansen S, Perry TL., Jr Dominantly inherited apathy, central hypoventilation, and Parkinson's syndrome: clinical, biochemical, and neuropathologic studies of 2 new cases. Neurology. 1990;40(12):1882–7. doi: 10.1212/wnl.40.12.1882. [DOI] [PubMed] [Google Scholar]
- 9.Purdy A, Hahn A, Barnett HJ, Bratty P, Ahmad D, Lloyd KG, McGeer EG, Perry TL. Familial fatal Parkinsonism with alveolar hypoventilation and mental depression. Ann Neurol. 1979;6(6):523–31. doi: 10.1002/ana.410060611. [DOI] [PubMed] [Google Scholar]
- 10.Roy EP, 3rd, Riggs JE, Martin JD, Ringel RA, Gutmann L. Familial parkinsonism, apathy, weight loss, and central hypoventilation: successful long-term management. Neurology. 1988;38(4):637–9. doi: 10.1212/wnl.38.4.637. [DOI] [PubMed] [Google Scholar]
- 11.Lechevalier B, Schupp C, Fallet-Bianco C, Viader F, Eustache F, Chapon F, Morin P. Familial parkinsonian syndrome with athymhormia and hypoventilation. Rev Neurol (Paris) 1992;148(1):39–46. [PubMed] [Google Scholar]
- 12.Lechevalier B, Chapon F, Defer G, Rivrain Y, Le Doze F, Schupp C, Viader F. Perry and Purdy's syndrome (familial and fatal parkinsonism with hypoventilation and athymhormia) Bull Acad Natl Med. 2005;189(3):481–90. discussion 490–2. [PubMed] [Google Scholar]
- 13.Bhatia KP, Daniel SE, Marsden CD. Familial parkinsonism with depression: a clinicopathological study. Ann Neurol. 1993;34(6):842–7. doi: 10.1002/ana.410340614. [DOI] [PubMed] [Google Scholar]
- 14.Tsuboi Y, Wszolek ZK, Kusuhara T, Doh-ura K, Yamada T. Japanese family with parkinsonism, depression, weight loss, and central hypoventilation. Neurology. 2002;58(7):1025–30. doi: 10.1212/wnl.58.7.1025. [DOI] [PubMed] [Google Scholar]
- 15.Elibol B, Kobayashi T, Atac F, Hattori N, Sahin G, Gurer G. Familial parkinsonism with apathy, depression and central hypoventilation (Perry's syndrome) Kluwer Academic/Plenum Publishers; Boston, MA: 2002. [Google Scholar]
- 16.Wider C, Dachsel JC, Farrer MJ, Dickson DW, Tsuboi Y, Wszolek ZK. Elucidating the genetics and pathology of Perry syndrome. J Neurol Sci. 2010;289(1–2):149–54. doi: 10.1016/j.jns.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohshima S, Tsuboi Y, Yamamoto A, Kawakami M, Farrer MJ, Kira J, Shii H. Autonomic failures in Perry syndrome with DCTN1 mutation. Parkinsonism Relat Disord. 2010;16(9):612–4. doi: 10.1016/j.parkreldis.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Chung EJ, Hwang JH, Lee MJ, Hong JH, Ji KH, Yoo WK, Kim SJ, Song HK, Lee CS, Lee MS, Kim YJ. Expansion of the clinicopathological and mutational spectrum of Perry syndrome. Parkinsonism Relat Disord. 2014;20(4):388–93. doi: 10.1016/j.parkreldis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Tacik P, Fiesel FC, Fujioka S, Ross OA, Pretelt F, Castaneda Cardona C, Kidd A, Hlavac M, Raizis A, Okun MS, Traynor S, Strongosky AJ, Springer W, Wszolek ZK. Three families with Perry syndrome from distinct parts of the world. Parkinsonism Relat Disord. 2014;20(8):884–8. doi: 10.1016/j.parkreldis.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pretelt F, Castaneda Cardona C, Tacik P, Ross OA, Wszolek ZK. Latin America's first case of Perry syndrome and a new treatment option for respiratory insufficiency. J Neurol. 2014;261(3):620–1. doi: 10.1007/s00415-014-7262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wider C, Wszolek ZK. Rapidly progressive familial parkinsonism with central hypoventilation, depression and weight loss (Perry syndrome)--a literature review. Parkinsonism Relat Disord. 2008;14(1):1–7. doi: 10.1016/j.parkreldis.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Mishima T, Fujioka S, Kurisaki R, Yanamoto S, Higuchi MA, Tsugawa J, Fukae J, Neshige R, Tsuboi Y. Impulse control disorders and punding in Perry syndrome. Parkinsonism Relat Disord. 2015;21(11):1381–2. doi: 10.1016/j.parkreldis.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Orimo S, Suzuki M, Inaba A, Mizusawa H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson's disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2012;18(5):494–500. doi: 10.1016/j.parkreldis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Felicio AC, Dinelle K, Agarwal PA, McKenzie J, Heffernan N, Road JD, Appel-Cresswell S, Wszolek ZK, Farrer MJ, Schulzer M, Sossi V, Stoessl AJ. In vivo dopaminergic and serotonergic dysfunction in DCTN1 gene mutation carriers. Mov Disord. 2014;29(9):1197–201. doi: 10.1002/mds.25893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saka E, Topcuoglu MA, Demir AU, Elibol B. Transcranial sonography in Perry syndrome. Parkinsonism Relat Disord. 2010;16(1):68–70. doi: 10.1016/j.parkreldis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Quinn N. The "round the houses" sign in progressive supranuclear palsy. Ann Neurol. 1996;40(6):951. doi: 10.1002/ana.410400630. [DOI] [PubMed] [Google Scholar]
- 27.Wider C, Dickson DW, Stoessl AJ, Tsuboi Y, Chapon F, Gutmann L, Lechevalier B, Calne DB, Personett DA, Hulihan M, Kachergus J, Rademakers R, Baker MC, Grantier LL, Sujith OK, Brown L, Calne S, Farrer MJ, Wszolek ZK. Pallidonigral TDP–43 pathology in Perry syndrome. Parkinsonism Relat Disord. 2009;15(4):281–6. doi: 10.1016/j.parkreldis.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuboi Y, Dickson DW, Nabeshima K, Schmeichel AM, Wszolek ZK, Yamada T, Benarroch EE. Neurodegeneration involving putative respiratory neurons in Perry syndrome. Acta Neuropathol. 2008;115(2):263–8. doi: 10.1007/s00401-007-0246-1. [DOI] [PubMed] [Google Scholar]
- 29.Newsway V, Fish M, Rohrer JD, Majounie E, Williams N, Hack M, Warren JD, Morris HR. Perry syndrome due to the DCTN1 G71R mutation: a distinctive levodopa responsive disorder with behavioral syndrome, vertical gaze palsy, and respiratory failure. Mov Disord. 2010;25(6):767–70. doi: 10.1002/mds.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aji BM, Medley G, O'Driscoll K, Larner AJ, Alusi SH. Perry syndrome: a disorder to consider in the differential diagnosis of Parkinsonism. J Neurol Sci. 2013;330(1–2):117–8. doi: 10.1016/j.jns.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Araki E, Tsuboi Y, Daechsel J, Milnerwood A, Vilarino-Guell C, Fujii N, Mishima T, Oka T, Hara H, Fukae J, Farrer MJ. A novel DCTN1 mutation with late-onset parkinsonism and frontotemporal atrophy. Mov Disord. 2014;29(9):1201–4. doi: 10.1002/mds.25833. [DOI] [PubMed] [Google Scholar]
- 32.Caroppo P, Le Ber I, Clot F, Rivaud-Pechoux S, Camuzat A, De Septenville A, Boutoleau-Bretonniere C, Mourlon V, Sauvee M, Lebouvier T, Bonnet AM, Levy R, Vercelletto M, Brice A, French C, Genetic S. Research Network on Frontotemporal Dementia/Frontotemporal Dementia-Amyotrophic Lateral, DCTN1 mutation analysis in families with progressive supranuclear palsy-like phenotypes. JAMA Neurol. 2014;71(2):208–15. doi: 10.1001/jamaneurol.2013.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustavsson EK, Trinh J, Guella I, Szu-Tu C, Khinda J, Lin CH, Wu RM, Stoessl J, Appel-Cresswell S, McKeown M, Rajput A, Rajput AH, Petersen MS, Jeon BS, Aasly JO, Farrer MJ. DCTN1 p.K56R in progressive supranuclear palsy. Parkinsonism Relat Disord. 2016;28:56–61. doi: 10.1016/j.parkreldis.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Barreto R, Lopes M, Roriz J, Magalhães M. Perry syndrome – Characteristics of the first Portuguese family. Mov Disord. 2015;30:S304–S305. [Google Scholar]
- 35.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 36.van der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson's disease. Mov Disord. 2013;28(11):1587–96. doi: 10.1002/mds.25658. [DOI] [PubMed] [Google Scholar]
- 37.Ross OA, Rutherford NJ, Baker M, Soto-Ortolaza AI, Carrasquillo MM, DeJesus-Hernandez M, Adamson J, Li M, Volkening K, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, Lippa C, Woodruff BK, Knopman DS, White CL, 3rd, Van Gerpen JA, Meschia JF, Mackenzie IR, Boylan K, Boeve BF, Miller BL, Strong MJ, Uitti RJ, Younkin SG, Graff-Radford NR, Petersen RC, Wszolek ZK, Dickson DW, Rademakers R. Ataxin-2 repeat-length variation and neurodegeneration. Hum Mol Genet. 2011;20(16):3207–12. doi: 10.1093/hmg/ddr227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson AM, Rademakers R. What we know about TMEM106B in neurodegeneration. Acta Neuropathol. 2016;132(5):639–651. doi: 10.1007/s00401-016-1610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, Kennedy WR, Wendelschafer-Crabb G, Vortmeyer A, Powers R, Finnegan K, Holzbaur EL, Fischbeck KH, Ludlow CL. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol. 2005;57(5):687–94. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nam SH, Hong YB, Hyun YS, Nam da E, Kwak G, Hwang SH, Choi BO, Chung KW. Identification of Genetic Causes of Inherited Peripheral Neuropathies by Targeted Gene Panel Sequencing. Mol Cells. 2016;39(5):382–8. doi: 10.14348/molcells.2016.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munch C, Sedlmeier R, Meyer T, Homberg V, Sperfeld AD, Kurt A, Prudlo J, Peraus G, Hanemann CO, Stumm G, Ludolph AC. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology. 2004;63(4):724–6. doi: 10.1212/01.wnl.0000134608.83927.b1. [DOI] [PubMed] [Google Scholar]
- 42.Munch C, Rosenbohm A, Sperfeld AD, Uttner I, Reske S, Krause BJ, Sedlmeier R, Meyer T, Hanemann CO, Stumm G, Ludolph AC. Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Ann Neurol. 2005;58(5):777–80. doi: 10.1002/ana.20631. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi Y, Seki N, Ishiura H, Mitsui J, Matsukawa T, Kishino A, Onodera O, Aoki M, Shimozawa N, Murayama S, Itoyama Y, Suzuki Y, Sobue G, Nishizawa M, Goto J, Tsuji S. Development of a high-throughput microarray-based resequencing system for neurological disorders and its application to molecular genetics of amyotrophic lateral sclerosis. Arch Neurol. 2008;65(10):1326–32. doi: 10.1001/archneur.65.10.1326. [DOI] [PubMed] [Google Scholar]
- 44.Liu ZJ, Li HF, Tan GH, Tao QQ, Ni W, Cheng XW, Xiong ZQ, Wu ZY. Identify mutation in amyotrophic lateral sclerosis cases using HaloPlex target enrichment system. Neurobiol Aging. 2014;35(12):2881e11–5. doi: 10.1016/j.neurobiolaging.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Steele JC, Guella I, Szu-Tu C, Lin MK, Thompson C, Evans DM, Sherman HE, Vilarino-Guell C, Gwinn K, Morris H, Dickson DW, Farrer MJ. Defining neurodegeneration on Guam by targeted genomic sequencing. Ann Neurol. 2015;77(3):458–68. doi: 10.1002/ana.24346. [DOI] [PubMed] [Google Scholar]
- 46.Cady J, Allred P, Bali T, Pestronk A, Goate A, Miller TM, Mitra RD, Ravits J, Harms MB, Baloh RH. Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Ann Neurol. 2015;77(1):100–13. doi: 10.1002/ana.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein CJ, Middha S, Duan X, Wu Y, Litchy WJ, Gu W, Dyck PJ, Gavrilova RH, Smith DI, Kocher JP, Dyck PJ. Application of whole exome sequencing in undiagnosed inherited polyneuropathies. J Neurol Neurosurg Psychiatry. 2014;85(11):1265–72. doi: 10.1136/jnnp-2013-306740. [DOI] [PubMed] [Google Scholar]
- 48.Schabhuttl M, Wieland T, Senderek J, Baets J, Timmerman V, De Jonghe P, Reilly MM, Stieglbauer K, Laich E, Windhager R, Erwa W, Trajanoski S, Strom TM, Auer-Grumbach M. Whole-exome sequencing in patients with inherited neuropathies: outcome and challenges. J Neurol. 2014;261(5):970–82. doi: 10.1007/s00415-014-7289-8. [DOI] [PubMed] [Google Scholar]
- 49.Daud D, Griffin H, Douroudis K, Kleinle S, Eglon G, Pyle A, Chinnery PF, Horvath R. Whole exome sequencing and the clinician: we need clinical skills and functional validation in variant filtering. J Neurol. 2015;262(7):1673–7. doi: 10.1007/s00415-015-7755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vilarino-Guell C, Wider C, Soto-Ortolaza AI, Cobb SA, Kachergus JM, Keeling BH, Dachsel JC, Hulihan MM, Dickson DW, Wszolek ZK, Uitti RJ, Graff-Radford NR, Boeve BF, Josephs KA, Miller B, Boylan KB, Gwinn K, Adler CH, Aasly JO, Hentati F, Destee A, Krygowska-Wajs A, Chartier-Harlin MC, Ross OA, Rademakers R, Farrer MJ. Characterization of DCTN1 genetic variability in neurodegeneration. Neurology. 2009;72(23):2024–8. doi: 10.1212/WNL.0b013e3181a92c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitsumoto H, Nagy PL, Gennings C, Murphy J, Andrews H, Goetz R, Floeter MK, Hupf J, Singleton J, Barohn RJ, Nations S, Shoesmith C, Kasarskis E, Factor-Litvak P. Phenotypic and molecular analyses of primary lateral sclerosis. Neurol Genet. 2015;1(1):e3. doi: 10.1212/01.NXG.0000464294.88607.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stockmann M, Meyer-Ohlendorf M, Achberger K, Putz S, Demestre M, Yin H, Hendrich C, Linta L, Heinrich J, Brunner C, Proepper C, Kuh GF, Baumann B, Langer T, Schwalenstocker B, Braunstein KE, von Arnim C, Schneuwly S, Meyer T, Wong PC, Boeckers TM, Ludolph AC, Liebau S. The dynactin p150 subunit: cell biology studies of sequence changes found in ALS/MND and Parkinsonian syndromes. J Neural Transm (Vienna) 2013;120(5):785–98. doi: 10.1007/s00702-012-0910-z. [DOI] [PubMed] [Google Scholar]
- 53.Omoto M, Suzuki S, Ikeuchi T, Ishihara T, Kobayashi T, Tsuboi Y, Ogasawara J, Koga M, Kawai M, Iwaki T, Kanda T. Autosomal dominant tauopathy with parkinsonism and central hypoventilation. Neurology. 2012;78(10):762–4. doi: 10.1212/WNL.0b013e318248e531. [DOI] [PubMed] [Google Scholar]
- 54.Ligon LA, Shelly SS, Tokito M, Holzbaur EL. The microtubule plus-end proteins EB1 and dynactin have differential effects on microtubule polymerization. Mol Biol Cell. 2003;14(4):1405–17. doi: 10.1091/mbc.E02-03-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moughamian AJ, Holzbaur EL. Dynactin is required for transport initiation from the distal axon. Neuron. 2012;74(2):331–43. doi: 10.1016/j.neuron.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lloyd TE, Machamer J, O'Hara K, Kim JH, Collins SE, Wong MY, Sahin B, Imlach W, Yang Y, Levitan ES, McCabe BD, Kolodkin AL. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 2012;74(2):344–60. doi: 10.1016/j.neuron.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayloo S, Lazarus JE, Dodda A, Tokito M, Ostap EM, Holzbaur EL. Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nat Commun. 2014;5:4807. doi: 10.1038/ncomms5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci U S A. 1995;92(5):1634–8. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, Finley J, Liu ZJ, Qiu SH, Chen H, Luan CH, Carson M, Tsao J, Johnson D, Lin G, Zhao J, Thomas W, Nagy LA, Sha B, DeLucas LJ, Wang BC, Luo M. Crystal structure of the cytoskeleton-associated protein glycine-rich (CAP-Gly) domain. J Biol Chem. 2002;277(50):48596–601. doi: 10.1074/jbc.M208512200. [DOI] [PubMed] [Google Scholar]
- 60.Levy JR, Sumner CJ, Caviston JP, Tokito MK, Ranganathan S, Ligon LA, Wallace KE, LaMonte BH, Harmison GG, Puls I, Fischbeck KH, Holzbaur EL. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J Cell Biol. 2006;172(5):733–45. doi: 10.1083/jcb.200511068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai C, Lin X, Chandran J, Shim H, Yang WJ, Cai H. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J Neurosci. 2007;27(51):13982–90. doi: 10.1523/JNEUROSCI.4226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD, Griffin J, Price DL, Martin LJ, Wong PC. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci. 2008;28(9):1997–2005. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chevalier-Larsen ES, Wallace KE, Pennise CR, Holzbaur EL. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin. Hum Mol Genet. 2008;17(13):1946–55. doi: 10.1093/hmg/ddn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dixit R, Levy JR, Tokito M, Ligon LA, Holzbaur EL. Regulation of dynactin through the differential expression of p150Glued isoforms. J Biol Chem. 2008;283(48):33611–9. doi: 10.1074/jbc.M804840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honnappa S, Okhrimenko O, Jaussi R, Jawhari H, Jelesarov I, Winkler FK, Steinmetz MO. Key interaction modes of dynamic +TIP networks. Mol Cell. 2006;23(5):663–71. doi: 10.1016/j.molcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196(4):407–17. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia Q, Wang H, Hao Z, Fu C, Hu Q, Gao F, Ren H, Chen D, Han J, Ying Z, Wang G. TDP-43 loss of function increases TFEB activity and blocks autophagosome-lysosome fusion. EMBO J. 2016;35(2):121–42. doi: 10.15252/embj.201591998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37(7):771–6. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]