Abstract
Background
Etravirine (ETR), an NNRTI approved for 200 mg BID dosing in conjunction with other antiretrovirals (ARVs), has pharmacokinetic properties which support once-daily dosing.
Methods
In this single arm, open-label study, 79 treatment-naïve HIV-infected adults were assigned to receive ETR 400 mg plus tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) 300/200mg once daily to assess antiviral activity, safety, and tolerability. Antiretroviral activity at 48 weeks was determined by proportion of subjects with HIV-1 RNA <50 copies/mL (intention-to-treat, missing = failure).
Results
Of 79 eligible subjects, 90% were men, 62% African-American and 29% Caucasian. At baseline, median (Q1, Q3) age was 29 years (23, 44) and HIV-1 RNA 4.52 log10 copies/mL (4.07, 5.04). Sixty-nine (87%) completed a week 48 visit and 61 (77%, 95% CI: 66 – 86%) achieved HIV-1 RNA <50 copies/mL at week 48. At time of virologic failure, genotypic resistance-associated mutations were detected in 3 participants, 2 with E138K (1 alone and 1 with additional mutations). Median (95% CI) CD4+ cell count increase was 163 (136, 203) cells/uL. Fifteen (19.0%) participants reported a new sign/symptom or lab abnormality ≥ Grade 3 and 3 participants (3.8 %) permanently discontinued ETR due to toxicity. Two participants had psychiatric symptoms of any grade. There were no deaths.
Conclusions
In this study of ARV-naïve HIV+ adults, once daily ETR with TDF/FTC had acceptable antiviral activity and was well-tolerated. Once daily ETR may be a plausible option as part of a combination ARV regimen for treatment-naïve individuals.
Introduction
The non-nucleoside reverse transcriptase inhibitor (NNRTI) etravirine (ETR) has demonstrated potency against HIV-1 and has been studied predominantly in treatment-experienced patients, including those who have HIV-1 resistance to other drugs in its class. In the Phase III DUET-1 and DUET-2 trials in which treatment-experienced HIV-1-infected patients with multi-class resistance, including NNRTI resistance-associated mutations (RAMs) were randomly assigned to receive ETR or placebo twice daily, together with an optimized background that included ritonavir-boosted darunavir and optional enfuvirtide, 57% of ETR-treated patients had HIV-1 viral load <50 copies/mL compared to 36% of patients in the placebo arm at Week 96.(1)
Etravirine is currently approved at a dose of 200 mg twice daily as a component of combination antiretroviral therapy. However, existing pharmacokinetic data support plausibility of once-daily dosing. The mean terminal elimination half-life of ETR is 30 – 40 hours, and systemic exposures comparing pharmacokinetics of once- and twice-daily administration of an equivalent dose per day have been similar.(2, 3) In the SENSE trial, ETR 400 mg once daily with two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) achieved similar rates of virologic suppression among treatment-naïve HIV-infected patients when compared to efavirenz and two NRTIs (76% on ETR and 74% on efavirenz, meeting study defined non-inferiority criteria with delta of −12%).(4)
Etravirine has demonstrated good tolerability and safety profiles, with the majority of adverse events (AEs) being mild or moderate. In the DUET trials the safety and tolerability profiles in the ETR and placebo groups were similar.(1) Nervous system and psychiatric adverse events did not differ between the two groups, however, rash was more frequent among participants in the ETR group, occurring most often in the first 2 weeks of treatment (21% vs 12%). (5, 6) Occurrence of new Grade 3 or 4 elevations in total cholesterol and triglyceride, while low in frequency, were more common in the ETR group.(7) The prevalence of ongoing neuropsychiatric events in the SENSE trial was lower in the ETR group (6.3%) compared to the efavirenz group (21.5%, p =0.011).(4)
This single-arm, open-label study aimed to assess antiretroviral activity, safety and tolerability of etravirine administered 400 mg once daily, together with tenofovir/emtricitabine 300/200 mg once daily, among treatment-naïve HIV-infected adults for up to 96 weeks. This paper presents the protocol-specified 48-week results.
METHODS
This study was conducted at the University of North Carolina at Chapel Hill School of Medicine, Wake Forest University School of Medicine, and Carolinas Medical Center, Charlotte, NC. The primary outcome measure was the proportion of patients with HIV-1 RNA <50 copies/mL at Week 24 (intention-to-treat [ITT] analysis, with missing counted as failure). Secondary outcome measures included proportion of participants with HIV RNA <50 copies/mL at Week 48 and Week 96, proportion with HIV RNA <200 copies/mL at all three time points, and change in CD4+ cell count from baseline to Weeks 24, 48 and 96. Changes in the lipid profile, glucose metabolism, and limb and trunk fat distribution, as measured by DEXA scan were assessed in a substudy of participants. The institutional review boards at each participating study site reviewed and approved the research protocol and all participants provided written informed consent prior to study entry. The study was conducted in compliance with Good Clinical Practices and the principles of the Declaration of Helsinki and its amendments. This trial was registered with ClinicalTrials.gov [NCT00959894].
Participants
This study enrolled participants with documented HIV-1 infection and HIV-1 RNA >1,000 copies/mL who were age 18 years or older, ARV treatment-naïve (≤10 days of cumulative prior ARV therapy and had never taken etravirine, dapivirine, or rilpivirine), had no TDF/FTC or ETR RAMs on screening genotype, and were not pregnant. Additionally, participants in the metabolic substudy had no history of diabetes mellitus and were not taking lipid-lowering medications. For sample size calculation we postulated that 80% of participants would achieve viral load <50 copies/mL at Week 24, based on published data on the efficacy of tenofovir/emtricitabine/efavirenz treatment of antiretroviral-naïve adults.(8) Assuming an observed virologic response rate of 80%, a sample size of 80 participants would provide a Clopper-Pearson 95% confidence interval with a width of 18.5% (69.6% to 88.1%).
All participants were assigned to receive 400 mg once daily together with one fixed-dose TDF/FTC 300/200 mg tablet (Truvada®, Gilead Sciences) once daily, and were instructed to take ETR with a meal. Substitution of alternative nucleosides, i.e. fixed dose zidovudine/lamivudine or abacavir/lamivudine (HLA B5701 negative), for TDF/FTC was permitted when treatment limiting clinical or laboratory AEs were considered related to TDF/FTC.
Antiretroviral activity and safety assessments
Participants were assessed during study visits at screening, baseline, and at weeks 2, 4, 8, 12, 24, and 48, with planned follow-up for a total of 96 weeks. Plasma HIV-1 RNA was measured using the Abbott RealTime HIV-1 assay (Abbott Molecular, Des Plaines, Illinois, USA). Viral genotype was determined using the TRUGENE® HIV-1 assay (Siemens Healthcare Diagnostics, Tarrytown, NY). Genotype testing was performed at screening and repeated on study for patients who experienced virologic failure while prescribed ETR and had viral load ≥500 copies/mL (the laboratory-specified threshold). Self-reported medication adherence was assessed at each study visit using the Adult AIDS Clinical Trials Group Antiretroviral Adherence Questionnaire. (9)
Safety and tolerability were assessed by evaluation of clinical events and laboratory abnormalities classified by using the Division of AIDS Grading Scale. Investigators recorded the occurrence and duration of AEs and judged relationship to the study treatment. Safety assessments included all AEs, discontinuation due to AEs, and laboratory abnormalities, including change from baseline. An independent data and safety monitoring board assessed safety and antiretroviral activity throughout the study period.
Assessment of metabolic effects
Assessment of lipid profiles, insulin resistance, and body fat distribution was conducted among a pre-specified substudy of up to 40 participants. Blood was obtained for measurement of lipid profiles (total, low-density lipoprotein and high-density lipoprotein cholesterol [LDL-C, HDL-C] and triglycerides), glucose, and insulin levels at baseline and at Weeks 12, 24, and 48. Analyses of LDL, triglycerides, glucose, and insulin values were restricted to measurements where the participant had been fasting for at least 8 hours at the time of phlebotomy. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [fasting insulin (µU/mL) × fasting glucose (mmol/L)]/22.5.(10) Lipid and glucose levels from the full study sample are also evaluated at entry and weeks 12 and 48. Whole body Dual X-ray Absorptiometry (DEXA) scans (Hologic Discovery W, Hologic Inc., Bedford, MA) were conducted at baseline and Week 24 to assess body fat distribution. Change from baseline to week 24 in limb fat, trunk fat, total body fat, lean mass, and fat mass ratio were calculated. Fat mass ratio was calculated as the ratio of trunk fat percentage and lower limb fat percentage (% trunk fat mass/% lower limb fat mass).
Statistical Analysis
Measurements from study entry to week 48 were included for analysis; the week 24 and 48 HIV-1 RNA analysis windows included +/− 6 weeks. The proportion of participants who achieved viral load <50 and <200 copies/mL was estimated with a corresponding Clopper-Pearson exact 95% confidence interval (CI). The primary efficacy analysis approach was conducted intention-to-treat (ITT); a missing evaluation was counted (i.e. imputed) as failure unless the viral loads both immediately before and after the missing value were below the respective threshold (<50 or <200 copies/mL). As-treated analyses included evaluations from the start of ETR and TDF/FTC through the time of permanent discontinuation of ETR or TDF/FTC (ignoring temporary holds and dose reductions). Missing observations were excluded in as-treated analyses.
Virologic failure was defined as confirmed viral load reduction of less than 2 log10 copies/mL compared to baseline and viral load ≥200 copies/mL after Week 8 or confirmed failure to achieve viral load <50 copies/mL at or after Week 24. Participants who experienced confirmed virologic failure were counted as a virologic failure at the initial failure week and all subsequent analysis weeks.
The safety/tolerability endpoint was defined as the first grade 3 (severe) or higher sign, symptom or laboratory abnormality that was at least one grade higher than baseline, or permanent discontinuation of ETR due to any toxicity (regardless of grade). Safety/tolerability analyses included participant follow-up at and after exposure to study treatment, and the Kaplan-Meier method was used to estimate the proportion of participants who remained event-free through weeks 24 and 48, with a 95% CI using Greenwood’s variance estimate and a log-log transformation. Time was handled as continuous (weeks from treatment start to event or censoring). Metabolic data analyses were conducted as-treated. Change from baseline was calculated using the measurement closest to schedule and within the analysis window, and quantified with an estimated median and distribution-free 95% CI. Statistical analyses were performed in SAS version 9.3 (Cary, North Carolina) or R version 2.15.1 (http://www.r-project.org/).
RESULTS
Participants
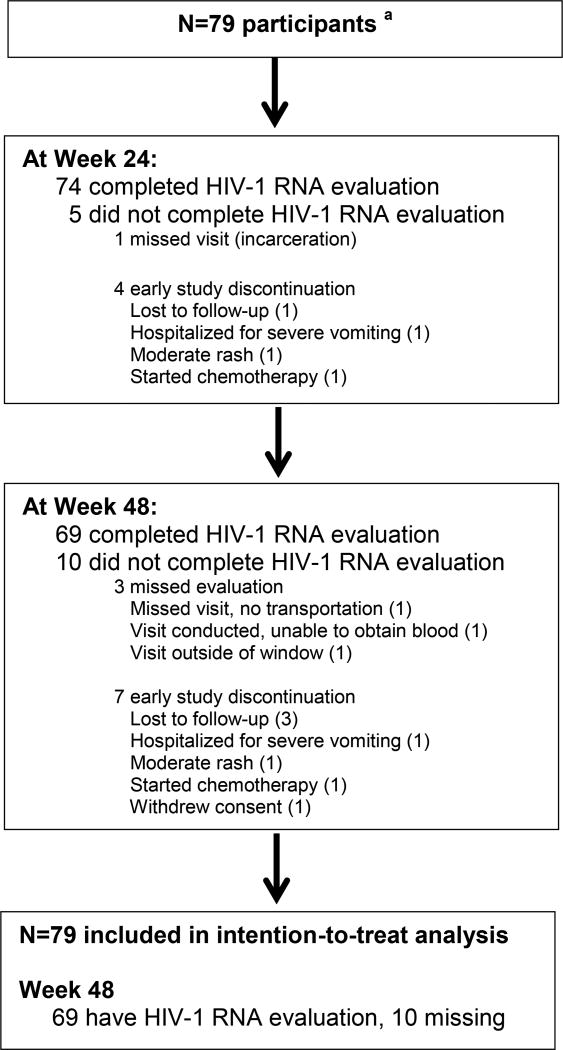
Of 117 individuals screened, 80 were enrolled, one of whom was ineligible and discontinued participation before beginning study treatment due to acute renal failure noted on labs at entry visit (patient had normal renal function at screening visit) and is therefore excluded from the analyses. Among the 37 screen failures, the most common reason for failure was presence of an exclusionary RAM at screening (n = 19; most common were K103N [5] and V179E/I/V [4]). Baseline characteristics of the participants are shown in (Table 1). Of the 79 participants who initiated study treatment, the majority (90%) were men and 62% were Black, non-Hispanic; median (Q1, Q3) age was 29 (23, 44) years. At study entry, the median (Q1, Q3) HIV-1 RNA and CD4 count were 4.52 (4.07, 5.04) log10 copies/mL and 383 (248, 472) cells/uL, respectively. Sixty-nine participants (87%) had data available for the Week 48 visit (Figure 1). Seven participants were discontinued from study follow-up, and 3 other participants missed the Week 48 evaluation. Occurrence of an AE was the reason for early study discontinuation for 2 of the discontinued participants (moderate rash for 1 and severe vomiting for the other) and 3 were discontinued because of loss to follow-up.
Table 1.
Baseline characteristics
| All Participants N = 79 |
||
|---|---|---|
| Male (n, %) | 71 (90%) | |
| Race/ethnicity (n, %) | Black, non-Hispanic | 49 (62%) |
| White, non-Hispanic | 23 (29%) | |
| Hispanic (of any race) | 5 (6%) | |
| Other, non-Hispanica | 2 (3%) | |
| Age, years | 29 (23, 44) | |
| HIV-1 RNA, log10 copies/mL | 4.52 (4.07, 5.04) | |
| HIV-1 RNA category (n, %) | ≤ 100,000 copies/mL | 59 (75%) |
| > 100,000 copies/mL | 20 (25%) | |
| CD4+ cell count, cells/uL | 383 (248, 472) | |
| CD4+ cell count category (n, %) | <200 cells/uL | 14 (18%) |
| 200–499 cells/uL | 49 (62%) | |
| ≥500 cells/uL | 16 (20%) | |
| Lipid levels, mg/dLb | Total cholesterol | 159 (141, 182) |
| LDL-cholesterol | 99 (81, 126) | |
| HDL-cholesterol | 39 (31, 48) | |
| Triglycerides | 93 (68, 118) |
Values are median (Q1, Q3) except where otherwise indicated.
Other race or ethnic group: African, Sudanese (n=1), African, Liberian (n=1)
Results are available for total cholesterol and HDL-cholesterol levels for 78 participants and for LDL-cholesterol and triglycerides for 73 participants.
Figure 1.
Participant Disposition
a n=79 eligible participants; 1 additional individual was determined to be ineligible on the day of entry due to acute renal failure and was excluded from all analyses.
Virologic and immunologic outcomes
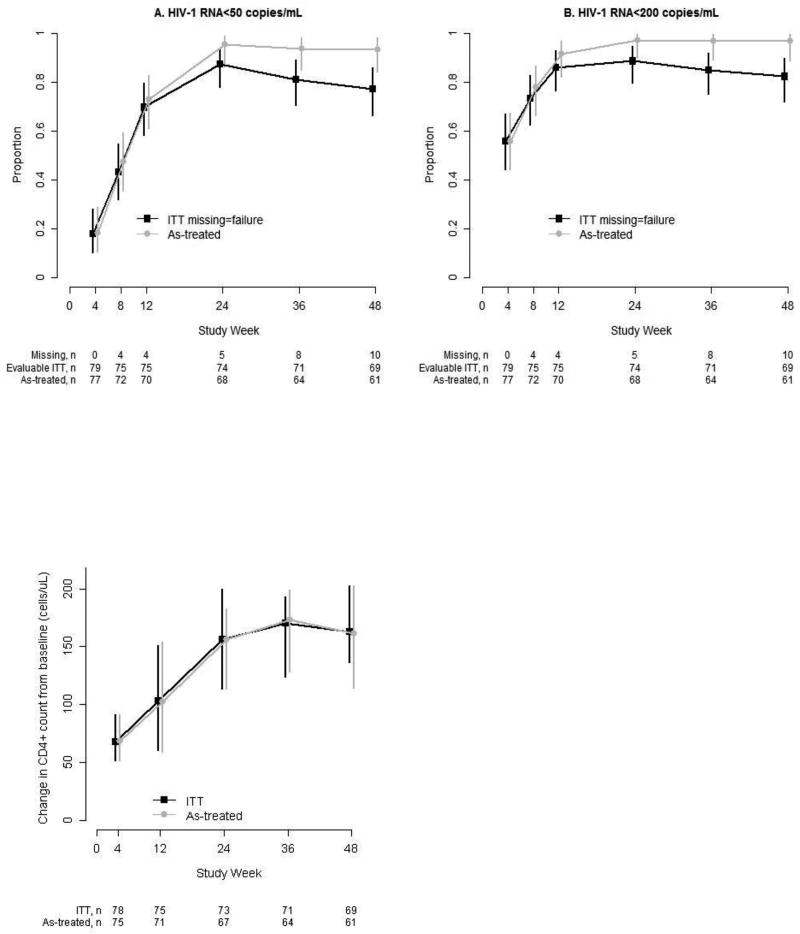
At Week 48, 61 of 79 participants achieved HIV-1 RNA < 50 copies/mL (ITT, estimated proportion: 0.77 [95% CI: 0.66, 0.86]), (Figure 2). Ten participants did not have a Week 48 viral load result because of premature study discontinuation (n = 7) or missed evaluation (n = 3). Excluding the 10 missing participants, 61 of 69 had HIV-1 RNA < 50 copies/mL at Week 48 (0.88 [0.78, 0.95]). The proportion achieving HIV-1 RNA < 200 copies/mL at Week 48 was 0.82 (0.72, 0.90), and if missing evaluations were excluded, was 0.94 (0.86, 0.98).
Figure 2.
Virologic and immunologic response
Proportion of participants with HIV-1 RNA <50 and <200 copies/mL: Vertical bars are 95% Clopper-Pearson binomial confidence intervals. Intention-to-treat (ITT) analysis includes all participants regardless of treatment status, with missing HIV-1 RNA counted as failure. The as-treated denominator excludes participants who permanently discontinued ETR + TDF/FTC or had a missing HIV-1 RNA result.
Change in CD4+ count from baseline: Estimated median and 95% distribution-free confidence interval (vertical bars) are plotted at each study week. The intention-to-treat (ITT) analysis includes evaluable participants regardless of treatment status. The as-treated analysis excludes participants who permanently discontinued ETR + TDF/FTC before CD4+ count evaluation.
Through Week 48, with missing viral load counted as failure unless the results immediately before and after were both <50 copies/mL, 11 participants (14%) experienced virologic failure. Two of 4 participants who had HIV-1 RNA < 200 copies/mL at confirmation of virologic failure, and therefore did not meet protocol criteria for discontinuation of study drug at that time, had subsequent viral load <50 copies/mL at Week 48. (Table 2) shows details of the 11 participants who had virologic failure. Genotype testing was performed for 3 of 5 participants with HIV-1 RNA ≥ 500 copies/mL at confirmed virologic failure (the laboratory-specified threshold for genotype testing); the remaining 2 did not have genotype samples available, 1 of whom had discontinued study treatment before virologic failure. All 3 participants who had genotype testing showed RAMs; two had isolated RAMs (one E138K and the other Y181C), and the third had multiple RAMs (V75I, E138K, Y181C, M184I, K219E, and M230L). None of these RAMs was present on the screening genotype for these participants at baseline.
Table 2.
Resistance testing among virologic failures through Week 48
| Subject ID | VF Week |
VL at baseline, copies/mL |
VL at VF confirmation, copies/mL |
Genotype status |
Resistance associated mutations |
|---|---|---|---|---|---|
| 1 | 8 | 19,356 | 422 | Below test thresholda | |
| 2 | 8 | 195,146 | 190,339 | Discontinued study treatmentb | |
| 3 | 8 | 848,845 | 82,432 | Tested | E138K, Y181C, M230L, M184I, K219E, V75I |
| 4 | 24 | 442,363 | 104 | Below test thresholda | |
| 5 | 24 | 10,823 | 110 | Below test thresholda | |
| 6 | 24 | 65,193 | 727 | Missingc | |
| 7 | 36 | 78,146 | 834 | Tested | Y181C |
| 8 | 36 | 83,096 | 244 | Below test thresholda | |
| 9 | 36 | 260,951 | 64 | Below test thresholda | |
| 10 | 48 | 37,177 | 53 | Below test thresholda | |
| 11 | 48 | 21,942 | 3129 | Tested | E138K |
VL: viral load, VF: virologic failure
Viral load below 500 copies/mL threshold specified by laboratory for genotype assay.
Participant developed rash and discontinued study treatment during Week 2.
Participant discontinued study participation, no sample available for genotype.
A CD4+ cell count measurement was available for 73 participants at Week 24; median (Q1, Q3) CD4+ cell count was 497 (357, 674) cells/µL and median (95% CI) change from baseline was 156 (95% CI: 113, 200) cells/µL. At Week 48, results were available for 69 participants; median (Q1, Q3) CD4+ cell count was 506 (389, 752) cells/µL, and the median (95% CI) change from baseline was 163 (136, 203) cells/µL.
Safety and tolerability
Study medication adherence was assessed by self-report, using a standardized interview. At Week 24, 28% of participants who completed the interview (18 of 65) reported having missed medications within the prior month, and at Week 48 non-adherence was reported by 23% (14 of 60). The most common reasons given for missed study medications were that the participant forgot, was busy with other things, or was traveling away from home. Among participants who experienced virologic failure while prescribed ETR, 56% (5 of 9) reported missed doses in the month prior to virologic failure.
Adverse events were generally mild or moderate (Table 3). Among the 79 participants who initiated the study treatment, 18 (22.8) had Grade 2 or higher clinical adverse events. The most common Grade 2 or higher signs/symptoms experienced were rash (5 participants, 6.3%) and transaminase elevation (5 participants, 6.3%). All rashes were Grade 2 and were maculopapular in nature. One participant who had chronic hepatitis B infection developed Grade 3 alanine aminotransferase and Grade 2 aspartate aminotransferase elevations within 12 weeks of starting study drugs and had subsequent normalization of transaminase levels with no change in study treatment. The highest grade of transaminase elevations in all other patients was Grade 2.
Table 3.
Clinical adverse events and laboratory abnormalities through Week 48
| N = 79 | ||
|---|---|---|
| Frequency | Percent | |
| Clinical adverse events | ||
| Rash (any grade)a | 5 | 6.3 |
| Any Grade 2 or higher sign/symptom | 18 | 22.8 |
| Individual Grade 2 or higher signs/symptoms | ||
| Psychiatric symptoms | 2 | 2.5 |
| Headache | 2 | 2.5 |
| Nausea/vomiting | 1 | 1.3 |
| Diarrhea | 1 | 1.3 |
| New/worsened Grade 3 or 4 sign/symptom | 10 | 12.7 |
| Grade 3 or 4 sign/symptom at least possibly related to ETRb | 3 | 3.8 |
| Laboratory abnormalities | ||
| Aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation (Grade 2 or higher)c | 5 | 6.3 |
| Grade 2 creatinine elevation | 2 | 2.5 |
| New/worsened grade 3 or 4 laboratory abnormalities | 6 | 7.6 |
| Grade 3 or 4 lab abnormalities at least possibly related to ETRb | 3 | 3.8 |
| ETR discontinued due to toxicity | 3 | 3.8 |
| Grade 2 rash | 1 | 1.3 |
| Grade 2 rash concurrent with Grade 1 AST elevation | 1 | 1.3 |
| Grade 2 elevations in AST, ALT, and total bilirubin | 1 | 1.3 |
Number of participants in each event category (and percent among n=79) is displayed; the 1 participant who never started treatment was excluded.
All rashes were Grade 2
Judged by investigator to be possibly, probably, or definitely related to ETR
Four participants had Grade 2 AST/ALT elevations, and 1 had Grade 2 and Grade 3 elevations.
Seven participants (8.9% of 79) had abnormal creatinine levels during follow-up, 2 of which were moderate (Grade 2) severity and 5 of which were ≤ Grade 1 severity. Five participants switched from TDF/FTC to another NRTI backbone during study follow-up: 3 due to abnormal creatinine and 2 due to other tolerability events (1 rash, 1 vomiting). Four of the participants who discontinued TDF/FTC switched to abacavir/lamivudine and 1 to zidovudine/lamivudine.
Fifteen (18.9%) participants reported a new sign/symptom or lab abnormality ≥ Grade 3, and three participants (3.8 %) permanently discontinued ETR due to toxicity. At weeks 24 and 48, the estimated probability of remaining free of a safety/tolerability event was 0.82 (95% CI: 0.72, 0.89) and 0.79 (0.69, 0.87), respectively. Safety/tolerability events that were at least possibly related to ETR were experienced by 8 (10.1%) participants. Results were similar with follow-up censored at discontinuation of ETR for reasons other than toxicity.
Among 15 participants who had a new safety event ≥ Grade 3, 10 experienced a clinical sign or symptom and 6 had laboratory abnormalities (1 experienced both). Three of the 10 participants with clinical sign or symptoms had a grade 4 event during the study period: one participant who had a history of bipolar disorder reported worsened symptoms of depression and vivid dreams after initiation of study treatment and made a suicide attempt, but had subsequent improvement in symptoms following dose adjustment of his antidepressant medication without stopping ETR; the second participant developed colitis (together with fever and sore throat) reported at Week 17 which resolved with no change in study treatment; and the third participant had discontinued ETR at 8 weeks, 30 weeks before expressing suicidal ideation. Three participants permanently discontinued ETR due to toxicity: one had Grade 2 rash alone; one had Grade 2 rash and concomitant Grade 1 aspartate aminotransferase elevation; and the third had elevated aspartate aminotransferase and alanine aminotransferase (Grade 2) as well as total bilirubin (Grade 1) levels. There were no deaths.
Metabolic parameters
In the overall study population there were no significant changes in total cholesterol, LDL-C, triglycerides, or fasting glucose at Week 48 relative to baseline. Levels of HDL-C increased by a median (95% CI) of 5 (2 to 8) mg/dL at Week 48 (n=58). Among participants in the metabolic substudy, insulin levels increased from baseline to Week 48, by a median of 4.3 (0.3 to 8.4) µU/mL (n=24). Median (Q1, Q3) HOMA-IR was 1.56 (0.90, 2.54) µU/ml.mmol/L at baseline and 1.62 (1.24, 5.60) at Week 48. Among participants with both a baseline and week 48 assessment, the estimated median (95%CI) increase was 0.71 (0.09 to 1.91) µU/ml.mmol/L (n=23). DEXA scans for substudy participants showed no significant changes from baseline to Week 24 (n=37) in percent total body fat (median change +0.43, 95% CI: −0.63 to 1.10), percent trunk fat (+0.32, 95% CI: −0.41 to 1.66), or percent limb fat (+0.48, 95% CI: −0.64 to 0.99). Similarly, increase in fat mass ratio over the first 24 weeks was not significant (median change +0.02, 95% CI: −0.01 to 0.09).
DISCUSSION
Simplification of antiretroviral medication schedules by use of once-daily regimens is an important factor in enhancing patient acceptability of HIV treatment. The more recent availability of a 200 mg tablet formulation of ETR reduces this pill burden and facilitates the possibility of once-daily ETR dosing. In addition, the ability to disperse ETR in water allows for the option of further decreasing the number of pills swallowed daily.
In this single-arm study, once-daily ETR demonstrated acceptable antiviral activity at 48 weeks when taken in combination with fixed-dose TDF/FTC by antiretroviral naïve HIV-infected patients. Most treatment-emergent adverse events were mild to moderate in severity and discontinuation of ETR due to adverse events was uncommon. Our findings show that, at 48 weeks, 77% of ARV-naive participants taking ETR 400 mg once daily achieved HIV-1 RNA < 50 copies/mL. The immunologic response was robust, with a median increase in CD4+ cell count of 163 cells/µL. At 48 weeks, 11 participants in our study met criteria for confirmed virologic failure by the ITT analysis. Of these, 2 participants who had HIV-1 RNA <200 copies/mL, and therefore did not meet criteria for stopping study drug, subsequently achieved virologic suppression on study treatment, suggesting transient low-level viremia as opposed to true virologic failure. The number of virologic failures in our study was higher than that of the SENSE trial, also conducted in a treatment-naïve study population, in which 4 of 79 participants in the etravirine arm had virologic failure. In our study, over half of the participants who experienced virologic failure while prescribed etravirine reported having missed doses of study medication in the month prior to virologic failure, suggesting the possibility that non-adherence played a role.
Three participants who experienced virologic failure with HIV-1 RNA > 500 copies/mL at the time of failure had RAMs, 1 of whom had multiple RAMs, including E138K and M184I, none of which were present on the screening genotype at baseline. The emergence of multiple RAMs on treatment in this participant is in contrast to the SENSE trial, in which no patients in the ETR arm developed RAMs. (4) In the DUET studies, however, participants all had at least one baseline NNRTI mutation and treatment-emergent mutations at position E138 in the reverse transcriptase enzyme were also observed, in addition to the more frequent V179F/I and Y181C mutations.(11). The E138K mutation contributes phenotypic resistance to ETR and rilpivirine, and has most often been reported in clinical trials of rilpivirine, commonly accompanied by the M184I mutation, as was seen in 1 of our participants.(12, 13) Both of these HIV-1 reverse transcriptase mutations result from guanine-to-adenine substitutions in APOBEC3 contexts and emerging data suggest that they may in fact be archived in pro-viral DNA at a high frequency prior to initiation of ARV, and may co-emerge following exposure to certain ARV drug combinations.(14–16) Existing data raise concern that the E138K and M184I mutations can interact to increase viral replication capacity, offsetting the decreased viral fitness usually associated with the M184I/V mutations.(17, 18) Additional studies would be needed to better assess the frequency of co-emergent E138K and M184I mutations in ARV-naïve patients treated with etravirine.
Etravirine was well-tolerated through 48 weeks in this study. Rash, although the most common symptom reported, occurred in only a small minority of participants (6.3%) and was of moderate severity, with no occurrence of vesicular or bullous exanthema and no mucous membrane involvement. One participant had Grade 2 rash in conjunction with mild elevation in aspartate aminotransferase, which resolved rapidly after discontinuation of study medication. The incidence of rash observed in this study was lower than the 20% incidence reported in the larger DUET trials, however it is possible that in those trials, concomitant darunavir-ritonavir and enfuvirtide may have contributed in part to the overall incidence of rash, notwithstanding higher rates of rash in the ETR arm compared to the placebo group.(7) Hepatic AEs occurred infrequently in our study, in keeping with data reported in the SENSE and DUET trials.(4, 7) Incidence of psychiatric events with ETR was low in this study (2 participants, 2.5%) and occurred in participants with a prior history of depression. The 1 patient who developed worsening of depressive symptoms while still taking ETR was also on antidepressant medications, including a selective serotonin reuptake inhibitor, and experienced improvement in symptoms without stopping ETR after adjustment of his antidepressant medications, suggesting the possibility that ETR may have reduced the levels of that antidepressant. In the SENSE trial, a low rate of psychiatric AEs was also seen in the etravirine arm (5%), compared to a significantly higher rate among participants in the efavirenz arm (15%).(4)
Consistent with previous data, triglycerides, total cholesterol and LDL-C levels did not change significantly with use of ETR.(4, 19–22) In several prior studies in which virologically suppressed patients switched from protease inhibitor (PI) and efavirenz-based therapy to ETR-based ARV, significant improvement in the lipid profile, and in some studies glycemic control, was observed after switching.(19–23) Notably, participants in our study did experience an increase in HDL-C, an improvement that has been reported with the NNRTIs nevirapine and efavirenz.(24–26) A similar increase in HDL-C was seen in a study in which virologically suppressed patients were switched from NRTI- and PI-based therapy to ETR, but this has not been consistently observed in other studies of ETR.(7, 19, 22) Analysis of fasting insulin and glucose levels in our study showed a modest increase in HOMA-IR but no change in glycemia compared to baseline. Our study is the first to assess body fat distribution by DEXA scan in patients initiating therapy with ETR, and found that total body fat, as well as truncal and limb fat, remained stable after 24 weeks on ETR. However, our ability to draw clear conclusions about changes in body fat distribution from the current analysis is limited because we have data only from a single early time-point, when slowly progressive changes in body fat may not yet be evident. In this study, DEXA imaging is repeated at Week 96, which will allow for a more definitive assessment of body fat distribution after initiation of ARV treatment with etravirine.
The absence of a comparator arm prevents us from drawing conclusions about efficacy of once-daily ETR in ARV-naïve patients. However, in this single-arm study, ETR achieved virologic suppression in 77% of 79 patients by ITT analysis with missing counted as failure. The occurrence of RAMs in some of the patients who experienced virologic failure, and in particular the co-emergence of E138K and M184I mutations in 1 patient, does raise concern about the potential for evolution of resistance if patients experience treatment failure with this regimen. Given the modest size of our study, it is not possible for us to adequately assess the likelihood of emergence of these RAMS with use of once-daily etravirine. Interpretation of results of metabolic assessments are similarly limited by our small sample size of the substudy, yet our observations regarding lipids and glucose levels are, in general, consistent with findings in other larger studies.(4, 20, 23)
In summary, in this single-arm study, ETR demonstrated acceptable antiviral activity through 48 weeks when given at the 400 mg once daily dose together with fixed-dose TDF/FTC. Etravirine was well-tolerated in this study, with mostly mild to moderate AEs and few toxicities that led to treatment discontinuation. As such, once daily ETR may be a plausible option as part of a combination ARV regimen for treatment-naïve individuals for whom other NNRTIs are contraindicated.
Acknowledgments
Acknowledgement statement
Funding for this trial was provided by an investigator-initiated grant from Janssen Services, LLC. Truvada® was provided by Gilead Sciences. The trial was conducted by the authors and statistical analyses performed by the study statisticians [KM, OF]. The corresponding author had the final responsibility for submitting the manuscript for publication. The 24-week results of this study were presented at the IDWeek™ 2013 Joint Meeting of the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America in San Francisco, CA.(27) The authors wish to thank the study participants for their generous contribution to this research. We also thank study site staff: David Ragan, Clara Zelasky, David Currin, Donna Pittard, Susan Pedersen, Dania Munson, Linda Manor (UNC), Ann Boye, Jessica Kearney-Bryan (Carolinas Medical Center), and Libbyada Moseley (Wake Forest University School of Medicine). We acknowledge the National Institutes of Health-funded programs supporting the Center for AIDS Research (CFAR) [P30 AI50410], the Clinical and Translational Science Award program of the Division of Research Resources [NIH - 1UL1TR001111], and the AIDS Clinical Trials Unit [UM1 AI069423] at the University of North Carolina at Chapel Hill.
DISCLOSURE STATEMENT
Michelle Floris-Moore: Grant support for this research from Janssen
Joseph Eron: Grant support for other research from ViiV Healthcare, Merck and Bristol Myers-Squibb. Consultant income from Janssen, Merck, Bristol Myers-Squibb, ViiV Healthcare, Glaxo SmithKlein, Abbvie and Gilead.
Angela Kashuba: Grant support for other research from Merck and Gilead; Consultant income from Merck.
Katie Mollan: Grant support for other research from Gilead, Abbvie, and Merck
Kristine Patterson: Grant support for other research from Abbvie, Glaxo SmithKlein and Merck. Consultant income from Glaxo SmithKlein.
Aimee Wilkin: Grant support for other research from Janssen, Pfizer, Gilead, and Merck.
David Wohl: Grant support for other research from Merck and Gilead. Advisory Board income from Gilead and Janssen.
Footnotes
Trial Registration: ClinicalTrials.gov NCT00959894
References
- 1.Katlama C, Clotet B, Mills A, Trottier B, Molina JM, Grinsztejn B, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther. 2010;15(7):1045–52. doi: 10.3851/IMP1662. [DOI] [PubMed] [Google Scholar]
- 2.Schöller-Gyüre M, Kakuda TN, De Smedt G, Woodfall B, Lachaert R, Beets G, Peeters M, Hoetelmans RM. 47th Interscience Conference of Antimicrobial Agents and Chemotherapy. Chicago, IL: 2007. Pharmacokinetics of TMC125 in once- and twice-daily regimens in HIV-1 negative volunteers. [Google Scholar]
- 3.Di Perri G, Green B, Morrish G, Hill A, Faetkenheuer G, Bickel M, et al. Pharmacokinetics and pharmacodynamics of etravirine 400 mg once daily in treatment-naive patients. HIV clinical trials. 2013;14(3):92–8. doi: 10.1310/hct1403-92. [DOI] [PubMed] [Google Scholar]
- 4.Gazzard B, Duvivier C, Zagler C, Castagna A, Hill A, van Delft Y, et al. Phase 2 double-blind, randomized trial of etravirine versus efavirenz in treatment-naive patients: 48-week results. AIDS. 2011;25(18):2249–58. doi: 10.1097/QAD.0b013e32834c4c06. [DOI] [PubMed] [Google Scholar]
- 5.Madruga JV, Cahn P, Grinsztejn B, Haubrich R, Lalezari J, Mills A, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370(9581):29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]
- 6.Lazzarin A, Campbell T, Clotet B, Johnson M, Katlama C, Moll A, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370(9581):39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]
- 7.Girard PM, Campbell TB, Grinsztejn B, Hartikainen J, Rachline A, Nijs S, et al. Pooled week 96 results of the phase III DUET-1 and DUET-2 trials of etravirine: further analysis of adverse events and laboratory abnormalities of special interest. HIV Med. 2012;13(7):427–35. doi: 10.1111/j.1468-1293.2012.00994.x. [DOI] [PubMed] [Google Scholar]
- 8.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Tambuyzer L, Vingerhoets J, Azijn H, Daems B, Nijs S, de Bethune MP, et al. Characterization of genotypic and phenotypic changes in HIV-1-infected patients with virologic failure on an etravirine-containing regimen in the DUET-1 and DUET-2 clinical studies. AIDS research and human retroviruses. 2010;26(11):1197–205. doi: 10.1089/aid.2009.0302. [DOI] [PubMed] [Google Scholar]
- 12.Rimsky L, Vingerhoets J, Van Eygen V, Eron J, Clotet B, Hoogstoel A, et al. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J Acquir Immune Defic Syndr. 2012;59(1):39–46. doi: 10.1097/QAI.0b013e31823df4da. [DOI] [PubMed] [Google Scholar]
- 13.Molina JM, Cahn P, Grinsztejn B, Lazzarin A, Mills A, Saag M, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378(9787):238–46. doi: 10.1016/S0140-6736(11)60936-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim EY, Lorenzo-Redondo R, Little SJ, Chung YS, Phalora PK, Maljkovic Berry I, et al. Human APOBEC3 induced mutation of human immunodeficiency virus type-1 contributes to adaptation and evolution in natural infection. PLoS Pathog. 2014;10(7):e1004281. doi: 10.1371/journal.ppat.1004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fourati S, Malet I, Lambert S, Soulie C, Wirden M, Flandre P, et al. E138K and M184I mutations in HIV-1 reverse transcriptase coemerge as a result of APOBEC3 editing in the absence of drug exposure. AIDS. 2012;26(13):1619–24. doi: 10.1097/QAD.0b013e3283560703. [DOI] [PubMed] [Google Scholar]
- 16.McCallum M, Oliveira M, Ibanescu RI, Kramer VG, Moisi D, Asahchop EL, et al. Basis for early and preferential selection of the E138K mutation in HIV-1 reverse transcriptase. Antimicrobial agents and chemotherapy. 2013;57(10):4681–8. doi: 10.1128/AAC.01029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu HT, Colby-Germinario SP, Asahchop EL, Oliveira M, McCallum M, Schader SM, et al. Effect of mutations at position E138 in HIV-1 reverse transcriptase and their interactions with the M184I mutation on defining patterns of resistance to nonnucleoside reverse transcriptase inhibitors rilpivirine and etravirine. Antimicrobial agents and chemotherapy. 2013;57(7):3100–9. doi: 10.1128/AAC.00348-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z, Kuritzkes DR. Interaction of reverse transcriptase (RT) mutations conferring resistance to lamivudine and etravirine: effects on fitness and RT activity of human immunodeficiency virus type 1. Journal of virology. 2011;85(21):11309–14. doi: 10.1128/JVI.05578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Echeverria P, Bonjoch A, Puig J, Molto J, Paredes R, Sirera G, et al. Randomised study to assess the efficacy and safety of once-daily etravirine-based regimen as a switching strategy in HIV-infected patients receiving a protease inhibitor-containing regimen. Etraswitch study. PLoS One. 2014;9(2):e84676. doi: 10.1371/journal.pone.0084676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casado JL, de Los Santos I, Del Palacio M, Garcia-Fraile L, Perez-Elias MJ, Sanz J, et al. Lipid-lowering effect and efficacy after switching to etravirine in HIV-infected patients with intolerance to suppressive HAART. HIV clinical trials. 2013;14(1):1–9. doi: 10.1310/hct1401-1. [DOI] [PubMed] [Google Scholar]
- 21.Waters L, Fisher M, Winston A, Higgs C, Hadley W, Garvey L, et al. A phase IV, double-blind, multicentre, randomized, placebo-controlled, pilot study to assess the feasibility of switching individuals receiving efavirenz with continuing central nervous system adverse events to etravirine. AIDS. 2011;25(1):65–71. doi: 10.1097/QAD.0b013e328341685b. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro P, Perez I, Laguno M, Martinez-Rebollar M, Gonzalez-Cordon A, Lonca M, et al. Dual therapy with etravirine plus raltegravir for virologically suppressed HIV-infected patients: a pilot study. J Antimicrob Chemother. 2014;69(3):742–8. doi: 10.1093/jac/dkt406. [DOI] [PubMed] [Google Scholar]
- 23.Nelson M, Hill A, van Delft Y, Moecklinghoff C. Etravirine as a Switching Option for Patients with HIV RNA Suppression: A Review of Recent Trials. AIDS research and treatment. 2014;2014:636584. doi: 10.1155/2014/636584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med. 2004;1(1):e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisac C, Fumero E, Crespo M, Roson B, Ferrer E, Virgili N, et al. Metabolic benefits 24 months after replacing a protease inhibitor with abacavir, efavirenz or nevirapine. AIDS. 2005;19(9):917–25. doi: 10.1097/01.aids.0000171405.46113.bf. [DOI] [PubMed] [Google Scholar]
- 26.Negredo E, Ribalta J, Ferre R, Salazar J, Rey-Joly C, Sirera G, et al. Efavirenz induces a striking and generalized increase of HDL-cholesterol in HIV-infected patients. AIDS. 2004;18(5):819–21. doi: 10.1097/00002030-200403260-00017. [DOI] [PubMed] [Google Scholar]
- 27.Floris-Moore MMK, Wilkin AM, Johnson M, Kashuba A, Wohl DA, Patterson KB, Ragan D, Zelasky C, Currin D, Pittard D, Eron JJ. Antiretroviral Activity and Safety of Once-daily Etravirine in Treatment-naïve HIV-infected Adults. IDWeek™ 2013; Joint Meeting of the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America; San Francisco, CA. 2013. [Google Scholar]