Abstract
The molecular mechanisms underlying Major Depressive Disorder (MDD) are largely unknown. Limited success of previous genetics studies may be attributable to heterogeneity of MDD, aggregating biologically different subtypes. We examined the polygenic features of MDD and two common clinical subtypes (typical and atypical) defined by symptom profiles in a large sample of adults with established diagnoses. Data were from 1,530 patients of the Netherlands Study of Depression and Anxiety (NESDA) and 1,700 controls mainly from the Netherlands Twin Register (NTR). Diagnoses of MDD and its subtypes were based on DSM-IV symptoms. Genetic overlap of MDD and subtypes with psychiatric (MDD, bipolar disorder, schizophrenia) and metabolic (BMI, C-reactive protein, triglycerides) traits was evaluated via genomic profile risk scores (GPRS) generated from meta-analysis results of large international consortia. SNP-heritability of MDD and subtypes was also estimated. MDD was associated with psychiatric GPRS, while no association was found for GPRS of metabolic traits. MDD subtypes had differential polygenic signatures: typical was strongly associated with schizophrenia GPRS (OR=1.54, p=7.8e-9), while atypical was additionally associated with BMI (OR=1.29, p=2.7e-4) and triglycerides (OR=1.21, p=0.006) GPRS. Similar results were found when only the highly discriminatory symptoms of appetite/weight to were used to define subtypes. SNP-heritability was 32% for MDD, 38% and 43% for subtypes with, respectively, decreased (typical) and increased (atypical) appetite/weight. In conclusion, MDD subtypes are characterized by partially distinct polygenic liabilities and may represent more homogeneous phenotypes. Disentangling MDD heterogeneity may help the psychiatric field moving forward in the search for molecular roots of depression.
Keywords: Major depression, heterogeneity, typical and atypical, polygenic, genetics
INTRODUCTION
Major Depressive Disorder (MDD) is highly prevalent(1) and one of the main contributors to disability worldwide(2). Though the heritability of MDD has been estimated to be 37%(3), the search for specific genetic variants has not yet been successful: the largest GWAS mega-analysis to date detected no significant associations with common polymorphisms(4). Statistical hints suggest that depression liability is polygenic, with the majority of variance due to joint effects of multiple loci with small effects scattered across the genome(5;6). Failure to detect single effects is attributable to underpowered sample sizes and to depression’s clinical heterogeneity, that additionally compromises the power of association studies(5–9). Patients with the same diagnosis of MDD (any five out of nine DSM-5 accessory symptoms(10)) may endorse very different symptom profiles. From clinical observations, criteria to identify two severe subtypes – each present in ~25–35% of patients - based on more homogenous symptom profiles have been proposed: typical/melancholic and atypical(11), reflected in the DSM-5 specifiers for melancholic and atypical depression. However, not all DSM criteria have been justified by research, and recent studies based on data-driven techniques highlighted the importance of vegetative symptoms (particularly appetite and weight) in distinguishing subtypes (decreased in typical, increased in atypical)(12–17). Increasing evidence suggests that subtypes are associated with different pathophysiological correlates: environmental stress (e.g. childhood trauma), smoking and HPA-axis hyperactivity appear more specific for typical depression, while obesity, metabolic dysregulations (e.g. abdominal adiposity, hypertriglyceridemia) and inflammation up-regulations appear more specific for atypical depression(11;18;19). In line with this observation, we recently showed(20) that the association between a variant in the FTO gene and MDD was completely driven by the atypical subtype. Based on these findings we hypothesized that MDD subtypes may be characterized by a partially distinct genetic liability, with genetic profiles for stress-related and psychiatric traits more specifically linked with typical MDD, and those for obesity-related metabolic dysregulations with atypical MDD. We tested our hypothesis in a sample of 3,230 Dutch adults with established psychiatric diagnoses and GWAS data. We examined for MDD and its subtypes: 1) the genetic overlap with major psychiatric disorders (MDD, bipolar-disorder, schizophrenia) and metabolic traits (BMI, C-reactive protein and triglycerides, capturing central metabolic dysregulations found to be strongly linked with atypical MDD(11;18;19)) using genomic profile risk scores (GPRS); 2) the proportion of variance in liability explained by the joint effect of all common SNPs using genomic-relationship-matrix restricted maximum likelihood (GREML) methods. MDD subtypes were identified by using both data-driven techniques and a parsimonious sub-phenotyping strategy focusing on appetite and weight symptoms.
METHODS AND MATERIALS
Study Population
The sample consisted of 3,230 participants (median year of birth 1967, range 1926–1994; 64.7% females) of North-European ancestry from the Netherlands Study of Depression and Anxiety (NESDA, n=1,846) and from the Netherlands Twin Register (NTR, n=1,384). Unrelated participants were selected applying a cut-off threshold of 0.025 (i.e., no closer relationships than third or fourth cousin) to a relationship matrix measuring genetic similarity calculated using GCTAv.1.24.1(21). Detailed descriptions of the rationale, design and methods for both studies are given elsewhere(22;23). Briefly, NESDA is an ongoing cohort study into the long-term course and consequences of depressive and anxiety disorders. In 2004–2007 2,981 participants aged 18 to 65 years were recruited from the community (19%), general practice (54%) and secondary mental health care (27%) and were followed-up during three biannual assessments. The NTR study has been collecting data on Dutch twin families since 1991 and comprises longitudinal data on nearly 40,000 adult participants. The research protocols from both studies were approved by the ethical committee of participating universities, and all participants provided written informed consent.
MDD ascertainment
The sample included 1,530 patients with a lifetime diagnosis of MDD and 1,700 screened healthy controls. All cases were drawn from NESDA. Presence of DSM-IV lifetime diagnosis of MDD was assessed using the Composite Interview Diagnostic Instrument (CIDI, version 2.1)(24) administered by specially trained research staff at baseline or one of the biannual follow-up assessments. From NESDA, 316 healthy controls were also selected, including participants without any lifetime psychiatric disorder. The majority of controls (n=1,384) were drawn from NTR participants who had no reports of MDD, no known first-degree relatives with MDD and a low factor score based on a multivariate analyses of depressive complaints, anxiety, neuroticism and somatic anxiety(25). Case-control selection criteria in the present study are the same as previously applied to include NESDA and NTR participants in the Genetic Association Information Network (GAIN) MDD dataset(25), which was used in previous studies(26–29) including the largest MDD GWAS available to date by the Psychiatric Genomics Consortium(4). Of note, 1,452 cases and 99 controls from the current study were previously included in the larger (1,943 cases, 1,807 controls) GAIN-MDD dataset. In GAIN, cases and controls were genotyped on a different platform (Perlegen-Affymetrix 5.0) as compared to the current study (Affymetrix 6.0).
Determination of MDD subtypes
Among cases, 1,477 subjects had MDD symptom-level data ascertained by the CIDI interview for the most severe episode in lifetime. Data on neurovegetative symptoms (appetite, weight, sleep and psychomotor disturbances) were disaggregated to code separately for increase, decrease and both increase/decrease. Subtypes of MDD were derived using two strategies. First, lifetime depression symptoms were used as input variables in a latent class analysis (LCA; Supplemental Methods) clustering persons on the basis of their endorsed symptom profiles. A 3-class model was found to fit the data best, similarly to previous results obtained applying LCA to NESDA patients with current MDD (extensive descriptions of subtypes and their correlates have been previously published (15;16;18)). Two classes were characterized by high severity and were labeled “severe typical” and “severe atypical” based on symptom profiles. Consistent with other latent modeling studies(12–16;18) the most discriminating symptoms were appetite and weight, decreased in typical and increased in atypical. Of note, LCA-subtypes do not necessarily overlap with DSM classification of melancholic and atypical. The third class was labeled “moderate” and was characterized by lower severity. For the analyses based on LCA-subtypes we initially included 1,176 patients (~80% of those available) whose class could be assigned with confidence (average posterior probability > 0.7, indicating adequate separation and classification precision(30)). Proportions of the subtypes were 19.4% for severe typical, 21.3% for severe atypical and 59.3% for moderate.
In large collaborative studies symptom-level data necessary to apply more sophisticated data-driven techniques may not be available in all involved cohorts. For this reason we additionally tested an alternative parsimonious sub-phenotyping strategy using only information on the direction of change in appetite and weight, as these were the symptoms with the highest discriminative power between subtypes (Supplemental Methods). Among the 1,477 patients with available data, two subtypes, namely “decreased appetite/weight” (39.7% of sample) and “increased appetite/weight” (26.2%), were defined by the presence of, respectively, decrease or increase in at least one of the two symptoms. The proportion of MDD cases with lifetime anxiety disorder did not differ across typical and atypical subtypes (respectively, 84.7% and 81.3%; p=0.33), nor across decreased and increased appetite/weight subtypes (respectively, 76.3% and 80.3%; p=0.15). Supplemental eFigure 1 summarizes the number of subjects included/excluded for both sub-phenotyping strategies.
Genotyping, quality control and genetic relationship matrix
Methods for biological sample collection and DNA extraction have been described previously(25). Autosomal SNPs were genotyped on the Affymetrix 6.0 Human Genome-Wide SNP Array in three separate batches. Main QC steps have been previously described(31;32). Primary analyses included 497,347 SNPs. Additional stringent QC was performed to build a genetic-relationship-matrix (GRM) in order to reduce the possibility that estimates from GRM-based analyses could be inflated by artifacts. The remaining 435,579 SNPs were used to build the GRM using GCTAv.1.24.1(21). All QC steps are described in supplemental methods.
Genomic profile risk scores (GPRS)
GPRS for psychiatric and metabolic traits were generated based on discovery GWAS meta-analysis results from large international consortia (see supplemental methods for detailed description). Results from the Psychiatric Genomics Consortium (PGC) were used to derive GPRS for MDD(4) (~8K cases, ~8K controls), bipolar disorder(33) (BIP; ~7K, ~9K controls) and schizophrenia(34) (SCZ2; ~36K cases, ~113K controls). Discovery GWAS meta-analyses for metabolic traits were from GIANT Consortium(35) for BMI (~120K samples), Dehghan et al.(36) for C-reactive protein (CRP; ~70K samples) and Teslovich et al.(37) for triglycerides (TR; ~100K). Since NESDA and NTR samples contributed to MDD and BMI discovery GWAS, meta-analyses for these traits were performed with the Dutch GWAS cohort excluded in order to remove any chance of overlap between discovery and target samples. For all traits, eight sets of independent SNPs were selected based on significance thresholds (Pt <0.0001, <0.001, <0.005, <0.01, <0.05, <0.1, <0.5, <1) of the discovery samples associations. GPRS were calculated as the number of scores alleles weighted by effect sizes from the discovery statistics using PLINKv1.07(38) and were standardized to aid interpretation of the results. Number of SNPs included in the GPRS according to Pts are reported in Table 1.
Table 1.
Number of SNPs included in the GPRS according to eight significance thresholds of the discovery samples associations
| Pt | MDD | BIP | SCZ2 | BMI | CRP | TG |
|---|---|---|---|---|---|---|
|
|
||||||
| Pt < 0.0001 | 37 | 79 | 807 | 398 | 156 | 235 |
| Pt < 0.001 | 286 | 479 | 2229 | 1276 | 550 | 591 |
| Pt < 0.005 | 1258 | 1654 | 5196 | 3399 | 1830 | 1688 |
| Pt < 0.01 | 2296 | 2911 | 7659 | 5212 | 3197 | 2910 |
| Pt < 0.05 | 9854 | 10974 | 20344 | 15778 | 12158 | 11667 |
| Pt < 0.1 | 18170 | 19161 | 31789 | 26090 | 21875 | 21761 |
| Pt < 0.5 | 71525 | 68578 | 91650 | 83256 | 81720 | 86364 |
| Pt < 1 (all SNPs) | 112018 | 104488 | 134114 | 125070 | 127280 | 134401 |
Statistical analyses
Differences in year of birth and gender across MDD cases and controls were tested using Wilcoxon-Mann-Whitney and chi-square statistics. Cross-correlations between GPRS were evaluated with Pearson’s coefficient. The association of GPRS with MDD (subtypes) was estimated by (multivariate) logistic regressions with controls as the reference category. The proportion of variance explained by GPRS on the liability scale for MDD (subtypes) was estimated using the R2 coefficient proposed by Lee at al.(39), which is directly comparable with heritability and robust against ascertainment bias. Linear transformation on the liability scale was based on prevalence (K) of 0.18 for MDD (Dutch lifetime prevalence(40)); Ks for subtypes were empirically derived based on subtypes proportions among cases. In order to examine the overlap between subtypes obtained with different sub-phenotyping strategies, the performance of appetite/weight subtypes to predict the corresponding LCA-subtypes (decreased appetite/weight→typical and increased appetite/weight→atypical) was evaluated using receiver operating characteristic (ROC) analyses. All analyses were performed with SAS (v. 9.2, SAS Institute, Inc., Cary, NC) and R (v. 3.0.1, R Project for Statistical Computing). Finally, the total variance in liability to MDD (subtypes) explained by the joint effect of all SNPs (SNP-heritability, h2SNP) was estimated using genomic-relationship-matrix restricted maximum likelihood (GREML) analyses(41) implemented in GCTAv.1.24.1(21). H2SNP is estimated in a linear mixed model in which the measure of genetic similarity (based on the GRM) is included as a random effect to predict the phenotype. All analyses were corrected for year of birth, gender and three ancestry-informative principal components(32) to take possible population stratification into account. Significance level was set at p<0.05, two-tailed.
RESULTS
Descriptives
The study sample included 2,085 women and 1,145 men. The 1,530 participants with lifetime MDD, as compared to the 1,700 controls, were younger (year of birth: 1962[IQR:1952–1973] vs 1972[IQR:1958–1979], p<0.0001) and more likely to be female (68.1% vs 61.4, p<0.0001).
Psychiatric traits GPRS analyses
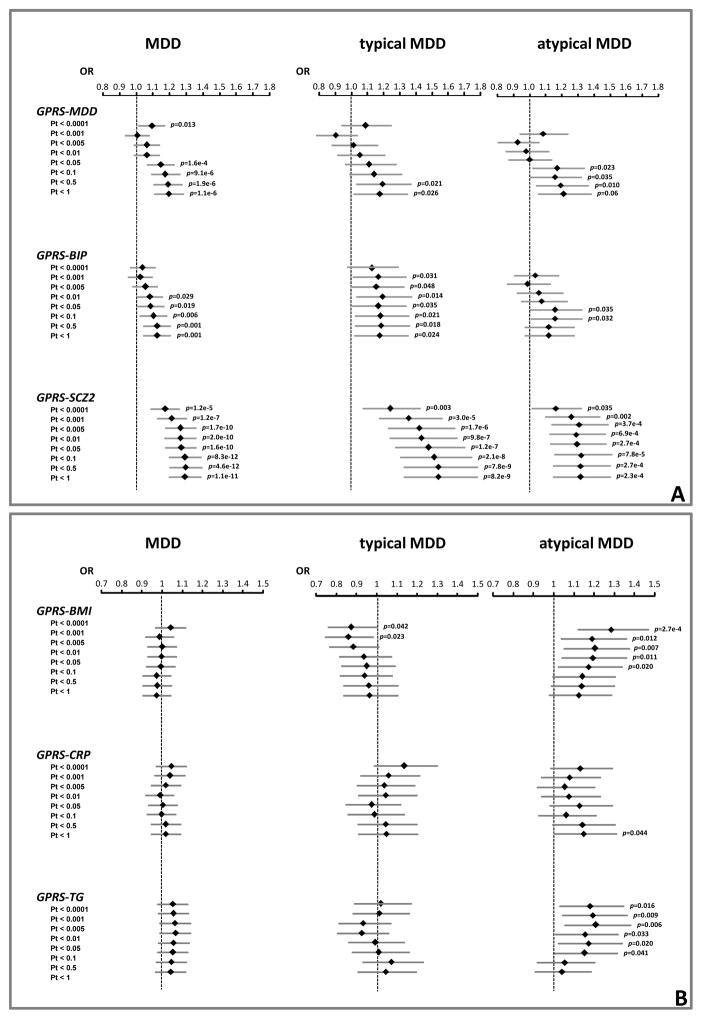
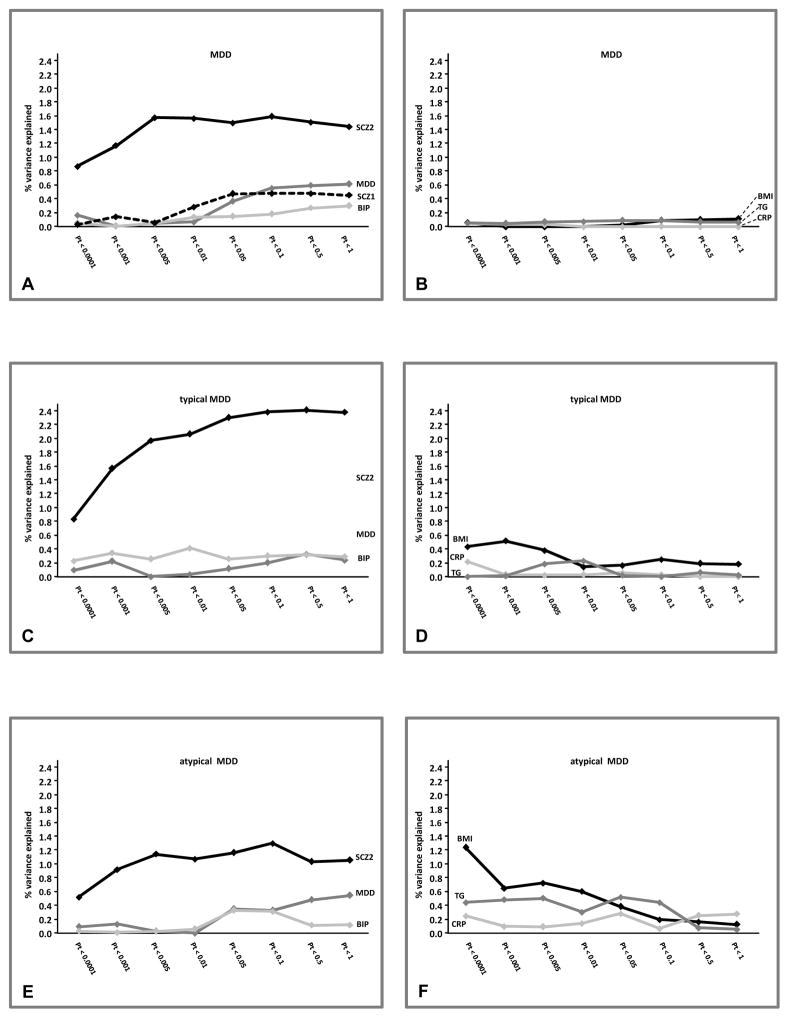
MDD case/control status (Figure 1A; full results available in eTable 1) was significantly predicted by the GPRS of psychiatric traits, especially at liberal Pts. Psychiatric GPRS including all independent SNPs (Pt<1) were significantly, although not strongly, correlated (MDD-BIP r=0.22; MDD-SCZ2 r=0.16; BIP-SCZ2 r=0.27; all p<0.0001). When including all three scores in the same model for Pt bins <0.05, <0.01, <0.5 and <1 only GPRS-MDD and GPRS-SCZ2 remained independently associated with MDD. Analyses were repeated including dummy covariates indexing the different genotyping batches and results were essentially unchanged (data not shown). The variance explained on the liability scale for MDD is shown in Figure 2A. GPRS-SCZ2 explained the higher proportion of variance, up to 1.6%, while GPRS-MDD and GPRS-BIP explained up to, respectively, 0.6% and 0.3%. The figure includes also GPRS-SCZ1 derived from the first PGC-GWAS on schizophrenia(42) (~9K cases, ~12K controls) in order to highlight the importance of discovery sample size in GPRS predictive performance(43): GPRS-SCZ1 predicted at best up to 0.5% of the variance on MDD liability. In order to further confirm these findings, we used the equations based on genetic quantitative theory developed by Dudbridge(43) in order to estimate the expected predictive accuracy of GPRS Pt<0.5 (the best performing threshold) as a function of parameters such as discovery sample size (Supplemental Methods). The theoretical estimates of explained variance were highly convergent (GPRS-MDD 0.5%, GPRS-BIP 0.3%, GPRS-SCZ2 1.2%, GPRS-SCZ1 0.3%) with the empirical values of explained variance obtained in the present study. More interestingly, a model assuming GPRS-MDD based on a discovery sample of the same size of SCZ2 indicated that an even larger amount of variance explained in MDD liability could be expected (3%).
Figure 1.
Associations of GPRS for psychiatric and metabolic traits with MDD and subtypes (severe typical and severe atypical).
Results (Odds Ratios and 95% Confidence Intervals) from binary (MDD: 1,530 cases) and multinomial (subtypes: 228 severe typical, 251 severe atypical) logistic regressions (reference: 1,700 controls) adjusted for year of birth, gender and three ancestry-informative principal components
Figure 2.
Proportions of variance explained on the liability scale for MDD and subtypes (severe typical and severe atypical) by the GPRS for psychiatric and metabolic traits. Explained variance based on R2 coefficient proposed by Lee at al.(39); prevalences for linear transformation into liability scale: MDD K=0.18, severe typical K=0.035, severe atypical K=0.038
Figure 1 graphically depicts the associations between GPRS with LCA-based typical and atypical subtype estimated by multinomial logistic regressions (associations with MDD are also depicted as benchmark for comparison; full results including moderate subtype are available in eTables 2,3). In general, GPRS for psychiatric traits were also associated with both severe subtypes, but the associations were stronger with typical, especially for GPRS-SCZ2 (Pt<0.5:OR=1.54,95%CI=1.33–1.78, p=7.8e-9). GPRS-SCZ2 explained up to 1.3% of the variance for atypical, similar to MDD, and up to 2.4% for typical MDD (Figure 2C,E)
Metabolic traits GPRS analyses
No association was found for GPRS of metabolic traits with MDD (Figure 1; eTable 1). Among the metabolic traits GPRS-BMI (Pt<0.0001:OR=1.29,95%CI=1.12–1.47, p=2.7e-4) and GPRS-TG (Pt<0.005:OR=1.21,95%CI=1.06–1.38, p=0.006) were associated with atypical MDD, particularly at stringent Pts. Cross-correlation between GPRS-BMI and GPRS-TG was low even at Pt<1 including all independent SNPs (r=0.04;p=0.02). When including both GPRS in the same model they remained independently associated with atypical in Pt bins <0.0001, <0.001, <0.005,<0.01 and 0.05. Figure 1F shows that GPRS-BMI explained up to 1.2% and GPRS-TG up to 0.5% of the variance for atypical MDD. GPRS for metabolic traits were not associated with typical MDD (Figure 1, eTable 3) Association analyses between LCA-based subtypes with all GPRS were repeated after lowering the cut-off for classification precision (average posterior probability > 0.6, including up to ~90% of the available subjects with symptom-level data) and results were very similar (data not shown).
Alternative sub-phenotyping
Subtypes defined by increase/decrease in appetite/weight were tested next. Increased appetite/weight almost perfectly predicted LCA-atypical (Area Under the Curve [AUC]:0.99; 95%CI=0.98–1.00; sensitivity 98.4%; specificity 99.5%), while the prediction of LCA-typical by decreased appetite/weight subtype was less accurate (AUC:0.81; 95%CI=0.78–0.83; sensitivity 87.8%; specificity 72.8%), suggesting that other symptoms beyond appetite/weight may be relevant to reliably identify this subtype. The decreased appetite/weight subtype captured indeed a large proportion of participants classified in LCA moderate class (Supplemental Methods). Multinomial logistic regression analyses testing the associations between GPRS and appetite/weight subtypes resulted in profiles (eFigure 2) similar to LCA-subtypes. In a sensitivity analysis addressing the impact of the symptoms increased weight when testing the association with GPRS-BMI we identified the atypical-like cases (N=364) using only the symptom increased appetite. The strength of the association (OR=1.19, 95%CI=1.06–1.34, p=0.004) with GPRS-BMI Pt<0.0001 was similar to that found when combining the two symptoms in the increased appetite/weight sub-phenotype (OR=1.20,95%CI=1.06–1.33, p=0.003; eFigure 2).
GREML analyses
Results from GREML analyses showed that common SNPs significantly captured a substantial part of the heritability of MDD (estimate=0.31; se=0.13; p=0.006). We estimated also h2SNP for the subtypes defined by appetite/weight symptoms since they allowed us to include a higher number of cases (587 decreased, 385 increased) as compared to LCA-subtypes (228 typical, 251 atypical). With the available sample size, for both subtypes 80% power to detect a significant (>0) h2SNP could be reached only assuming heritability estimates higher than those for MDD (eFigure 3). Estimates of h2SNP were significant for both decreased (K=0.072; estimate=0.38; se=0.17; p=0.01) and increased (K=0.047; estimate=0.43; se=0.20; p=0.01) appetite/weight, and higher than those for MDD, although with large standard errors due to restricted sample sizes.
DISCUSSION
In a large sample of depressed adults and controls with GWAS data, we examined the polygenic features of MDD and two common subtypes, typical and atypical, defined on the basis of symptom profiles.
We confirmed that MDD disease liability reflects the combined small effects of a large number of genetic variants across the genome(5;6). MDD case/control status was significantly predicted by GPRS-MDD based on the largest dataset to date(4), especially by scores including SNPs associated with MDD at liberal significance thresholds. This pattern indicates that the explanatory power of the scores is increased by the addition of many variants of small effect scattered across the genome. The score including all independent SNPs explained 0.6% of the variance in MDD liability. Consistently with previous cross-disorders analyses by PGC(28;29), we also confirmed that MDD shares genetic risk with major psychiatric disorders such as bipolar disorder and schizophrenia. Among the GPRS for psychiatric disorders, schizophrenia scores explained the highest proportions of variance in MDD liability (1.6%). This higher explanatory power is attributable to the larger training dataset (43), leading to smaller sampling variance on the individual SNP effects. For GPRS based on the latest schizophrenia PGC-GWAS(34) the discovery sample size was ~150K samples, whereas this was ~16K for GPRS based on MDD(4) and bipolar disorder(44). In a seminal paper, Dudbrige(43) elegantly showed that the accuracy of GPRS predictions depends on the size of the training samples. Since PGC cross-disorder analyses showed(28) that MDD could be predicted by GPRS for the other psychiatric disorders, we could expect that - due to a training sample that was almost 10-times larger - the schizophrenia GPRS may explain a portion of MDD variance even larger than GPRS for the same trait. Using Dudbrige’s equations(43) estimating the predictive accuracy of GPRS as a function of parameters such as discovery sample size, we confirmed the empirical values of explained variance obtained in the current study. More interestingly, results indicated that the availability of a discovery GWAS for MDD of the same size as that for schizophrenia, may lead to an even larger amount of variance explained in MDD liability by MDD GPRS. These results confirmed that the predictive accuracy of GPRS should be always interpreted in light of the genetic characteristics of the specific trait and the size of the discovery sample; any attempt to frame findings from different GPRS in terms of simple direct comparisons between each other should be avoided.
The evidence of shared genetic risk factors between different psychiatric disorders has been previously interpreted as a first step in moving beyond descriptive syndromes toward a biology-informed nosology(28). A project pursuing this aim is represented by the National Institute of Mental Health’s Research Domain Criteria (RDoC), which support research examining fundamental biobehavioral dimensions that cut across current heterogeneous disorder categories(45). The present study used a similar approach and results are the first to show partially different polygenic signatures across MDD subtypes. While typical had a stronger genetic overlap with psychiatric traits, particularly with schizophrenia (2.4% explained variance), atypical MDD showed an additional contribution of genetic signals from the metabolic traits of BMI (1.2% explained variance) and triglycerides (0.5% explained variance). These findings show intriguing consistencies with previous clinical and research observations. The overlap between schizophrenia genetic risk and typical MDD is consistent with the common presence of psychotic symptoms in patients with melancholic depression(46). Furthermore, previous findings from NESDA cohort showed that patients categorized as severe typical were more likely to be smoker(15;16;18); smoking is highly comorbid with psychiatric disorders, especially with schizophrenia, although the underlying biology is not well understood(47). For the atypical subtype, converging epidemiological evidence suggests a correlation with obesity and immuno-metabolic alterations(11;18;19). In the current study, atypical was associated with GPRS for BMI and triglycerides, particularly when based on SNPs strongly associated with traits at stringent significance threshold, suggesting the presence of loci of moderate effect. This is consistent with our previous findings showing the strong association between the FTO rs9939609-A variant and atypical MDD(20). When tested, the FTO-atypical association was also independent from BMI; similarly, in the current study the association of the best performing GPRS (Pt<0.0001) for BMI and triglycerides with atypical was reduced in effect size after controlling for BMI, but was still evident. Nevertheless, we decided not to adjust these analyses for BMI: our results of a shared genetic basis sustain indeed the hypothesis that atypical depression and BMI-related metabolic dysregulations may represent epiphenomenon stemming from the same pathophysiological mechanism, and adjusting for BMI may therefore represent an overadjustment. Nevertheless, the association with GPRS-BMI was unchanged when using only the increased appetite symptom to define the atypical-like subtype. Finally, no genetic overlap was found between MDD or the atypical subtype with CRP. This is in line with a recent large mendelian-randomization study showing that genetically elevated CRP is not associated with increased risk of depression(48).
Intriguingly, the differential polygenic signatures were found when deriving subtypes by LCA applied to all endorsed symptoms or by simply coding the direction of change (increase/decrease) in the highly discriminatory(12–16;18) symptoms of appetite and weight. It should be highlighted that while increased appetite/weight almost perfectly predicted LCA-atypical, the decreased appetite/weight→LCA-typical prediction was less accurate, suggesting that other symptoms beyond appetite/weight may be relevant to reliably identify this subtype. This should be carefully evaluated in specifically dedicated diagnostic-accuracy studies. Nevertheless, the possibility of using parsimonious and effective sub-phenotyping strategies may be relevant for large collaborative studies for which symptom-level data necessary to apply more sophisticated data-driven techniques may not be available in all involved cohorts. Moreover, symptom endorsement profiles may be highly variable across cohorts, reflecting differences such as settings (e.g. clinical, population-based), ascertainment (e.g. psychiatric interviews, medical records) or diagnosis timeframe (e.g. lifetime, current). When applying typical/atypical sub-phenotyping strategies an important aspect to consider may be the possible impact of antidepressant medications (AD), some of which may affect weight change and other metabolic disturbances(49). For the current study, previous results from NESDA point toward a reduced likelihood of AD impact. The AD classes more commonly used were not cross-sectionally associated with metabolic dysregulations(50) nor with 2-year trajectories of weight change(51). In another study(18) focusing on a subset of patients with current chronic MDD, AD used were similar across typical and atypical subtypes; moreover, the differential associations across subtypes of characteristics such as waist circumference, BMI, triglycerides and CRP was unchanged after adjustment for antidepressant use.
Results of the present study also showed that 31% of the variance in MDD liability was explained by the joint effect of all common SNPs. This perfectly replicates the h2SNP estimate (0.32) obtained by a previous study based on the GAIN MDD dataset (26), including overlapping cases with the current study and different controls genotyped on a different platform. Estimates of h2SNP were higher for the subtypes: 38% for MDD with decreased and 43% for MDD with increased appetite/weight. This suggests that subtypes may be genetically more homogeneous. It is important to remark, however, that due to the limited sample size the standard errors around the estimates were large. Nevertheless, our results are in line with recent twin-based estimates(52) showing higher heritability for atypical depression defined according to DSM-III (0.51) as compared to MDD (0.43). Major strengths of the current study include the availability of GWAS data in a large sample well-characterized in terms of psychiatric diagnoses, the use of different sub-phenotyping strategies to identify MDD subtypes and GPRS based on large international consortia. An important limitation is that the sample size was still largely underpowered to perform bivariate-GREML analyses to calculate the SNP correlation (average genome-wide relationship)(41) between the subtypes.
In summary, our results suggest that MDD subtypes based on symptom profiles are characterized by partially distinct polygenic liabilities and may represent more homogeneous phenotypes. Similarly to other complex diseases MDD may represent a diagnostic aggregation of biologically different subtypes. As shown by recent simulations studies(7;8), this heterogeneity could severely compromise the power of association studies. Moreover, the finding of partially distinct genetic signature across more homogenous subtypes suggest translational implication in the long term: it could be hypothesized that distinct subtypes may specifically respond to different treatments. Our results provide proof of principle evidence that should stimulate further studies scaling up the dissection of MDD heterogeneity in larger samples. As we demonstrated, the use of cost-effective sub-phenotyping strategies to identify subtypes, in particular atypical, may be a successful strategy to harmonize phenotypes across different cohorts. While we dissected MDD along symptom-profiles, other clinical features (e.g.; age of onset, post-partum onset, sensitivity to environmental stressors) may be also tested in future studies. Disentangling MDD heterogeneity (provided a constant parallel effort in increasing samples size of genetic studies) may help the psychiatric field moving forward in the search for molecular roots of depression.
Supplementary Material
Acknowledgments
Netherlands Study of Depression and Anxiety and Netherland Twin Register: funding was obtained from the Netherlands Organization for Scientific Research (NWO) and MagW/ZonMW grants Middelgroot-911-09-032, Spinozapremie 56-464-14192, Geestkracht program of the Netherlands Organization for Health Research and Development (ZonMW 10-000-1002), Center for Medical Systems Biology (CSMB, NWO Genomics), Genetic influences on stability and change in psychopathology from childhood to young adulthood (ZonMW 912-10-020), NBIC/BioAssist/RK (2008.024), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI–NL, 184.021.007), VU University’s Institute for Health and Care Research (EMGO+) and Neuroscience Campus Amsterdam (NCA); the European Science Council (ERC Advanced, 230374). Part of the genotyping and analyses were funded by the Genetic Association Information Network (GAIN) of the Foundation for the National Institutes of Health, Rutgers University Cell and DNA Repository (NIMH U24 MH068457-06), the Avera Institute, Sioux Falls, South Dakota (USA) and the National Institutes of Health (NIH R01 HD042157-01A1, MH081802, Grand Opportunity grants 1RC2 MH089951 and 1RC2 MH089995). Computing was supported by BiG Grid, the Dutch e-Science Grid, which is financially supported by NWO.
Funding for this research is also provided by EU FP7 MooDFOOD Project ‘Multi-country cOllaborative project on the rOle of Diet, FOod-related behaviour, and Obesity in the prevention of Depression’, Grant agreement no. 613598.
The CHARGE inflammation working group is supported by the following grants: N01-HC 25195 1RO1 HL64753; R01 HL076784; 1 R01 AG028321
F Lamers is supported by a FP7-Marie Curie Career Integration Grant (PCIG12-GA-2012-334065)
We are grateful to the Psychiatric Genomics Consortium (MDD group) and to GIANT consortium for providing the summary statistics for meta-analyses of, respectively, MDD and BMI after removal of the Dutch GWAS cohort.
Footnotes
Potential Conflicts of Interest: The authors do not have any conflict of interest in the publication of the manuscript.
Supplemental materials are available at the journal website.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003 Jun 18;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000 Oct;157(10):1552–62. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 4.Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, Breen G, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013 Apr;18(4):497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flint J, Kendler KS. The genetics of major depression. Neuron. 2014 Feb 5;81(3):484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray NR, et al. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol Psychiatry. 2014 Oct 1;76(7):510–2. doi: 10.1016/j.biopsych.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manchia M, Cullis J, Turecki G, Rouleau GA, Uher R, Alda M. The impact of phenotypic and genetic heterogeneity on results of genome wide association studies of complex diseases. PLoS One. 2013;8(10):e76295. doi: 10.1371/journal.pone.0076295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wray N, Maier R. The Contribution of Disease Heterogeneity to Missing Heritability. Current Epidemiology Reports. 2014;1(4) [Google Scholar]
- 9.Lubke GH, Laurin C, Amin N, Hottenga JJ, Willemsen G, Van GG, et al. Genome-wide analyses of borderline personality features. Mol Psychiatry. 2014 Aug;19(8):923–9. doi: 10.1038/mp.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 11.Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. doi: 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan PF, Kessler RC, Kendler KS. Latent class analysis of lifetime depressive symptoms in the national comorbidity survey. Am J Psychiatry. 1998 Oct;155(10):1398–406. doi: 10.1176/ajp.155.10.1398. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan PF, Prescott CA, Kendler KS. The subtypes of major depression in a twin registry. J Affect Disord. 2002 Apr;68(2–3):273–84. doi: 10.1016/s0165-0327(00)00364-5. [DOI] [PubMed] [Google Scholar]
- 14.Kendler KS, Eaves LJ, Walters EE, Neale MC, Heath AC, Kessler RC. The identification and validation of distinct depressive syndromes in a population-based sample of female twins. Arch Gen Psychiatry. 1996 May;53(5):391–9. doi: 10.1001/archpsyc.1996.01830050025004. [DOI] [PubMed] [Google Scholar]
- 15.Lamers F, de JP, Nolen WA, Smit JH, Zitman FG, Beekman AT, et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA) J Clin Psychiatry. 2010 Dec;71(12):1582–9. doi: 10.4088/JCP.09m05398blu. [DOI] [PubMed] [Google Scholar]
- 16.Lamers F, Rhebergen D, Merikangas KR, de JP, Beekman AT, Penninx BW. Stability and transitions of depressive subtypes over a 2-year follow-up. Psychol Med. 2012 Oct;42(10):2083–93. doi: 10.1017/S0033291712000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold PW, Chrousos GP. Melancholic and atypical subtypes of depression represent distinct pathophysiological entities: CRH, neural circuits, and the diathesis for anxiety and depression. Mol Psychiatry. 2013 Jun;18(6):632–4. doi: 10.1038/mp.2013.5. [DOI] [PubMed] [Google Scholar]
- 18.Lamers F, Vogelzangs N, Merikangas KR, de JP, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013 Jun;18(6):692–9. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- 19.Lasserre AM, Glaus J, Vandeleur CL, Marques-Vidal P, Vaucher J, Bastardot F, et al. Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: a prospective, population-based study. JAMA Psychiatry. 2014 Aug;71(8):880–8. doi: 10.1001/jamapsychiatry.2014.411. [DOI] [PubMed] [Google Scholar]
- 20.Milaneschi Y, Lamers F, Mbarek H, Hottenga JJ, Boomsma DI, Penninx BW. The effect of FTO rs9939609 on major depression differs across MDD subtypes. Mol Psychiatry. 2014 Sep;19(9):960–2. doi: 10.1038/mp.2014.4. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011 Jan 7;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penninx BW, Beekman AT, Smit JH, Zitman FG, Nolen WA, Spinhoven P, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17(3):121–40. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boomsma DI, De Geus EJ, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, et al. Netherlands Twin Register: from twins to twin families. Twin Res Hum Genet. 2006 Dec;9(6):849–57. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Thw Composite Interview Diagnostic Instrument. Geneva: WHO; 1997. [Google Scholar]
- 25.Boomsma DI, Willemsen G, Sullivan PF, Heutink P, Meijer P, Sondervan D, et al. Genome-wide association of major depression: description of samples for the GAIN Major Depressive Disorder Study: NTR and NESDA biobank projects. Eur J Hum Genet. 2008 Mar;16(3):335–42. doi: 10.1038/sj.ejhg.5201979. [DOI] [PubMed] [Google Scholar]
- 26.Lubke GH, Hottenga JJ, Walters R, Laurin C, De Geus EJ, Willemsen G, et al. Estimating the genetic variance of major depressive disorder due to all single nucleotide polymorphisms. Biol Psychiatry. 2012 Oct 15;72(8):707–9. doi: 10.1016/j.biopsych.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan PF, De Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009 Apr;14(4):359–75. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013 Apr 20;381(9875):1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013 Sep;45(9):984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roeder K, Lynch K, Nagin K. Modeling Uncertainty in Latent Class Membership: A Case Study in Criminology. Journal of the American Statistical Association. 1999;94(447):766–76. [Google Scholar]
- 31.Peyrot WJ, Milaneschi Y, Abdellaoui A, Sullivan PF, Hottenga JJ, Boomsma DI, et al. Effect of polygenic risk scores on depression in childhood trauma. Br J Psychiatry. 2014 Aug;205(2):113–9. doi: 10.1192/bjp.bp.113.143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdellaoui A, Hottenga JJ, de KP, Nivard MG, Xiao X, Scheet P, et al. Population structure, migration, and diversifying selection in the Netherlands. Eur J Hum Genet. 2013 Nov;21(11):1277–85. doi: 10.1038/ejhg.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011 Oct;43(10):977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014 Jul 24;511(7510):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010 Nov;42(11):937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011 Feb 22;123(7):731–8. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010 Aug 5;466(7307):707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007 Sep;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SH, Goddard ME, Wray NR, Visscher PM. A better coefficient of determination for genetic profile analysis. Genet Epidemiol. 2012 Apr;36(3):214–24. doi: 10.1002/gepi.21614. [DOI] [PubMed] [Google Scholar]
- 40.de Graaf R, ten Have M, van Gool C, van Dorsselaer S. Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Soc Psychiatry Psychiatr Epidemiol. 2012 Feb;47(2):203–13. doi: 10.1007/s00127-010-0334-8. [DOI] [PubMed] [Google Scholar]
- 41.Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, Middeldorp CM. Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014 Oct;55(10):1068–87. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- 42.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011 Oct;43(10):969–76. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013 Mar;9(3):e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011 Oct;43(10):977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker G, Fink M, Shorter E, Taylor MA, Akiskal H, Berrios G, et al. Issues for DSM-5: whither melancholia? The case for its classification as a distinct mood disorder. Am J Psychiatry. 2010 Jul;167(7):745–7. doi: 10.1176/appi.ajp.2010.09101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008 Dec;10(12):1691–715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 48.Wium-Andersen MK, Orsted DD, Nordestgaard BG. Elevated C-reactive protein, depression, somatic diseases, and all-cause mortality: a mendelian randomization study. Biol Psychiatry. 2014 Aug 1;76(3):249–57. doi: 10.1016/j.biopsych.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010 Oct;71(10):1259–72. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 50.van Reedt Dortland AK, Giltay EJ, van VT, Zitman FG, Penninx BW. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr Scand. 2010 Jul;122(1):30–9. doi: 10.1111/j.1600-0447.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 51.Gibson-Smith D, Bot M, Milaneschi Y, Twisk JW, Visser M, Brouwer IA, et al. Major depressive disorder, antidepressant use and subsequent 2-year weight change patterns in the Netherlands Study of Depression and Anxiety. J Clin Psychiatry. 2015 doi: 10.4088/JCP.14m09658. in press. [DOI] [PubMed] [Google Scholar]
- 52.Edvardsen J, Torgersen S, Roysamb E, Lygren S, Skre I, Onstad S, et al. Unipolar depressive disorders have a common genotype. J Affect Disord. 2009 Sep;117(1–2):30–41. doi: 10.1016/j.jad.2008.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.