Abstract
Laboratory mouse strains carry approximately 35 major urinary protein (MUP) genes per haploid genome, tightly clustered together on chromosome 4. Most belong to two main groups (Groups 1 and 2). The available evidence strongly suggests that the Group 1 genes are active while the Group 2 genes are pseudogenes. Here we present the complete sequence of a Group 1 gene and a Group 2 gene and 700 bp of flanking sequence. The sequence of the Group 1 gene is consistent with its being active. The Group 2 gene contains two stop codons and a frame-shift mutation in the reading frame defined by the Group 1 gene, and would code for a signal peptide 25 rather than 19 amino acids long. The Group 2 gene differs from the Group 1 gene in other ways: a deletion upstream of the TATA box and another in intron 3, a base change in the TATA box itself, a 2 bp duplication at the splice acceptor boundary of intron 6, an altered poly(A) addition signal and a 1-base deletion 5' to the initiation codon. Some of these differences may explain the 10- to 20-fold higher level of Group 1 mRNA in mouse liver, and the fact that Group 1 and Group 2 transcripts are mainly spliced differently. The presence of the stop codon means that the Group 2 gene is a pseudogene in the context of the Group 1 gene. However, there is some evidence that the mature hexapeptide that it would code for may have biological activity. The 12 acceptor splice sites of the two genes all contain the identical sequence ACAG at the exon boundary.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

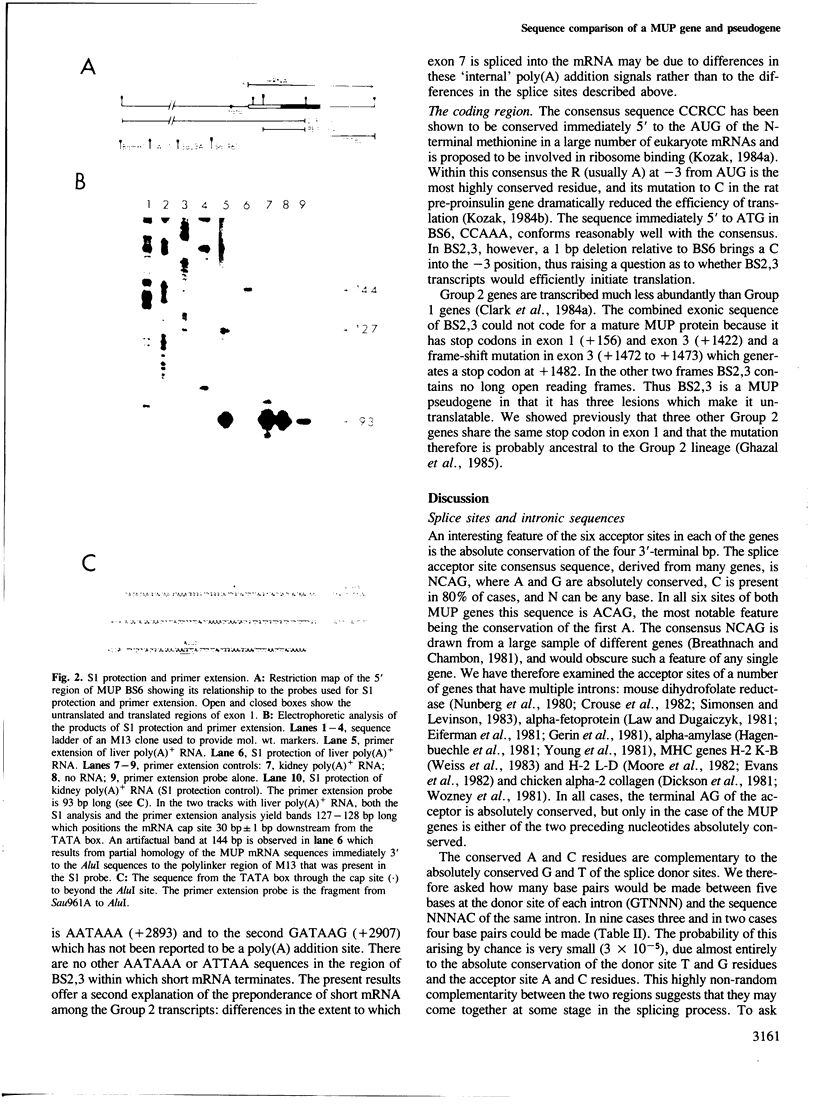

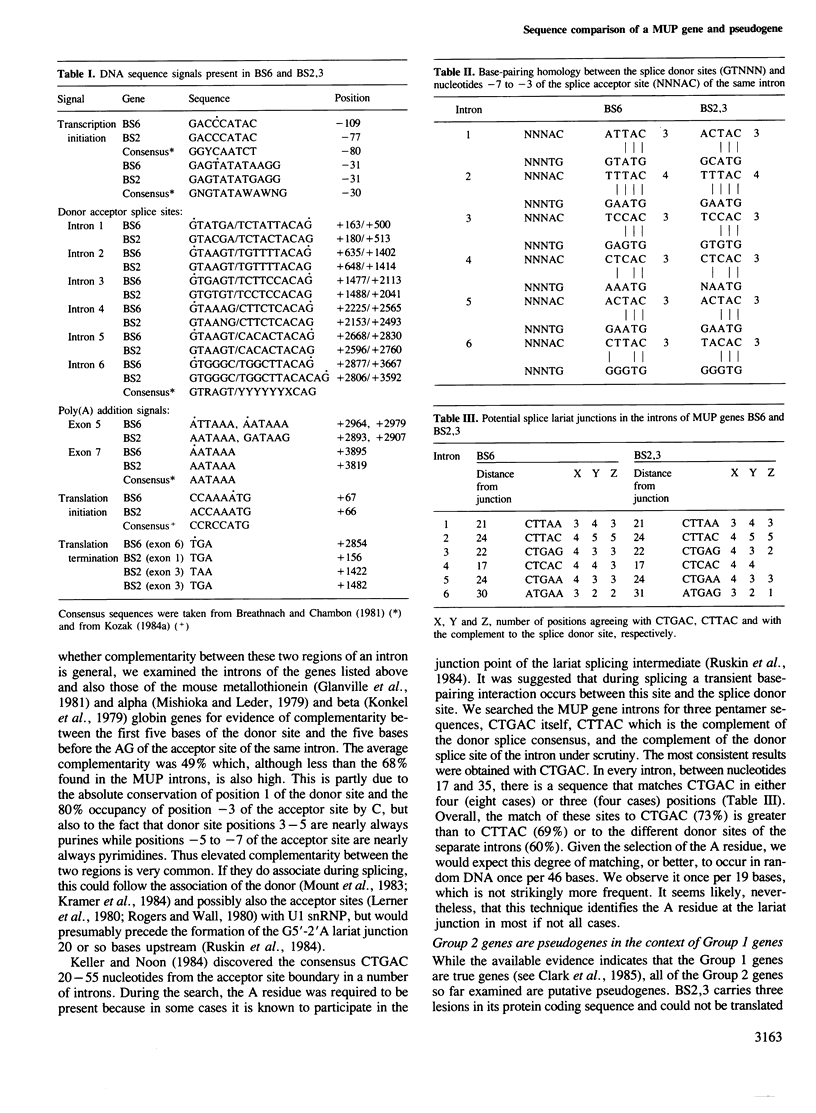


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Gait M. J., Mayol L., Young I. G. A short primer for sequencing DNA cloned in the single-stranded phage vector M13mp2. Nucleic Acids Res. 1980 Apr 25;8(8):1731–1743. doi: 10.1093/nar/8.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Clark A. J., Clissold P. M., Hainey S., Francke U. Two main groups of mouse major urinary protein genes, both largely located on chromosome 4. EMBO J. 1982;1(5):615–620. doi: 10.1002/j.1460-2075.1982.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Selman G. G., Hickman J., Black L., Saunders R. D., Clark A. J. The 45-kb unit of major urinary protein gene organization is a gigantic imperfect palindrome. Mol Cell Biol. 1985 Jul;5(7):1591–1600. doi: 10.1128/mcb.5.7.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Chave-Cox A., Ma X., Bishop J. O. Analysis of mouse major urinary protein genes: variation between the exonic sequences of group 1 genes and a comparison with an active gene out with group 1 both suggest that gene conversion has occurred between MUP genes. EMBO J. 1985 Dec 1;4(12):3167–3171. doi: 10.1002/j.1460-2075.1985.tb04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Clissold P. M., Al Shawi R., Beattie P., Bishop J. Structure of mouse major urinary protein genes: different splicing configurations in the 3'-non-coding region. EMBO J. 1984 May;3(5):1045–1052. doi: 10.1002/j.1460-2075.1984.tb01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Clissold P. M., Bishop J. O. Variation between mouse major urinary protein genes isolated from a single inbred line. Gene. 1982 Jun;18(3):221–230. doi: 10.1016/0378-1119(82)90159-7. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Hickman J., Bishop J. A 45-kb DNA domain with two divergently orientated genes is the unit of organisation of the murine major urinary protein genes. EMBO J. 1984 Sep;3(9):2055–2064. doi: 10.1002/j.1460-2075.1984.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clissold P. M., Bishop J. O. Variation in mouse major urinary protein (MUP) genes and the MUP gene products within and between inbred lines. Gene. 1982 Jun;18(3):211–220. doi: 10.1016/0378-1119(82)90158-5. [DOI] [PubMed] [Google Scholar]
- Dickson L. A., Ninomiya Y., Bernard M. P., Pesciotta D. M., Parsons J., Green G., Eikenberry E. F., de Crombrugghe B., Vogeli G., Pastan I. The exon/intron structure of the 3'-region of the pro alpha 2(I) collagen gene. J Biol Chem. 1981 Aug 25;256(16):8407–8415. [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Eiferman F. A., Young P. R., Scott R. W., Tilghman S. M. Intragenic amplification and divergence in the mouse alpha-fetoprotein gene. Nature. 1981 Dec 24;294(5843):713–718. doi: 10.1038/294713a0. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Clark A. J., Bishop J. O. Evolutionary amplification of a pseudogene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4182–4185. doi: 10.1073/pnas.82.12.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., Saedler H. DNA sequence of the mini-insertion IS2--6 and its relation to the sequence of IS2. Nature. 1978 Oct 19;275(5681):611–617. doi: 10.1038/275611a0. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Gorin M. B., Cooper D. L., Eiferman F., van de Rijn P., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. I. A comparison of the primary amino acid sequences of mammalian alpha-fetoprotein and albumin. J Biol Chem. 1981 Feb 25;256(4):1954–1959. [PubMed] [Google Scholar]
- Grabowski P. J., Padgett R. A., Sharp P. A. Messenger RNA splicing in vitro: an excised intervening sequence and a potential intermediate. Cell. 1984 Jun;37(2):415–427. doi: 10.1016/0092-8674(84)90372-6. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Tosi M., Schibler U., Bovey R., Wellauer P. K., Young R. A. Mouse liver and salivary gland alpha-amylase mRNAs differ only in 5' non-translated sequences. Nature. 1981 Feb 19;289(5799):643–646. doi: 10.1038/289643a0. [DOI] [PubMed] [Google Scholar]
- Hong G. F. A systemic DNA sequencing strategy. J Mol Biol. 1982 Jul 5;158(3):539–549. doi: 10.1016/0022-2836(82)90213-3. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Maizel J. V., Jr, Leder P. The evolution and sequence comparison of two recently diverged mouse chromosomal beta--globin genes. Cell. 1979 Nov;18(3):865–873. doi: 10.1016/0092-8674(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Woodworth-Gutai M., Gross K. W., Held W. A. Subfamilies of the mouse major urinary protein (MUP) multi-gene family: sequence analysis of cDNA clones and differential regulation in the liver. Nucleic Acids Res. 1984 Aug 10;12(15):6073–6090. doi: 10.1093/nar/12.15.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. W., Dugaiczyk A. Homology between the primary structure of alpha-fetoprotein, deduced from a complete cDNA sequence, and serum albumin. Nature. 1981 May 21;291(5812):201–205. doi: 10.1038/291201a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Chang A. C., Cohen S. N., Schimke R. T. Structure and genomic organization of the mouse dihydrofolate reductase gene. Cell. 1980 Feb;19(2):355–364. doi: 10.1016/0092-8674(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J. G., Finlayson J. S., Dobrogosz W. J., Dills S. S., Kost T. A. Chromatographic separation of puberty accelerating pheromone from male mouse urine. Biol Reprod. 1976 Sep;15(2):260–265. doi: 10.1095/biolreprod15.2.260. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J. G., Whitsett J. M., Lombardi J. R. Partial isolation of a pheromone accelerating puberty in female mice. J Reprod Fertil. 1975 Jun;43(3):515–523. doi: 10.1530/jrf.0.0430515. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Tate V., Boedtker H., Doty P. Structure of the pro alpha 2 (I) collagen gene. Nature. 1981 Nov 12;294(5837):129–135. doi: 10.1038/294129a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]