Abstract
Understanding the prognostic capacity of a simple measure of self-rated health (SRH) by older people becomes increasingly important as the population ages. SRH has been shown to predict survival, functional status and service use. The relationship with cognitive impairment has not been widely investigated. This paper investigates SRH as a predictor of death, functional impairment (inability to perform activities of daily living) and cognitive impairment (MMSE < 18) over a 10-year follow-up of participants in the MRC Cognitive Function and Ageing Study. A stratified random sample of 13,004 people aged 65 or over resident in five areas in England and Wales were interviewed. Analysis used data from interviews at baseline, 2, 6 and 10 year follow-up. Hazard ratios for risk of death, functional and cognitive impairment were estimated, unadjusted and adjusted for potential confounding baseline factors. Of the 13,004 participants recruited, 6,882 had died by 10 years and 1,252 and 481 new cases of functional and cognitive impairment respectively were recorded. SRH was associated with a higher risk of death, functional and cognitive impairment. The associations remained after adjustment for age, gender, functional ability and MMSE at baseline: comparing those who rated their health as excellent and good, hazard ratios for risk of death, functional and cognitive impairment were 0.8 (95% CI 0.8–0.9), 0.6 (95% CI 0.5–0.7) and 0.7(95% CI 0.5–0.9), respectively. In-depth qualitative study designs are needed to investigate why the meaning older people give to their health status predicts long-term outcomes.
Keywords: Self-rated health, Survival, Cognitive function, Activities of daily living, Epidemiology
Introduction
Self-rated health (SRH) or self-perceived health has frequently been considered in studies of older people during the last four decades. It has long been recognised that better SRH is a good predictor of better survival (Baron-Epel et al. 2004; Han et al. 2005; Idler et al. 1990, 2000; Idler and Benyamini 1997; Ishizaki et al. 2006; Kaplan et al. 1988; Long and Marshall 1999; McGee et al. 1999; Mossey and Shapiro 1982; Nybo et al. 2003; Siegel et al. 2003; Vuorisalmi et al. 2005; Wolinsky and Tierney 1998) better functional status (Atchley and Scala 1998; Dening et al. 1998; Hillen et al. 2003; Idler et al. 2000; Spiers et al. 1996) and reported lower service use (Dening et al. 1998; Long and Marshall 1999). The potential of SRH to predict cognitive decline has not been widely investigated in longitudinal studies.
SRH is usually inferred from participants’ responses to a single question using closed response categories in the form of a rating scale. Studies differ in the number of responses offered and also in the frame of reference used. Studies assessing global SRH have asked participants to rate their health in general, while other studies have assessed comparative SRH by offering a frame of reference, since study participants often spontaneously compare their health to reference groups (Kaplan and Baron-Epel 2003). In studies of older people this is most often the health of age peers (Bowling 2005).
The prognostic capacity of SRH for predicting death has been well reported (Idler and Benyamini 1997). Of 27 community studies reviewed 22 focus on people aged 60 years or over; 14 report studies from North America; 19 report on the role of functional ability and three on cognitive ability. The mean sample size is 2,878 (range 534–7,725) and the mean follow-up is 6.4 years (range 2–28). The purpose of this paper is to evaluate, in a large (N = 13004) longitudinal study (follow up over 10 years) of older people (aged 65 or over) in five centres in the UK, the capacity of SRH for predicting not only death and functional impairment but also cognitive impairment. It extends previous analysis (up to 5 years follow-up) of the CFAS data set of survival (MRC Cognitive Function and Ageing Study 2001) and risk factors for dementia (MRC Cognitive Function and Ageing Study 2006) and onset of disability which showed that SRH is associated with survival and that poor SRH increased incident risk of dementia and disability.
Methods
Details of the MRC Cognitive Function and Ageing study (http://www.cfas.medschl.cam.ac.uk) have been previously reported (MRC CFAS Medical Research Council Cognitive Function and Ageing Study Group 1998).
Sample
Briefly, 20,234 people in their sixty-fifth year and above were randomly selected from a sampling frame of 123,691 people on the Family Health Service Authority lists, which constitute a comprehensive population register including those in institutional care, in five areas of England and Wales (rural Cambridgeshire, Gwynedd, Newcastle, Nottingham and Oxford). Stratification ensured equivalent numbers in the 65–74 and ≥75 years age groups. After duplicate records, deaths and migrants from the areas had been omitted, a sample of 16,258 was eligible for approach. In all, 13,004 participants (80%) agreed to the first interview. Each centre received Local Research Ethics Committee approval. Informed consent was received from participants.
Interview schedule
The initial screening interview was carried out between 1991 and 1994 on the entire sample by trained interviewers using laptop computers in participants’ homes. Further interviews were conducted with all participants remaining in the study 2 and 10 years after the screening interview. Additionally, 6 years after the screening interview, all 717 respondents in Cambridge and a sample of respondents from the other centres were interviewed.
Attrition between interviews has been reported in detail (Matthews et al. 2004, 2006) and the numbers interviewed at each major wave (baseline, 2 and 10-year follow-up) are summarised in Table 1.
Table 1.
Number of participants at major waves of MRC CFAS study
| Number of participants | Reason for loss to follow-up | |||||
|---|---|---|---|---|---|---|
| Alive | Interviewed | Lost to follow-up | Died | Refused | Moved | |
| Screening interview (year 0) | – | 13,004 | – | – | – | |
| 2-year interview | 11,530 | 8,827 | 4,177 | 1,474 | 2,520 | 183 |
| 10-year interviewa | 6,171 | 3,145 | 5,707 | 3,992 | 1,373 | 342 |
a25 individuals re-entered after year 2
All interviews included items on sociodemographic variables, lifestyle and health factors, cognitive function and ability to perform activities of daily living.
Outcomes
Participants were flagged on the Office of National Statistics National Health Service Central Register, resulting in automatic notification of death to the researchers. Analysis used version 7.1 of the dataset, which included deaths ascertained up to the end of 2003.
Cognitive function was estimated using the Mini-Mental State Examination (MMSE) (Folstein et al. 1975). Cognitive impairment was defined as MMSE under 18. Functional ability was assessed by asking six questions about activities of daily living (ADL) and instrumental activities of daily living (IADL). Participants were assigned to one of four categories, those who: (1) needed help several times a week with washing, hot meals, putting on shoes and socks, or getting around outside (ADL disability); (2) needed help regularly with heavy housework or shopping and carrying heavy bags (IADL disability); (3) had no ADL or IADL disability; (4) were unclassifiable as they answered some but not all of these questions—this group included many cognitively frail people (Spiers et al. 2005). If participants appeared disoriented in time or space they were assigned to group (1). Functional impairment was defined as ADL disability.
Self-rated health
For SRH, participants were asked to rate their own health as excellent, good, fair or poor compared to others of the same age.
Potential confounders
Potential confounders of an association of SRH in older people with functional and cognitive impairment and death are summarised in Table 2.
Table 2.
Potential confounding factors: description, method of measurement, and evidence for association with death and functional impairment
| Covariates | Method of measurement | Categories: | D | FI | Evidence from | |
|---|---|---|---|---|---|---|
| No. | Description | |||||
| Socio-demographic characteristics | ||||||
| Age in years | Age at screening interview (calculated from date of birth and date of interview) | 6 | 64–69, 70–74, 75–79 80–84, 85–89, 90+ |
✓ | ✓ | Baron-Epel et al. (2004), Benyamini et al. (2004), Dasbach et al. (1994), Fenton et al. (1995), Helweg-Larsen et al. (2003), Hillen et al. (2003), and Wolinsky and Tierney (1998) |
| Gender | 2 | Male/female | ✓ | Baron-Epel et al. (2004), Benyamini et al. (2003), Dasbach et al, (1994), Deeg and Kriegsman (2003), Fenton et al. (1995), Helmer et al. (1999), Idler et al. (2000), Jylhä et al. (1998), Kim et al. (1997), Liang et al. (2003), Lichtman (1997), Long and Marshall (1999), and Wolinsky and Tierney (1998) | ||
| Marital status | Coded on basis of response to questions: “Are you married, single, widowed or divorced? (If NO are you separated or do you have a partner?)” | 3 | Married or cohabiting Never married Widowed or divorced |
✓ | Baron-Epel et al. (2004), Liang et al. (2003), Nybo et al, (2003), and Wolinsky and Tierney (1998) | |
| Years of full-time education | Coded on basis of response to question: “How many years did you spend in full-time education?” | 5 | Less than 9, 9,10,11,12+ | Jylhä et al. (1998), Liang et al. (2003), and Wolinsky and Tierney (1998) | ||
| Social class | Social class was assigned according to the Registrar General’s method on the basis of the participant’s occupation during most of working life, or for married women, her husband’s occupations | 7 | 1, 2 3 (non-manual) 3 (manual) 4, 5 armed forces |
Broese van Groenou et al. (2003), and OPCS (1990) | ||
| Townsend deprivation score | A composite measure of area-based socio-economic status, based on the proportion of: unemployed but economically active individuals of working age; households which do not possess a car; households which have more than one person per room; households which are not owner-occupied. Postcodes of the participants’ residence were mapped to 1991 census enumeration districts, which contain about 200 households and 400 individuals. Based on the 1991 census data, a Townsend deprivation score was calculated for each enumeration district | 5 | Quintiles | Fenton et al. (1995), Townsend et al. (1988), and Wolinsky and Tierney (1998) | ||
| Functional and cognitive ability | ||||||
| Activities of daily living | Coded on basis of response to six questions about: washing, preparing hot meals, putting on shoes and socks, getting around outside, doing heavy housework, shopping and carrying heavy bags | ✓ | ✓ | Hillen et al. (2003), Idler and Benyamini (1997), Jylhä et al. (1998), Kim et al. (1997), Lichtman (1997), Nybo et al. (2003), Spiers et al. (1996), and Wolinsky and Tierney (1998) | ||
| Cognitive function | MMSE | ✓ | Idler and Benyamini (1997), Liang et al. (2003), and Nybo et al. 2003 | |||
| Self-reported medical history | ||||||
| Heart attack | Coded on basis of response to question, “Have you ever suffered from a heart attack?” | 2 | Yes/No | Dasbach et al. (1994), and Wolinsky and Tierney (1998) | ||
| Stroke | Coded on basis of response to question, “Have you ever had a stroke that required medical attention?” | 2 | Yes/No | |||
| Diabetes | Coded on basis of response to question, “Have you ever had sugar diabetes?” | 2 | Yes/No | ✓ | Dasbach et al. (1994), and Wolinsky and Tierney (1998) | |
| Transient ischaemic attack | Coded on basis of response to three questions about transient problems with speech, sight, or weakness in arm or leg | 2 | Yes/No | |||
| Angina | Coded on basis of response to questions from Rose questionnaire | 2 | Yes/No | Rose (1962) | ||
| Intermittent claudication | As above | 2 | Yes/No | |||
| Depression | Coded on basis of response to two questions: “ Have you ever consulted a doctor about emotional problems, or problems with your nerves? What did the doctor say you had?” | 2 | Yes/No | Han (2000), Kim et al. (1997), and Williams (2000) | ||
| Anxiety | See above | 2 | Yes/No | Han (2000), Kim et al. (1997), and Williams (2000) | ||
| Medication use | Coded on basis of response to question: “do you take any medicine, tablets or injections of any kind, either that you buy yourself or that are prescribed by your doctor?” | 2 | Yes/No | |||
| Smoking history | Coded on basis of response to questions: “Do you smoke? Have you ever smoked?” | 3 | Current Past Never |
✓ | Dasbach et al. (1994), and Lichtman (1997) | |
D Death, FI Functional impairment
Potential confounding variables which were reported to be associated with both SRH and either survival or cognitive or functional impairment were considered. Table 2 presents a description of these factors, the methods used to measure and categorise them, and the evidence that they may confound the relationship between SRH and the outcomes of interest.
Statistical methods
The main analysis considered all deaths and diagnoses of functional and cognitive impairment made at those interviews which included all participants: the 2 and 10 year interviews and the 6 year interview by the Cambridge centre. Multivariable Cox regression was used to estimate the hazard ratio for death, functional and cognitive impairment in relation to SRH at the screening interview, both unadjusted and adjusted for possible confounding variables. Adjusted analyses are reported, firstly adjusting only for age and gender, secondly with further adjustment for functional and cognitive ability and, finally, with adjustment for all potential confounding baseline factors. This allows assessment of the additional prognostic information provided by SRH, after allowing for known prognostic factors (Simon and Altman 1994). Functional and cognitive ability were considered jointly as cognitive ability almost certainly affects ability to perform activities of daily living. All potential confounding variables were treated as categorical variables (see Table 2), because the primary aspect of interest was not the form of their relationship with the various outcomes but rather the extent to which their inclusion in the model changed the relationship between SRH and the outcomes. Treating them as continuous variables would have required careful modelling of the functional form of their relationship with each outcome and is unlikely to have made any substantive difference to the estimate of the adjusted relationship between SRH and the outcome.
Participants were regarded as entering the study at the date of the screening interview and were censored at 31-12-2003, the final date for ascertainment of deaths. The data were weighted to allow for the over-sampling of people over 75 years. The proportional hazards assumption was checked by testing whether the log hazard ratio was constant over time (Grambsch and Therneau 1994) and by examination of log(−ve log) survival curves and was found to be satisfactory in all analyses.
It is possible that IADL disability at baseline may have been not only an early indicator of subsequent ADL disability but also associated with poor self-reported health. Sensitivity analyses were therefore performed, excluding participants with IADL disability at baseline from the analysis of the relationship between SRH and functional impairment. Likewise, for cognitive impairment, sensitivity analyses were performed, excluding from analysis participants with an MMSE score below 21 at baseline.
It is likely that several participants developed cognitive or functional impairment after the screening interview but died or withdrew from the study before this was recorded at interviews considered in the main analysis. To evaluate the effect of these missed cases on the estimated relationship between SRH and the risk of developing cognitive or functional impairment, sensitivity analyses were performed which included data from interviews based on a sample of participants: (1) 6 years after screening in centres other than Cambridge and, for cognitive impairment only (2) assessment interviews a few months and 2 years after screening. These data were weighted to allow for the sampling strategy.
Analysis was performed using Stata version 8.
Results
Of the 13,004 participants, sixty per cent were women and ages ranged from 64 to 105 with a median of 75 years. Median follow-up time was 5.7 years (inter-quartile range 2.3–9.2 years). At the screening interview, SRH, functional and cognitive impairment were assessed for 12,623 (97%), 12,747(98%) and 12,699 (98%) participants respectively.
Distribution of SRH (see Fig. 1)
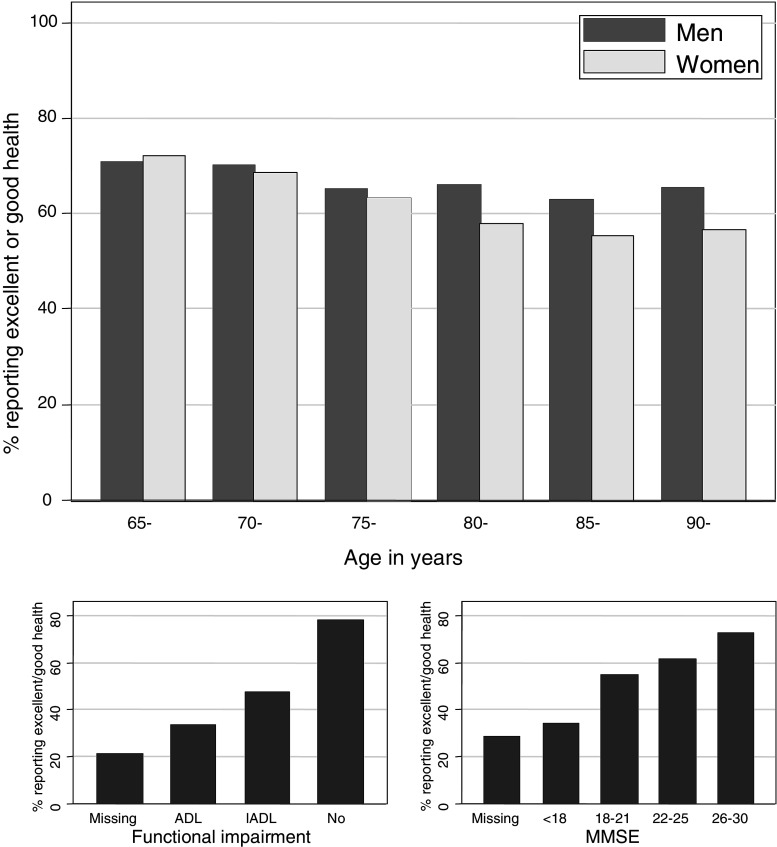
Fig. 1.
Percentage of participants reporting excellent or good self-related health in relation to baseline demographic characteristics
Overall, 19, 47, 26 and 6% of the participants rated their health as excellent, good, fair and poor respectively, compared to other people of a similar age. 3% of participants did not respond to this question; three quarters of the non-responders were over 80, over 60% were cognitively impaired at baseline and a further 30% did not have an assessment of cognitive impairment at baseline.
Figure 1 summarises how SRH varied with gender, age, functional and cognitive impairment. Men and women differed little in their perception of their health: 68% of men and 64% of women rated their health as good or excellent. There was a consistent trend of older people rating their health less highly: 70, 63 and 58% of those aged 65–74, 75–84 and 85+ rated their health as good or excellent. People who were more functionally impaired rated their health less highly: whereas about 80% of those with no functional impairment rated their health as excellent or good, only about one half and one third of those with IADL and ADL impairment respectively did so. Likewise, people who were more cognitively impaired rated their health less highly: whereas over 70% of those with MMSE between 26 and 30 rated their health as excellent or good, only about one third of those with MMSE under 18 did so. People whose functional and cognitive ability could not be assessed rated their health more poorly than others: only 21 and 28%, respectively, rated their health as good or excellent. Both functional and cognitive impairment were associated with increasing age and, within each 5-year age group, functional and cognitive impairment were associated (χ2 P < 0.0001). Poor SRH was associated with both anxiety and depression: 41% of those with depression and 36% of those with anxiety rated their health as fair or poor, compared to only 29 and 30%, respectively, without these diagnoses.
Relationship between SRH and death (see Fig. 2a; Tables 3, 4)
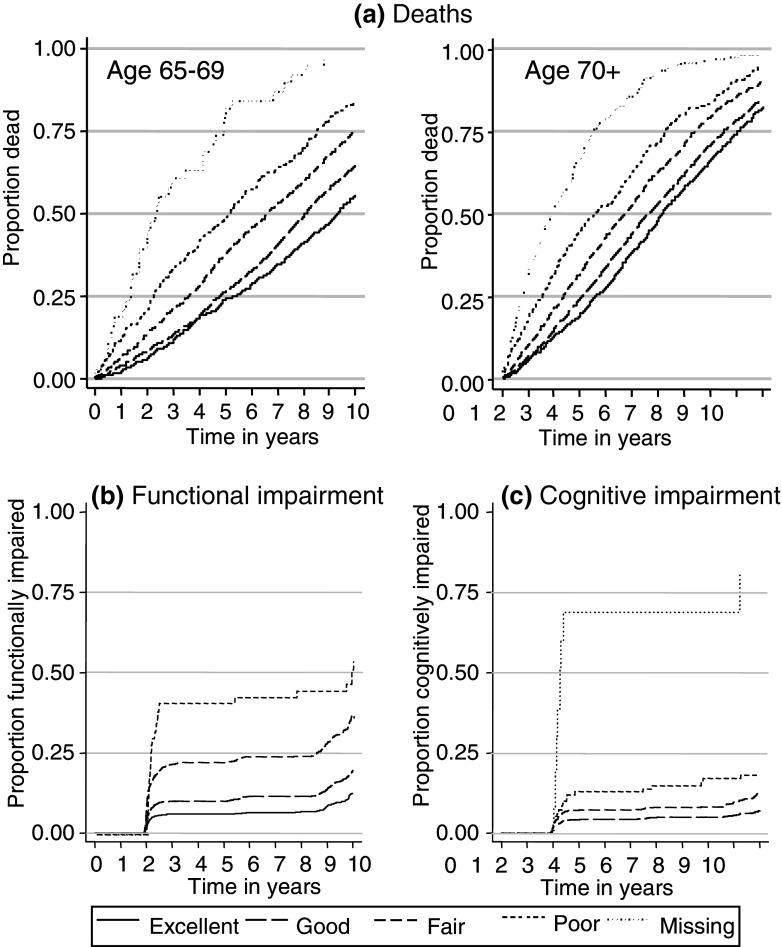
Fig. 2.
Kaplan–Meier curves showing all cause mortality and new diagnoses of functional and cognitive impairment over time since screening interview, by self-reported health status
Table 3.
Number of participants, number of deaths within 10 years of recruitment, and number of new cases by year 10 interview of functional and cognitive impairment, by strata of self-rated health
| Death | Functionally impaired at baseline | Cognitively impaired at baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Missing | No | Yes | Missing | ||||||
| No. participants | No. (%) deaths | No. participants | No. new cases | No. participants | No. participants | No. participants | No. new cases | No. participants | No. participants | ||
| Self-rated health | |||||||||||
| Excellent | 2,488 | 970 (39) | 2,354 | 165 | 122 | 12 | 2,417 | 58 | 55 | 16 | |
| Good | 6,077 | 2,865 (47) | 5,423 | 623 | 631 | 23 | 5,833 | 236 | 186 | 58 | |
| Fair | 3,335 | 2,132 (64) | 2,462 | 402 | 847 | 26 | 3,111 | 146 | 174 | 50 | |
| Poor | 723 | 552 (76) | 332 | 61 | 375 | 16 | 648 | 33 | 57 | 18 | |
| Missing | 381 | 363 (95) | 11 | 1 | 284 | 85 | 34 | 8 | 232 | 115 | |
| Total | 13,004 | 6,882 (53) | 10,582 | 1,252 | 2,259 | 163 | 12,043 | 481 | 704 | 257 | |
Percentages are not reported for functional and cognitive impairment as participants who dropped out could not be assessed on these outcomes
Table 4.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality, functional impairment and cognitive impairment in each stratum of self-reported health relative to good self-reported health, unadjusted and adjusted for baseline characteristics
| Mortality (n = 13,004) | Functional impairment | Cognitive impairment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary analysis—including all participants not functionally impaired at baseline | Sensitivity analysis—excluding participants with IADL impairment at baseline | Primary analysis—including all participants not cognitively impaired at baseline | Sensitivity analysis—excluding participants with MMSE < 21 at baseline | |||||||
| n = 8,452 | n = 6,375 | n = 8,395 | n = 7,977 | |||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Unadjusted | ||||||||||
| Excellent | 0.8 | 0.8–0.9 | 0.6 | 0.5–0.7 | 0.6 | 0.5–0.8 | 0.5 | 0.4–0.7 | 0.5 | 0.3–0.7 |
| Good | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| Fair | 1.3 | 1.2–1.4 | 2.0 | 1.8–2.3 | 1.9 | 1.6–2.3 | 1.7 | 1.4–2.1 | 1.6 | 1.3–2.2 |
| Poor | 1.6 | 1.5–1.8 | 3.9 | 2.9–5.1 | 3.8 | 2.5–5.9 | 2.7 | 1.8–3.9 | 3.1 | 2.0–4.8 |
| Missing | 4.0 | 3.6–4.4 | Only 1 new case | No new cases | 16.4 | 8.7–40.0 | 8.9 | 2.3–33.7 | ||
| Adjusted for age and gender | ||||||||||
| Excellent | 0.8 | 0.8–0.9 | 0.6 | 0.5–0.7 | 0.6 | 0.5–0.7 | 0.6 | 0.4–0.8 | 0.5 | 0.4–0.8 |
| Good | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| Fair | 1.3 | 1.3–1.4 | 2.1 | 1.8–2.4 | 1.9 | 1.6–2.2 | 1.6 | 1.3–2.0 | 1.6 | 1.2–2.1 |
| Poor | 1.8 | 1.7–2 | 4.7 | 3.5–6.1 | 4.8 | 3.2–7.0 | 2.1 | 1.4–3.1 | 2.6 | 1.7–4.2 |
| Missing | 3.1 | 2.8–3.5 | Only 1 new case | No new cases | 6.2 | 2.8–13.5 | Only 2 new cases | |||
| Adjusted for age, gender, functional ability and MMSE | ||||||||||
| Excellent | 0.8 | 0.8–0.9 | 0.6 | 0.5–0.7 | 0.6 | 0.5–0.8 | 0.7 | 0.5–0.9 | 0.7 | 0.5–0.9 |
| Good | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| Fair | 1.2 | 1.1–1.3 | 1.7 | 1.5–2.0 | 1.8 | 1.5–2.2 | 1.1 | 0.8–1.4 | 1.2 | 0.9–1.6 |
| Poor | 1.4 | 1.2–1.5 | 2.8 | 2.0–3.9 | 4.6 | 3.1–6.7 | 0.9 | 0.6–1.4 | 0.9 | 0.5–1.6 |
| Missing | 1.9 | 1.6–2.2 | Only 1 new case | No new cases | 3.8 | 2.0–7.3 | Only 2 new cases | |||
| Adjusted for all potential confounding baseline factors | ||||||||||
| Excellent | 0.9 | 0.8–0.9 | 0.6 | 0.5– 0.8 | 0.6 | 0.5–0.8 | 0.7 | 0.5–0.9 | 0.7 | 0.4–1.0 |
| Good | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| Fair | 1.1 | 1.1–1.2 | 1.6 | 1.4–1.9 | 1.7 | 1.4–2.1 | 1.1 | 0.8–1.4 | 1.2 | 0.9–1.6 |
| Poor | 1.2 | 1.1–1.4 | 2.6 | 1.9–3.6 | 4.3 | 2.9–6.6 | 1.0 | 0.6–1.5 | 0.9 | 0.5–1.5 |
| Missing | 1.0 | 0.5–1.9 | Only 1 new case | No new cases | 1.1 | 0.4–2.8 | Only 2 new cases | |||
After 10 years follow-up, 6,882 (53%) of the participants had died (see Table 3). There was a consistent trend that people who rated their health more highly were less likely to die. Compared with those who rated their health as good, the unadjusted hazard ratios for mortality among those who rated their health as excellent, fair and poor were 0.8 (95% CI 0.7–0.8), 1.6 (95% CI 1.5–1.7) and 2.3 (95% CI 2.1–2.5), respectively; the hazard ratio among those who did not respond to this question was much higher: 6.4 (95% CI 5.7–7.1).
The relationship between SRH and death was little changed after allowing for age and gender. The relationship was partly explained by confounding with functional and cognitive status: those with better functional and cognitive ability were less likely to die and also tended to report better health. The higher risk of death in participants who did not respond to the SRH question was largely explained by their age, functional and cognitive impairment. However, a significant relationship between SRH and mortality remained after multivariable adjustment for age, gender, functional impairment and MMSE score: the hazard ratios comparing those who rated their health as excellent, fair and poor with those who rated it as good were 0.8 (95% CI 0.8–0.9), 1.4 (95% CI 1.3–1.5) and 1.7 (95% CI 1.5–1.8), respectively; the adjusted hazard ratio among those who did not respond to this question was 1.8 (95% CI 1.6–2.2).
Non-smokers and participants who were married, had more years of education, a higher social class, lived in less deprived areas, did not take medication, did not suffer from anxiety or depression, and did not have a history of cardiovascular disease or diabetes were less likely to die than others. These factors had little effect—either separately or jointly—on the relationship between SRH and mortality.
Relationship between SRH and functional impairment (see Fig. 2b; Tables 3, 4)
At the screening interview 2,259 participants had functional impairment and a further 163 were not assessed (see Table 3). Of the remaining 10,582 participants, 7,473 were assessed for functional impairment at follow-up interviews which included all participants and 1,252 (17%) new cases were diagnosed: analysis was restricted to these participants.
People with poorer SRH were more likely to develop functional impairment. Compared with those who rated their health as good, the unadjusted hazard ratios for developing functional impairment among those who rated their health as excellent, and fair and poor were 0.6 (95% CI 0.5–0.7), 2.0 (95% CI 1.8–2.3) and 3.9 (95% CI 2.9–5.1), respectively.
Adjustment for age and gender had little effect on the relationship between SRH and risk of functional impairment. As for cognitive impairment, unmarried participants and those who had fewer years of education, lived in more deprived areas, had never smoked, used medication or reported a history of angina, heart attack, stroke or diabetes were more likely to develop functional impairment than others. However, adjustment for these factors—either separately or jointly—had little effect on the relationship between SRH and development of functional impairment. After multivariable adjustment for age, gender and baseline IADL and MMSE, the relationship between SRH and risk of functional impairment was attenuated but remained statistically significant.
Sensitivity analyses, excluding participants with IADL disability at baseline, yielded similar results (see Table 4).
Further sensitivity analysis was performed, including the sample of participants who were assessed 6 years after screening. This analysis allowed follow-up of 33 additional participants (interviewed at 6 years but not at 2 or 10 years or the 6-year assessment by the Cambridge centre) and identified 139 additional cases of functional impairment in participants who died or were lost to follow-up before their functional impairment was recorded at one of the interviews which included all participants. Using these data, the relationship between SRH and development of functional impairment was similar to that reported above.
Relationship between SRH and cognitive impairment (see Fig. 2c; Tables 3, 4)
At the screening interview 704 participants had cognitive impairment and a further 257 were not assessed (see Table 3). Of the remaining 12,043 participants, 8,395 were assessed for cognitive impairment at follow-up interviews which included all participants and 481 (6%) new cases were diagnosed: analysis was restricted to these participants.
There was a strong and consistent trend that people with better SRH were less likely to develop cognitive impairment. Compared with those who rated their health as good, the unadjusted hazard ratios for developing cognitive impairment among those who rated their health as excellent, fair and poor were 0.5 (95% CI 0.4–0.7), 1.7 (95% CI 1.4–2.1) and 2.7 (95% CI 1.8–3.9), respectively. The trend was more marked in the younger age group. Only 34 of the participants who were not cognitively impaired at baseline failed to respond to the SRH question; among these participants the unadjusted hazard ratio for developing cognitive impairment was 16 (95% CI 9–40).
Adjustment for age and gender slightly weakened the relationship between SRH and risk of cognitive impairment. Unmarried participants, those who had fewer years of education, lived in more deprived areas, had never smoked, used medication or reported a history of stroke or diabetes were more likely to develop cognitive impairment than others. However, adjustment for these factors—either separately or jointly—had little effect on the relationship between SRH and cognitive impairment. Those who had difficulty with ADL or IADL or lower MMSE scores tended to rate their health less highly and also to be at higher risk of cognitive impairment. Hence additional multivariable adjustment for functional impairment and MMSE partly—but not completely—explained the relationship between SRH and risk of cognitive impairment. Even after adjusting for all potential confounding baseline factors, those who rated their health as excellent had a lower risk of cognitive impairment than those who rated it as good: hazard ratio comparing the risk of cognitive impairment in these groups = 0.7, 95% CI 0.5–0.9. These factors also explained much, but not all, of the increased risk of cognitive impairment among the small group of participants who did not respond to the SRH question; the remaining excess risk was explained by cardiovascular risk factors.
Sensitivity analyses, excluding participants with MMSE score under 21 at baseline, yielded similar results (see Table 4).
Further sensitivity analysis was performed, including the samples of participants who were assessed at 1–6 months, 2 and 6 years after screening. This analysis allowed follow-up of 714 additional participants (interviewed at one or more of these assessments, but neither at 10 years nor at the 6-year assessment by the Cambridge centre) and identified 251 additional cases of cognitive impairment in participants who died or were lost to follow-up before their cognitive impairment was recorded at one of the interviews which included all participants. Using these data, the relationship between SRH and development of cognitive impairment was similar to that reported above.
Discussion
These analyses further confirm that SRH is a good predictor of mortality and functional impairment for up to 10 years after assessment. They also report that SRH is also a good predictor of cognitive impairment for up to 10 years after assessment. The marked trend of higher risk of death with lower SRH remained after adjustment for known prognostic indicators. In participants who rated their health as fair or poor, the associations between SRH and functional and cognitive impairment were weakened by adjustment for cognitive and functional status at baseline. This is not surprising, as these outcomes were defined as the lowest categories of the corresponding baseline measures. The findings indicate that SRH has prognostic capacity for functional and cognitive decline over and above the prognostic capacity of baseline status.
There are at least four possible interpretations of these findings. First, they may be an artefact of the study and the way that SRH was assessed. Second, SRH may be a proxy for a range factors not already included in the study which, if identified, might need a large battery of items or questions to assess with greater precision and accuracy. Third, SRH may be a distinct entity that is associated with outcome. Fourth, SRH may be partly explained by known prognostic indicators but add something over and above these known factors.
On balance the strengths of CFAS militate against the first explanation. CFAS is a well designed large population study of a prospective cohort of older people aged 65 or over at baseline. The findings of these analyses are consistent with earlier studies (Atchley and Scala 1998; Baron-Epel et al. 2004; Dening et al. 1998; Han et al. 2005; Hillen et al. 2003; Idler et al. 1990; Idler and Benyamini 1997; Kaplan et al. 1988; Long and Marshall 1999; Mossey and Shapiro 1982; Nybo et al. 2003; Siegel et al. 2003; Spiers et al. 1996; Wolinsky and Tierney 1998). They are based on a much larger sample than the majority of SRH studies and with longer follow up than most other longitudinal studies. There are limitations of the analyses. All SRH studies are recognised as having important bias due to non-response by a substantial proportion of the population due to incapacity or illness (Hoeymans et al. 1998). In this study some frail participants with cognitive or functional impairment did not respond to the question about SRH and were categorised separately (Matthews et al. 2004). Analysis by Cox regression allowed for the substantial loss to follow-up of participants but had the disadvantage that the date of diagnosis of functional and cognitive impairment was assumed to be the date when the participant was detected at a follow-up interview as having the condition. This probably introduces some error into the modelling, but is likely to obscure significant associations rather than introduce spurious findings. Sensitivity analysis, which used information from interviews between the major waves to provide more precise information about when functional and cognitive impairment were detected, yielded similar findings to the main analysis. Further, people of lower social class, education, cognitive ability, in residential care, with sight/hearing problems and poor/fair self-reported health were less likely to be seen after 10 years of follow-up (Matthews et al. 2006). This differential attrition probably led to under-estimation of the association between poor SRH and the various outcomes. Change in functional or cognitive status was missed for people who died between interviews, although a sensitivity analysis suggests that this had limited influence. Measures of functional and cognitive impairment are known to be of poor reliability at the individual level. However when averaged over a large population they are relatively robust. Functional and cognitive impairment are not independent since cognitive impairment as well as physical incapacity influences an individual’s ability to carry out activities of daily living. Although SRH variables are not insensitive to question wording (Manderbacka et al. 2003) the approach chosen in this study (in 1989) does not appear to have had a significant effect on these analyses given the confirmation of the known relationship between SRH and both mortality and functional impairment.
The second explanation also seems unlikely. There is remarkable consistency across studies in the variety of life course or lifestyle factors identified as influencing mortality, functional and cognitive decline. We considered a wide range of these potential risk factors and the associations were partly, but not completely, explained by adjustment for those identified as confounders. Few studies have included a full suite of biopsychosocial variables but there are no clear theoretical reasons for assuming that SRH would be a proxy for them. A greater understanding of the factors that affect SRH, however, would help clarify its role as a proxy variable.
The third explanation is more likely since it is consistent with different meanings of health captured in sociological and psychological research and the increasing understanding of the ‘disability paradox’ (Albrecht and Devlieger 1999) which highlights the apparent discrepancy between reports of personal well being and health status or levels of disability (Blaxter 1990; Blaxter and Paterson 1982; Charmaz 2000; Herzlich 1973; Herzlich and Pierret 1987; Stainton Rogers 1991; Williams 1983). However, the fourth explanation would appear to be most plausible: that SRH partly reflects other known prognostic indicators but also partly captures the unique way that individuals reflect on their own health. It may also be capturing milder levels of disability than those recorded by known prognostic markers (Spiers et al. 1996). SRH therefore explains some of the variability in health outcomes not explained by more objective prognostic indicators.
As a measure of health, SRH is not accepted as an objective measure or biomarker of disease. It is accepted as a proxy for objective health status in the absence of objective measures but domain specific measures are preferred (Kempen et al. 1998). Although SRH remains one of the simplest and robust questions to be included in health surveys there still remain a number of issues to resolve. First there remains some uncertainty as to whether global SRH or comparative SRH should be assessed. Survey item-response theory would suggest that global SRH and comparative SRH would elicit different responses (Schwarz 1998), but it has been suggested that SRH is too insensitive to semantic variations and that comparisons with one’s peers is implicit in self ratings of health (Idler et al. 1990; Idler and Benyamini 1997). However, when different question wordings for SRH were compared within a single study they were found not to be entirely comparable, exhibiting significant variation between age and educational groups (Kaplan and Baron-Epel 2003). Where global SRH and comparative SRH have been used in the same study global SRH was found to be a better predictor of mortality than an age-referential comparative SRH (Vuorisalmi et al. 2005). A meta analysis suggests that comparative SRH shows higher optimism among those aged 75 or over than those aged 65–74 but when global SRH is used the trend is reversed (Roberts 1999), suggesting that both should be included in future studies of the older population. Further studies like those of Jylhä (1994) and Manderbacka (1998) using cognitive interviewing techniques (Willis 2005) are needed to explain further what survey respondents understand by questions and mean by their answers when responding to questions about SRH.
A second issue concerns the influences on SRH. Whereas SRH is widely used as a covariate in longitudinal analyses it is less often seen to be an outcome in its own right. This is partly because we are not really clear about whether SRH is simply tapping an individual’s attitude toward their health, rather than being a more specific characteristic of health status and it is also partly because of concern about the simplicity of the single survey item that does not have the appropriate psychometric properties of more complex health status measures in the form of an attitude scale. It is also partly because of our lack of knowledge about the assessments’ stability in assessing change and how it responds to ‘response shift’ (Schwartz and Sprangers 2000). But an assessment of SRH as a dependent outcome variable and the factors that they predict SRH would have enormous practical, as well as theoretical value. If it could be shown that SRH performs robustly as an outcome variable then clinical trialists might be able to dispense with the use of some of the more complex health status and quality of life measures.
A third issue concerns the stability of the variable representing SRH across populations and particularly different generational cohorts. Do older people of the same age from different generational cohorts respond differently given the same levels of health and disability?
Finally what SRH means to individual older people remains a fundamental empirical question and further understanding about how older people perceive their own health would help clarify how SRH might be interpreted as either a covariate or as an outcome variable. Secondary analysis is required to explore information that older people bring to the rating of their health and whether these factors are weighted differently within subgroups of the population.
The new finding provided by these analyses is that SRH is not only a prognostic factor of mortality and functional impairment but also dementia and cognitive decline. What are the plausible and possible explanations for such a relationship? There are at least three, none of which are mutually exclusive. First it may be that older people recognise cognitive decline before it is readily recognisable to others and this is reflected in their rating of their own health. This explanation is supportive of the view that as individuals we understand our own bodies and are aware of changes within the context of our daily lives and life course, that others may not be able to readily observe. Second it may be that positive expectations about one’s own health contribute to more positive health outcomes while negative expectations are a form of self-fulfilling prophecy. Third and associated with both the first and second explanations is the long established idea that the level of control that we have over our daily lives and life course influences our health outcomes. There is limited evidence that such psychosocial factors do underpin the significance of SRH as a prognostic factor (Mackenbach et al. 2002; Menec et al. 1999). Therefore to have a fuller understanding of why SRH is a useful prognostic indicator of future cognitive function, further work is needed building on existing social and behavioural science understandings of self perceptions of health (and ageing).
Conclusion
Notwithstanding its limitations, an assessment of SRH remains one of the simplest and robust questions to be included in health surveys and in combination with other covariates provides additional prognostic capacity. This study confirms the prognostic capacity of SRH for death and onset of functional incapacity and provides new evidence that SRH is a predictor of cognitive impairment. Further research is required to understand factors that influence SRH; how SRH changes over time; how SRH differs between generational cohorts; how older people perceive their health and what they understand by SRH; and the relative value of assessing global SRH or comparative SRH.
Acknowledgments
The MRC CFA Study is supported by major awards from the UK Medical Research Council and the UK Department of Health.
References
- Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all the odds. Soc Sci Med. 1999;48:977–988. doi: 10.1016/S0277-9536(98)00411-0. [DOI] [PubMed] [Google Scholar]
- Atchley RC, Scala MA. Long-range antecedents of functional capability in later life. J Aging Health. 1998;10:3–19. doi: 10.1177/089826439801000101. [DOI] [PubMed] [Google Scholar]
- Baron-Epel O, Shemy G, Carmel S. Prediction of survival: a comparison between two subjective health measures in an elderly population. Soc Sci Med. 2004;58:2035–2043. doi: 10.1016/S0277-9536(03)00412-X. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in the self-rated health-mortality association: is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? Gerontologist. 2003;43:396–405. doi: 10.1093/geront/43.3.396. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Leventhal H, Leventhal EA. Self-rated oral health as an independent predictor of self-rated general health, self-esteem and life satisfaction. Soc Sci Med. 2004;59:1109–1116. doi: 10.1016/j.socscimed.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Blaxter M. Health and lifestyles. London: Routledge; 1990. [Google Scholar]
- Blaxter M, Paterson E. Mothers and daughters: a three generational study of health attitudes and behaviour. London: Heinemann Educational Books; 1982. [Google Scholar]
- Bowling A. Techniques of questionnaire design. In: Bowling A, Ebrahim S, editors. Handbook of health research methods: investigation, measurement and analysis. Maidenhead: Open University Press; 2005. pp. 394–427. [Google Scholar]
- van Broese Groenou MI, Deeg DJ, Penninx BW. Income differentials in functional disability in old age: relative risks of onset, recovery, decline, attrition and mortality. Aging Clin Exp Res. 2003;15:174–183. doi: 10.1007/BF03324497. [DOI] [PubMed] [Google Scholar]
- Charmaz K. Experiencing chronic illness. In: Albrecht GL, Fitzpatrick R, Scrimshaw SC, editors. Handbook of social studies in health and medicine. London: Sage; 2000. pp. 277–292. [Google Scholar]
- Dasbach EJ, Klein R, Klein BEK, Moss SE. Self-rated health and mortality in people with diabetes. Am J Public Health. 1994;84:1777–1779. doi: 10.2105/ajph.84.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg DJH, Kriegsman DMW. Concepts of self-rated health: specifying the gender difference in mortality risk. Gerontologist. 2003;43:376–386. doi: 10.1093/geront/43.3.376. [DOI] [PubMed] [Google Scholar]
- Dening TR, Chi L-Y, Brayne C, Huppert FA, Paykel ES, O’Connor DW. Changes in self-rated health, disability and contact with services in a very elderly cohort: a 6-year follow-up study. Age Ageing. 1998;27:23–33. doi: 10.1093/ageing/27.1.23. [DOI] [PubMed] [Google Scholar]
- Fenton S, Hughes AO, Hine CE. Self assessed health, economic status and ethnic origin. New Commun. 1995;21:55–68. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.2307/2337123. [DOI] [Google Scholar]
- Han B, Phillips C, Ferrucci L, Bandeen-Roche K, Jylha M, Kasper J, Guralnik JM. Change in self-rated health and mortality among community-dwelling disabled older women. Gerontologist. 2005;45:216–221. doi: 10.1093/geront/45.2.216. [DOI] [PubMed] [Google Scholar]
- Han H (2000) Depressive symptoms and self-rated health in community-dwelling older adults: a longitudinal study. Dissertation Abstracts International: Section B: The Sciences and Engineering 61:1863
- Helmer C, Barberger-Gateau P, Letenneur L, Dartigues JF. Subjective health and mortality in French elderly women and men. J Gerontol. 1999;54:S84–S92. doi: 10.1093/geronb/54b.2.s84. [DOI] [PubMed] [Google Scholar]
- Helweg-Larsen M, Kjoller M, Thoning H. Do age and social relations moderate the relationship between self-rated health and mortality among adult Danes? Soc Sci Med. 2003;57:1237–1247. doi: 10.1016/S0277-9536(02)00504-X. [DOI] [PubMed] [Google Scholar]
- Herzlich C. Health and illness. London: Academic; 1973. [Google Scholar]
- Herzlich C, Pierret J. Illness and self in society. Baltimore: Johns Hopkins; 1987. [Google Scholar]
- Hillen T, Davies S, Rudd AG, Kieselbach T, Wolfe CD. Self ratings of health predict functional outcome and recurrence free survival after stroke. J Epidemiol Comm Health. 2003;57:960–966. doi: 10.1136/jech.57.12.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeymans N, Feskens EJM, van den Bos GAM, Kromhout D. Non-response bias in a study of cardiovascular diseases, functional status and self-rated health among elderly men. Age Ageing. 1998;27:35–40. doi: 10.1093/ageing/27.1.35. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. doi: 10.2307/2955359. [DOI] [PubMed] [Google Scholar]
- Idler EL, Russell LB, Davis D. Survival, functional limitations, and self-rated health in the NHANES I Epidemiologic Follow-up Study, 1992. Am J Epidemiol. 2000;152:874–883. doi: 10.1093/aje/152.9.874. [DOI] [PubMed] [Google Scholar]
- Idler EL, Stanislav VK, Lemke JH. Self-evaluated health and mortality among the elderly in New Haven, Connecticut, and Iowa and Washington Counties, Iowa, 1982–1986. Am J Epidemiol. 1990;131:91–103. doi: 10.1093/oxfordjournals.aje.a115489. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Kai I, Imanaka Y. Self-rated health and social role as predictors for 6-year total mortality among a non-disabled older Japanese population. Arch Gerontol Geriatrics. 2006;42:91–99. doi: 10.1016/j.archger.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Jylhä M. Self-rated health revisited: exploring survey interview episodes with elderly respondents. Soc Sci Med. 1994;39:983–990. doi: 10.1016/0277-9536(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Jylhä M, Guralnik JM, Ferrucci L, Jokela J, Heikkinen E. Is self-rated health comparable across cultures and genders? J Gerontol. 1998;53B:S144–S152. doi: 10.1093/geronb/53b.3.s144. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Barell V, Lusky A. Subjective state of health and survival in elderly adults. J Gerontol. 1988;43:S114–S120. doi: 10.1093/geronj/43.4.s114. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Baron-Epel O. What lies behind the subjective evaluation of health status? Soc Sci Med. 2003;56:1669–1676. doi: 10.1016/S0277-9536(02)00179-X. [DOI] [PubMed] [Google Scholar]
- Kempen GIJM, Miedema I, van den Bos GAM, Ormel J. Relationship of domain-specific measures of health to perceived overall health among older subjects. J Clin Epidemiol. 1998;51:11–18. doi: 10.1016/S0895-4356(97)00234-5. [DOI] [PubMed] [Google Scholar]
- Kim SH, Wolde-Tsadik G, Reuben DB. Predictors of perceived health in hospitalized older persons: a cross-sectional and longitudinal study. J Am Geriatr Soc. 1997;45:420–426. doi: 10.1111/j.1532-5415.1997.tb05165.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Krause NM, Bennett JM, Blaum C, Shaw BA, Kobayashi E, Fukaya T, Sugihara Y, Sugisawa H. Changes in Functional Status among Older Adults in Japan: successful and usual aging. Psychol Aging. 2003;18:684–695. doi: 10.1037/0882-7974.18.4.684. [DOI] [PubMed] [Google Scholar]
- Lichtman JH (1997) Are depressive symptoms, social networks and social support, or self-evaluated health measures predictive of cancer incidence and stage at diagnosis? evidence from a prospective community-based sample of elderly New Haven residents. Dissertation Abstracts International: Section B: The Sciences and Engineering 57:6876
- Long MJ, Marshall BS. The relationship between self-assessed health status, mortality, service use, and cost in a managed care setting. Health Care Manage Rev. 1999;24:20–27. [PubMed] [Google Scholar]
- Mackenbach JP, Simon JG, Looman CW, Joung IM. Self-assessed health and mortality: could psychosocial factors explain the association? Int J Epidemiol. 2002;31:1162–1168. doi: 10.1093/ije/31.6.1162. [DOI] [PubMed] [Google Scholar]
- Manderbacka K. Examining what self-rated health question is understood to mean by respondents. Scand J Soc Med. 1998;26:145–153. doi: 10.1177/14034948980260020301. [DOI] [PubMed] [Google Scholar]
- Manderbacka K, Kareholt I, Martikainen P, Lundberg O. The effect of point of reference on the association between self-rated health and mortality. Soc Sci Med. 2003;56:1447–1452. doi: 10.1016/S0277-9536(02)00141-7. [DOI] [PubMed] [Google Scholar]
- Matthews FE, Chatfield M, Brayne C, Medical Research Council Cognitive Function and Ageing Study (2006) An investigation of whether factors associated with short-term attrition change or persist over ten years: data from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). BMC Public Health 6:185 [DOI] [PMC free article] [PubMed]
- Matthews FE, Chatfield M, Freeman C, McCracken C, Brayne C, MRC-CFAS (2004) Attrition and bias in the MRC cognitive function and ageing study: an epidemiological investigation. BMC Public Health 4:(http://www.biomedcentral.com/1471–2458/4/12) [DOI] [PMC free article] [PubMed]
- McGee DL, Liao Y, Cao G, Cooper RS. Self-reported health status and mortality in a multiethnic US cohort. Am J Epidemiol. 1999;149:41–46. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Cognitive Function, Ageing Study Writing Committee Cognition and survival: an exploration in a large multicentre study of the population aged 65 years and over. Int J Epidemiol. 2001;30:1383–1388. doi: 10.1093/ije/30.6.1383. [DOI] [PubMed] [Google Scholar]
- Menec VH, Chipperfield JG, Perry RP. Self-perceptions of health: a prospective analysis of mortality, control, and health. J Gerontol. 1999;54:P85–P93. doi: 10.1159/000006615. [DOI] [PubMed] [Google Scholar]
- Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72:800–808. doi: 10.2105/AJPH.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC CFAS Medical Research Council Cognitive Function, Ageing Study Group MRC CFAS Medical Research Council Cognitive Function and Ageing Study Group: cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA study. Psychol Med. 1998;28:319–335. doi: 10.1017/s0033291797006272. [DOI] [PubMed] [Google Scholar]
- MRC Cognitive Function, Ageing Study, Yip AG, Brayne C, Matthews FE (2006) Risk factors for incident dementia in England and Wales: The Medical Research Council Cognitive Function and Ageing Study. A population-based nested case-control study. Age Ageing 35:154–160 [DOI] [PubMed]
- Nybo H, Petersen HC, Gaist D, Jeune B, Andersen K, McGue M, Vaupel JW, Christensen K. Predictors of mortality in 2,249 nonagenarians - The Danish 1905-cohort survey. J Am Geriatr Soc. 2003;51:1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- OPCS (1990) Standard occupational classification, vol 1. HMSO, London
- Roberts G. Age effects and health appraisal: a meta analysis. J Gerontol. 1999;54B:S24–S30. doi: 10.1093/geronb/54b.1.s24. [DOI] [PubMed] [Google Scholar]
- Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Sprangers MAG (Eds) (2000) Adaptation to changing health: response shift in quality-of-life research. American Psychological Association, Washington
- Schwarz N, et al. Self-reports of behaviors and opinions: cognitive and communicative processes. In: Schwarz N, et al., editors. Cognition, aging, and self-reports. Philadelphia: Psychology Press; 1998. pp. 17–43. [Google Scholar]
- Siegel M, Bradley EH, Kasl SV. Self-rated life expectancy as a predictor of mortality: evidence from the HRS and AHEAD surveys. Gerontology. 2003;49:265–271. doi: 10.1159/000070409. [DOI] [PubMed] [Google Scholar]
- Simon R, Altman DG. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994;69:979–985. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers NA, Jagger C, Clarke M. Physical function and perceived health: cohort differences and interrelationships in older people. J Gerontol. 1996;51:S226–S233. doi: 10.1093/geronb/51b.5.s226. [DOI] [PubMed] [Google Scholar]
- Spiers NA, Matthews RJ, Jagger C, Matthews FE, Boult C, Robinson TG, et al. Diseases and impairment as risk factors for onset of disability in the older population in England and Wales: findings from the Medical Research Council Cognitive Function and Ageing Study. J Gerontol. 2005;60:248–254. doi: 10.1093/gerona/60.2.248. [DOI] [PubMed] [Google Scholar]
- Stainton Rogers W. Explaining health and illness. Hertfordshire: Harvester Wheatsheaf; 1991. [Google Scholar]
- Townsend P, Phillimore P, Beattie A. Health and deprivation. inequality and the North. London: Croom Helm; 1988. [Google Scholar]
- Vuorisalmi M, Lintonen T, Jylha M. Global self-rated health data from a longitudinal study predicted mortality better than comparative self-rated health in old age. J Clin Epidemiol. 2005;58:680–687. doi: 10.1016/j.jclinepi.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Williams RGA. Concepts of health: an analysis of lay logic. Sociol. 1983;17:185–205. doi: 10.1177/0038038583017002003. [DOI] [Google Scholar]
- Williams SA (2000) Psychosocial factors and risk of heart failure incidence among elderly: New Haven Epese, 1982–1996 (Connecticut). Dissertation Abstracts International: Section B: The Sciences and Engineering 61:2497
- Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks: Sage; 2005. [Google Scholar]
- Wolinsky FD, Tierney WM. Self-rated health and adverse health outcomes: an exploration and refinement of the trajectory hypothesis. J Gerontol. 1998;53B:S336–S340. doi: 10.1093/geronb/53b.6.s336. [DOI] [PubMed] [Google Scholar]