Abstract
Background and purpose
This study examines attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy (PBRT).
Material and methods
We examined 39 survivors (age 6–19 years) who were 3.61 years post-PBRT on average. Craniospinal (CSI; n = 21) and focal (n = 18) subgroups were analyzed. Attention, processing speed, and executive functioning scores were compared to population norms, and clinical/demographic risk factors were examined.
Results
As a group, survivors treated with focal PBRT exhibited attention, processing speed, and executive functioning that did not differ from population norms (all p > 0.05). Performance in the CSI group across attention scales was normative (all p > 0.05), but areas of relative weakness were identified on one executive functioning subtest and several processing speed subtests (all p < 0.01).
Conclusions
Survivors treated with PBRT may exhibit relative resilience in cognitive domains traditionally associated with radiation late effects. Attention, processing speed, and executive functioning remained intact and within normal limits for survivors treated with focal PBRT. Among survivors treated with CSI, a score pattern emerged that was suggestive of difficulties in underlying component skills (i.e., processing speed) rather than true executive dysfunction. No evidence of profound cognitive impairment was found in either group.
Keywords: Cognitive late effects, Pediatric brain tumor, Proton radiation therapy, Executive functioning, Processing speed
Neurocognitive late effects are commonly reported in long-term survivors of pediatric brain tumors. Domains of particular vulnerability to CNS-directed treatment include attention [1,2], processing speed [3–5], and executive functions, including working memory [2], task efficiency [6], inhibitory control [7], and organization [6]. Understanding the range of attention and executive function outcomes expected following treatment for pediatric brain tumors is important, as impairments in these neurocognitive domains are associated with lasting difficulties in social functioning, educational attainment, employment, and reduced income potential [6,8].
While cranial radiation therapy (RT) is often an essential treatment for pediatric brain tumors, it is associated with neurocognitive impairment [9], with higher radiation doses linked to greater cognitive decline [10,11]. In an effort to mitigate treatment late effects, proton beam radiation therapy (PBRT) is being used more frequently in the treatment of pediatric brain tumors. Compared to conventional photon RT (XRT), PBRT eliminates exit dose, thus minimizing irradiation of healthy brain tissue [12]. As such, there is optimism that PBRT will better preserve cognitive functioning in pediatric brain tumor patients while providing equivalent tumor control. Still, research examining neurocognitive outcomes in patients treated with PBRT is sparse given its historically limited availability.
Thus far, empirical support for the neuroprotective benefits of PBRT is not definitive. In a large retrospective comparison of survivors of pediatric brain tumors, we found no statistically significant IQ decline or impairment in survivors treated with PBRT (n = 90; mean (M) follow-up = 2.7 years), while survivors treated with XRT showed statistically significant IQ decline (n = 60; M follow-up = 5.4 years); yet, the IQ trajectories (i.e., the slope or the amount of IQ change over time) did not differ between groups [13]. Another recent study examining intellectual ability in 43 pediatric survivors of medulloblastoma treated with PBRT-based craniospinal irradiation (CSI; M follow-up = 5.2 years) found significant decline in processing speed and verbal comprehension, with global IQ decline observed only in patients younger than 8 years old at treatment [14]. Finally, Pulsifer et al. [15] identified significant decline in processing speed, but not in global IQ, in a sample of survivors of pediatric brain tumors treated with PBRT (n = 60; M follow-up = 2.5 years). Together, these early studies seem to show that patients treated with PBRT, as a group, are not exhibiting profound cognitive changes in early survivorship, although areas of cognitive vulnerability (e.g., processing speed) and clinical risk factors (e.g., younger age at treatment) are emerging.
In sum, uncertainty lingers with respect to the neurocognitive impact (and potential neuroprotective benefits) of PBRT, and the few available studies to date have focused on changes in global intelligence or broad intellectual domains. The aim of the current study was to expand our knowledge of the impact of PBRT by examining measures of attention, processing speed, and executive functioning in survivors of pediatric brain tumors treated with PBRT. Since attention, processing, and executive functions are cognitive domains known to be at particular risk following treatment with XRT, we hypothesized that survivors treated with PBRT would underperform in these cognitive domains relative to population norms.
Materials and methods
Procedure and participants
Data presented here are part of a larger ongoing study examining long-term outcomes in pediatric brain tumor survivors. With approval from the Institutional Review Board, eligible study participants were identified by medical chart review and consecutively enrolled. Informed written consent was obtained prior to participation.
Eligible survivors were between the ages of 6 and 19 years with a history of a brain tumor treated with PBRT at least one year prior to enrollment and with no evidence of active disease. Patients diagnosed with brain stem gliomas, high-grade gliomas, and atypical teratoid/rhabdoid tumors were excluded from participation due to our interest in long-term outcomes. The present study reports on the 39 survivors who completed the neuropsychological measures of interest at a single time point between 2011 and 2014 (88.9% participation rate). Two patients were excluded from analysis due to disease progression identified following enrollment.
Measures
Conners’ Continuous Performance Test-II (CPT-II)
The CPT-II [16] is a computerized assessment of sustained attention. Seven performance indicators were included in the present study: Omission Errors, Commission Errors, Mean Reaction Time, Reaction Time Standard Error, Variability, Attentiveness, and Perseverations. Age-norm derived T-scores (M =50, SD = 10) are reported. Higher scores indicate worse performance, with scores >65 considered to fall in the clinically significant range. On two scales, however, atypically low scores are also interpreted as problematic: Mean Reaction Time (low score = impulsive responding, high score = slow responding) and Perseverations (low score = slow responding, high score = impulsive or random responding). T-scores < 35 on these two scales were also categorized as clinically significant.
Delis–Kaplan Executive Function System (D-KEFS)
The D-KEFS [17] is a collection of standardized tests of executive functions using a cognitive process measurement approach. Three timed D-KEFS tests were included in the present study: Color-Word Interference, Trail Making Test, and Verbal Fluency. Each test includes several subtests. Some subtests measure higher level executive functioning while others measure processing speed, a lower level cognitive skill. Taken together, these scores provide a better understanding of whether weak performance on an executive functioning subtest is due to true executive dysfunction or due to a deficit in processing speed that also contributes to performance on the task. Age-normed scores are reported as scaled scores (M = 10, SD = 3), with lower scores indicating worse performance. Scaled scores <5.5 (or 1.5 SD below the normative mean) were considered clinically significant in the current study. Of note, since D-KEFS tests are normed for ages 8–89 years, the one patient in the sample who was less than 8 years old at testing was excluded from analyses of D-KEFS scores.
The D-KEFS Color-Word Interference test includes four timed subtests. Two subtests assess processing speed: Color Naming (speed of naming colors) and Word Reading (speed of reading words). Higher level executive functioning is assessed on two subtests: Inhibition (speed of naming the ink color while not reading the printed word), and Inhibition/Switching (speed of naming the ink color or reading the printed word depending on a rule). We also examined the Inhibition/Switching Errors score, which assesses the accuracy of performance on the executive functioning task.
We included three timed subtests from the D-KEFS Trail Making Test. Two subtests assess processing speed: Number Sequencing (speed of drawing a line to connect numbers in order) and Letter Sequencing (speed of drawing a line to connect letters in order). Higher level executive functioning is assessed on one subtest: Number-Letter Switching (speed of drawing a line to alternately connect numbers and letters in order). We also examined the Number-Letter Switching Errors score, which assesses accuracy of performance on the Number-Letter Switching executive functioning task.
The D-KEFS Verbal Fluency Test includes four timed subtests. Two subtests assess processing speed: Letter Fluency (the number of words produced beginning with a specified letter) and Category Fluency (the number of words produced from specified categories). Two subtests assess higher level executive functioning: Category Switching (the number of words produced that belong to alternating categories) and Category Switching Accuracy (the number of successful category switches). We also examined the Percent Switching Accuracy score, which assesses error frequency on the executive functioning task.
Beery-Buktenica Developmental Test of Visual-Motor Integration (VMI) – Sixth Edition
The VMI [18] examines visual-motor integration. Two subtests were included to explore the possible contribution of visual (Visual Perception) and/or fine motor functioning (Motor Coordination) on D-KEFS Trail Making Test performance. Age-normed scores are reported as standard scores (M = 100, SD = 15), with lower scores indicative of worse performance. We defined clinical scores on this measure as those <77.5 (or 1.5 SD below the normative mean).
Analyses
CSI and Focal groups were compared on demographic and medical characteristics using t-tests and Χ2 tests. These groups were analyzed separately for all remaining analyses. One-sample ttests compared normative means (i.e., the means from the tests’ standardization samples) to survivor group means across measures collected at a single time point for each participant. When survivor means differed significantly from normative means, we examined the distribution of survivors with clinically significant scores, which we defined as scores falling more than 1.5 standard deviations from the normative mean in the direction of impairment for each subtest. Additionally, given the visual scanning and fine motor demands of the D-KEFS Trail Making Test, we used Pearson’s correlations to explore whether visual perception and motor coordination skills, as assessed using the VMI, were significantly associated with performance on the Trail Making Test subtests. Finally, we conducted t-tests and analyses of variance (ANOVAs) to examine associations between test scores and demographic and medical variables, including sex, tumor location (infratentorial/supratentorial), tumor diameter, history of craniotomy, history of shunt, total RT dose, age at testing, age at RT, and time from RT. When two medical/demographic variables were found to be significantly associated with a given subtest, we also examined associations between those two medical/demographic variables. Of note, too little variability existed within RT groups on race/ethnicity and history of chemotherapy to reliably examine score associations with these variables. The significance level was set at an alpha level of 0.05 (two-tailed). Because of multiple comparisons, we employed the false discovery rate (FDR) approach [19] to examine our results for the proportion of false positives likely among significant associations (p < 0.05) using an FDR threshold of 10%. Associations with p values <0.05 but FDR values ≥0.1 are reported as statistical trends. Effect sizes are reported for all statistically significant comparisons. We used the following conventions to guide our interpretation of small, medium, and large effect sizes, respectively: Pearson’s r (0.1, 0.3, 0.5) and Cohen’s d (0.2, 0.5, 0.8) [20,21].
Results
Demographic and clinical characteristics for the sample are reported in Table 1. The sample was balanced in terms of the number of survivors treated with CSI (n = 21) versus focal irradiation (n = 18). In the CSI group, 95.2% received passive scatter and 4.8% received both passive scatter and scanning beam PBRT. In the Focal group, 72.2% received passive scatter and 27.8% received scanning beam PBRT. Survivors in this sample were 13.28 years old at testing (Range: 6.70–19.49 years) and were 3.51 years post-PBRT (Range: 1.07–7.30 years) on average. CSI and Focal groups were similar in composition by sex, race/ethnicity, history of craniotomy, and history of ventriculoperitoneal (VP) shunt (all p > 0.05). Groups were also similar in age at testing, age at diagnosis, age at PBRT, and time from PBRT at testing (all p > 0.05). There were more gliomas represented in the Focal group and more medulloblastomas/primitive neuroectodermal tumors (PNET) represented in the CSI group, p < 0.001. Also, the CSI group received significantly higher RT dose to tumor compared to the Focal group, p < 0.001. There were more infratentorial tumors represented in the CSI group than in the Focal group, p < 0.05. No other statistically significant differences were identified between groups.
Table 1.
Patient characteristics by PBRT group (CSI vs. Focal).
| CSI | Focal | CSI vs. Focal | |||
|---|---|---|---|---|---|
|
|
|
||||
| Characteristic | n | % | n | p | p |
| Total | 21 | 100.0 | 18 | 100.0 | – |
| Sex | |||||
| Male | 14 | 66.7 | 11 | 61.1 | 0.718 |
| Female | 7 | 33.3 | 7 | 38.9 | |
| Race/Ethnicity | |||||
| White | 11 | 52.4 | 14 | 77.8 | 0.400 |
| Black | 2 | 9.5 | 1 | 5.6 | |
| Hispanic/Latino | 4 | 19.0 | 2 | 11.1 | |
| Other | 4 | 19.0 | 1 | 5.6 | |
| Histology | |||||
| Glioma | 0 | 0.0 | 10 | 55.6 | 0.000*** |
| Medulloblastoma/PNET | 13 | 61.9 | 1 | 5.6 | |
| Germ Cell Tumor | 7 | 33.3 | 2 | 11.1 | |
| Craniopharyngioma | 0 | 0.0 | 4 | 22.2 | |
| Other | 1 | 4.8 | 1 | 5.6 | |
| Tumor Location | |||||
| Supratentorial | 9 | 42.9 | 14 | 77.8 | 0.026* |
| Infratentorial | 12 | 57.1 | 3 | 16.7 | |
| Both | 0 | 0.0 | 1 | 5.6 | |
| Craniotomy | |||||
| Yes | 17 | 81.0 | 16 | 88.9 | 0.493 |
| No | 4 | 19.0 | 2 | 11.1 | |
| VP Shunt | |||||
| Yes | 8 | 38.1 | 3 | 16.7 | 0.138 |
| No | 13 | 61.9 | 15 | 83.3 | |
| Median | Range | Median | Range | ||
|
|
|||||
| Total RT dose to tumor (Gy) | 55.80 | 45.00–55.80 | 50.40 | 45.00–60.00 | 0.000*** |
| Age at Testing (Years) | 13.08 | 8.55–18.70 | 12.31 | 6.70–19.49 | 0.548 |
| Age at RT (Years) | 10.90 | 3.01–15.54 | 9.91 | 1.56–16.27 | 0.767 |
| Time from RT (Years) | 2.92 | 1.15–7.30 | 2.20 | 1.07–6.91 | 0.656 |
CSI = craniospinal irradiation; PBRT = proton beam radiation therapy; PNET = primitive neuroectodermal tumor; RT = radiation therapy; VP = ventriculoperitoneal.
Nonparametric comparison was used rather than a means test because of the skewed distribution of values.
p < 0.05,
p < 0.01,
p < 0.001.
Attention, Processing Speed, and Executive Functioning among Survivors in the PBRT CSI group
Table 2 reports means, standard deviations, and ranges across cognitive tests. Figs. 1–5 illustrate group means across cognitive tests compared to the normative means of each test. In the PBRT CSI group, survivors’ attention scores did not differ from the normative mean on any CPT-II scale. A trend was found between higher Omission Errors scores (indicative of more frequent failure to respond to a stimulus) and history of craniotomy, t(17) = 3.068, p = 0.008, d = 1.12, FDR > 0.1, although scores fell solidly within normal limits for both subgroups (Craniotomy: M =51.78, SD = 9.23; None: M= 44.44, SD = 0.47). A trend was also identified between lower Perseverations scores (indicative of slower responding) and higher total RT dose, r = −0.555, p = 0.014, FDR > 0.1. Still, it should be noted that there was restricted variability in RT dose across survivors in the CSI group (Table 1), and only one survivor obtained a score falling in the clinical range on this scale. No other statistically significant associations were found between CPT-II scores and demographic or clinical variables.
Table 2.
Attention, processing speed, and executive functioning, and visual-motor scores by PBRT group (CSI vs. Focal).
| CSI (n = 21) | Focal (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Cognitive Test/Subtest | n | M | SD | p | n | M | SD | p |
| Conners’ CPT-II | ||||||||
| Omission Errors | 19 | 50.23 | 8.70 | 0.908 | 17 | 52.72 | 9.49 | 0.254 |
| Commission Errors | 19 | 49.82 | 11.76 | 0.947 | 17 | 44.85 | 11.70 | 0.089 |
| Mean Reaction Time | 19 | 47.57 | 12.44 | 0.407 | 17 | 51.75 | 11.11 | 0.525 |
| Reaction Time Standard Error | 19 | 51.17 | 10.43 | 0.632 | 17 | 53.86 | 10.16 | 0.137 |
| Variability | 19 | 50.01 | 12.56 | 0.998 | 17 | 53.68 | 11.51 | 0.206 |
| Attentiveness | 19 | 49.00 | 13.68 | 0.755 | 17 | 46.16 | 10.42 | 0.148 |
| Perseverations | 19 | 53.17 | 17.42 | 0.438 | 17 | 54.20 | 18.69 | 0.368 |
| D-KEFS Color Word Interference | ||||||||
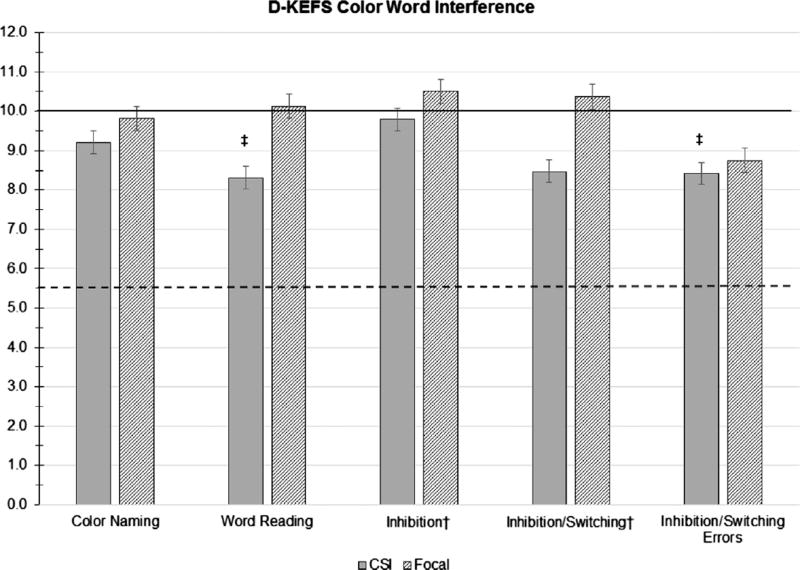
| Color Naming | 19 | 9.21 | 2.68 | 0.215 | 16 | 9.81 | 2.97 | 0.804 |
| Word Reading | 19 | 8.32 | 2.50 | 0.009‡ | 16 | 10.13 | 2.09 | 0.814 |
| Inhibition† | 19 | 9.79 | 2.53 | 0.721 | 16 | 10.50 | 3.61 | 0.588 |
| Inhibition/Switching† | 19 | 8.47 | 3.60 | 0.081 | 16 | 10.38 | 2.25 | 0.515 |
| Inhibition/Switching Errors | 19 | 8.42 | 3.25 | 0.049‡ | 16 | 8.75 | 4.12 | 0.244 |
| D-KEFS Trail Making Test | ||||||||
| Number Sequencing | 19 | 6.00 | 3.65 | 0.000* | 16 | 9.31 | 2.63 | 0.312 |
| Letter Sequencing | 19 | 6.68 | 3.40 | 0.000* | 16 | 8.81 | 3.71 | 0.220 |
| Number-Letter Switching† | 19 | 6.53 | 3.32 | 0.000* | 16 | 9.06 | 2.84 | 0.206 |
| Number-Letter Switching Errors | 19 | 9.58 | 2.85 | 0.528 | 16 | 10.88 | 1.02 | 0.004‡ |
| D-KEFS Verbal Fluency | ||||||||
| Letter Fluency | 19 | 8.16 | 2.48 | 0.005‡ | 16 | 9.75 | 3.96 | 0.804 |
| Category Fluency | 19 | 8.89 | 2.79 | 0.101 | 16 | 9.75 | 3.17 | 0.757 |
| Category Switching† | 19 | 8.89 | 2.38 | 0.058 | 16 | 10.50 | 2.48 | 0.432 |
| Category Switching Accuracy† | 19 | 9.26 | 2.56 | 0.225 | 16 | 10.63 | 2.53 | 0.338 |
| Percent Switching Accuracy | 19 | 9.58 | 3.32 | 0.587 | 16 | 10.25 | 2.84 | 0.730 |
| VMI | ||||||||
| Visual Perception | 21 | 92.62 | 15.31 | 0.039‡ | 17 | 95.88 | 10.06 | 0.111 |
| Motor Coordination | 21 | 80.43 | 12.40 | 0.000* | 17 | 92.82 | 13.29 | 0.041‡ |
Indicates primary executive functioning subtests.
The p-values are reported for tests comparing survivor group means to normative means.
CPT-II = Continuous Performance Test, Second Edition. CSI = craniospinal irradiation. D-KEFS = Delis–Kaplan Executive Function System. VMI = Beery–Buktenica Developmental Test of Visual-Motor Integration – Sixth Edition.
Indicates trend association (p < 0.05 and FDR > 0.1).
Indicates a statistically significant association (p < 0.001 and FDR < 0.1).
Fig. 1.
Mean scores by PBRT group (CSI vs. Focal) on the Conners’ CPT-II scales. The solid line indicates the mean of 50.0 for the CPT-II normative sample. Scores above the dashed line (1.5 standard deviations above the normative mean) are generally considered indicative of clinical impairment. ‡indicates trend association (p < 0.05 and FDR > 0.1). *indicates a statistically significant association (p < 0.001 and FDR < 0.1).
Fig. 5.
Mean scores by PBRT group (CSI vs. Focal) on the VMI subtests. The solid line indicates the mean of 100.0 for the VMI normative sample. Scores below the dashed line (1.5 standard deviations below the normative mean) are generally considered indicative of clinical impairment. ‡indicates trend association (p < 0.05 and FDR > 0.1). *indicates a statistically significant association (p < 0.001 and FDR < 0.1).
On the Color-Word Interference test, the PBRT CSI group did not differ significantly from the normative mean on executive functioning subtests (Inhibition and Inhibition/Switching). On processing speed tasks, a trend was identified where survivors had lower Word Reading scores compared to the normative mean, t(18) = 2.942, p = 0.009, d = 0.61, FDR > 0.1, indicating that they read fewer words than expected for their age within a time limit. Still, as a group, survivors’ performance on this task was solidly within normal limits, with only 15.79% of survivors in this group obtaining scores in the clinically significant range. In a similar trend, survivors exhibited more errors on the executive functioning Inhibition/Switching task compared to the normative sample, t(18) = 2.115, p = 0.049, d = 0.51, FDR > 0.1, although the group mean remained within normal limits. The group mean did not differ significantly from the normative mean on the other Color-Word Interference processing speed task (Color Naming).
A trend identified worse Word Reading scores among survivors with infratentorial (M =7.18, SD = 2.23) compared to supratentorial tumors (M = 9.88, SD = 2.03), t(17) = 2.697, p = 0.015, d = 1.27, FDR > 0.1. In a similar trend, worse Inhibition scores were associated with infratentorial (M =8.73, SD = 2.149) compared to supratentorial tumor location (M =11.25, SD = 2.375), t(17) = 2.418, p = 0.027, d = 1.11, FDR > 0.1, although subgroup means in this comparison were both solidly within normal limits. Of note, all infratentorial tumors in the PBRT CSI group were medulloblastoma. No other significant associations were found between Color-Word Interference scores and demographic or clinical variables.
On the Trail Making Test, survivors in the PBRT CSI group exhibited significantly weaker executive functioning compared to the normative mean on the Number-Letter Switching subtest, t(18) = 4.557, p = 0.000, d = 1.10, with 47.37% of survivors obtaining scores in the clinical range on this task. Survivor means were also significantly lower (worse) than normative means on both processing speed subtests: Number Sequencing, t(18) = 4.775, p = 0.000, d = 1.20, and Letter Sequencing, t(18) = 4.251, p = 0.000, d = 1.04, with 42.11% of survivors obtaining scores in the clinical range on each subtest. Given the visual-motor demands of the Trail Making Test, we examined correlations between scores on each of these subtests and the VMI Visual Perception and Motor Coordination subtests, but no statistically significant correlations were found. Further, the group mean did not significantly differ from the normative mean on the Number-Letter Switching Errors subtest, suggesting survivors’ performance was accurate on the Trail Making Test despite being significantly slow.
Trends were found between lower Number Sequencing scores and sex (Female: M = 3.50, SD = 1.975; Male: M = 7.15. SD = 3.716), t(17) = 2.243, p = 0.039, d = 1.23, FDR > 0.1, as well as having a history of a shunt (Shunt: M =3.86, SD = 2.734; No Shunt: M= 7.25, SD = 3.621), t(17) = 2.139, p = 0.047, d = 1.06, FDR > 0.1. It should be noted that the proportion of males who received shunts (21.4%) was significantly lower than females (71.4%) in this subgroup, Χ2 (1, N = 21) = 4.947, p = 0.026, d = 1.11. No other significant associations were found between Trail Making Test scores and demographic or clinical variables.
On the Verbal Fluency test, executive functioning performance on the Category Switching and the Category Switching Accuracy subtests did not differ between the PBRT CSI group and the normative mean. On processing speed subtests, a trend was identified, with survivors exhibiting lower (worse) Letter Fluency scores compared to the normative mean, t(18) = 3.24, p = 0.005, d = 0.67, FDR > 0.1; yet, only 5.26% of the sample obtained scores in the clinical range on this task, and the group mean fell solidly within normal limits. Group means did not differ from the normative mean on the other Verbal Fluency processing speed task (Category Fluency) or on the errors subtest (Percent Switching Accuracy).
Trends were identified between lower (worse) Category Fluency scores and older age at testing (r = −0.616, p = 0.005, FDR > 0.1) as well as older age at RT (r = −0.463, p = 0.046, FDR > 0.1). A trend was also found between lower Category Switching scores and older age at evaluation, r = −0.459, p = 0.048, FDR > 0.1. Of note, age at testing and age at RT were also significantly correlated, r = 0.841, p = 0.000. No other statistically significant associations were found between Verbal Fluency scores and demographic or clinical variables.
Group performance on the VMI was found to be significantly lower (worse) than the normative mean on the Motor Coordination subtest, t(20) = 7.235, p = 0.000, d = 1.42, while survivors’ performance trended lower on the Visual Perception subtest compared to the normative mean, t(20) = 2.210, p = 0.039, d = 0.49, FDR > 0.1. Only 14.3% of the CSI group participants obtained clinical scores on the Visual Perception subtest, while 38.1% of this subsample obtained scores in the clinical range on the Motor Coordination subtest. Trends were identified showing lower Motor Coordination scores in females (Female: M = 72.86, SD = 13.74; Male: M = 84.21, SD = 10.15; t(19) = 2.151, p = 0.045, d = 0.94, FDR > 0.1) and in survivors with a history of VP shunt (Shunt: M= 73.625, SD = 13.125; No Shunt: M= 84.615, SD = 10.284; t (19) = 2.143, p = 0.045, d = 0.93, FDR > 0.1). A trend also indicated lower Visual Perception scores among survivors with supratentorial tumors (M = 84.00, SD = 19.138) compared to infratentorial tumors (M = 99.08, SD = 7.292), t(19) = 2.245, p = 0.049, d = 1.041, FDR > 0.1. No other statistically significant associations were found between VMI scores and demographic or clinical variables.
Attention, Processing Speed, and Executive Functioning among Survivors in the PBRT Focal group
In the PBRT Focal group, survivors’ attention, processing speed, and executive functioning did not differ from normative means across all CPT-II and D-KEFS scales. In fact, these survivors exhibited a trend toward more accurate performance than the normative group on the D-KEFS Trail Making Test: Number-Letter Switching Errors t(15) = 3.416, p = 0.004, d = 0.39, FDR > 0.1. Even so, some clinical risk factors were identified on administered measures. A trend indicated higher Perseverations scores (indicative of impulsive or random responding) on the CPT-II were associated with younger age at RT, r = −0.491, p = 0.045, FDR > 0.1. Another trend was identified between lower (worse) Word Reading scores and history of a VP shunt, t(14) = 2.181, p = 0.047, d = 1.18, FDR > 0.1, although mean scores for both contrasted groups fell within normal limits for age (Shunt: M= 8.00, SD = 2.646; No Shunt: M= 10.62, SD = 1.710) on this task. No other statistically significant associations were found between CPT-II or D-KEFS scores and demographic or clinical variables.
A trend indicated lower (worse) performance on the VMI Motor Coordination subtest among survivors compared to the normative mean, t(16) = 2.226, p = 0.041, d = 0.51, FDR > 0.1, with 11.8% of this subsample obtaining scores in the clinical range on this measure. Performance on the Visual Perception subtest did not differ from the normative group. No statistically significant associations were found between VMI scores and demographic or clinical variables.
Discussion
While previous reports have identified some vulnerability in processing speed skills among pediatric brain tumor survivors treated with PBRT [13–15], to our knowledge, this is the first report on attention and executive functioning outcomes in this population. In our sample of survivors who were more than three years post-PBRT on average, sustained attention was found to fall within age-expectation. Survivors who received focal PBRT also exhibited normative performance across all executive functioning and processing speed tasks. In contrast, some areas of mild relative weakness were identified in the CSI PBRT group. Together, these results suggest that survivors treated with PBRT may exhibit resilience in cognitive domains traditionally known to be vulnerable to RT, although CSI emerges as a cognitive risk factor.
Attentional late effects are well documented in the pediatric brain tumor survivor population [1,2]. Evidence suggests that impaired attention is fundamental to associations found between RT-induced white matter damage and subsequent declines in intellectual functioning and academic achievement [22], although the literature to date has reported exclusively on such outcomes among survivors treated with XRT. The present study used the CPT-II [16] to assess attention, a measure that has been used extensively to assess attention in outcomes research and clinical practice with survivors of brain tumors. Although there is some variability across studies that have used the CPT, a recent meta-analysis examining CPT performance in pediatric brain tumor samples found worse Omission Errors scores in survivors compared to population norms, with a large effect size (d = 0.82) [23], suggesting that attention is a particularly radiosensitive domain in post-XRT outcomes studies. The solidly average performance on this and all other CPT-II scales in our sample is notable and may be indicative of cognitive sparing with PBRT. Still, while our sample did not show significant attention deficits at more than three years post-PBRT on average, it remains unknown whether deficits would be identified in a larger sample with lengthier follow-up.
Like attention, processing speed is another established domain of cognitive risk identified across studies of pediatric brain tumor survivors [3,4,24]. Recent studies have also shown survivors to be at risk for executive dysfunction post-treatment [2,6,7]. While some of the earliest cognitive outcomes studies of pediatric brain tumor survivors treated with PBRT have also found relative weaknesses in processing speed [14,15], post-PBRT executive functioning has not been reported to date in this population. In our sample, survivors treated with focal PBRT obtained group means across processing speed and executive functioning subtests (i.e., D-KEFS) that were solidly average and within normal limits relative to age norms.
In contrast, some relative weaknesses were identified among survivors treated with CSI PBRT in our sample. In particular, this group performed worse than age-based norms on all subtests of the D-KEFS Trail Making Test, including the task assessing executive functioning as well as the tasks assessing lower level processing speed skills. Despite completing these subtests more slowly than expected for age, survivors were appropriately accurate on the switching subtest. Trail Making Test performance was not better explained by co-occurring deficits in visual perception or motor skills. Together, this pattern of scores across Trail Making Test subtests suggests survivors treated with CSI, as a group, may have intact sequencing skills but weaker efficiency. This is in line with trends suggestive of isolated, but small, weaknesses on other processing speed subtests in this group (i.e., Word Reading and Letter Fluency), although group means were solidly within normal limits on these subtests. Further, in survivors with a history of CSI, trends suggested that higher total PBRT dose and history of craniotomy were associated with indicators of slow responding on the CPT-II (i.e., lower Perseverations scores and higher Omission Errors scores). Thus, while we did not find broadly impaired processing speed in this sample, subtle relative weaknesses identified on specific tasks indicate that processing speed may remain vulnerable under certain clinical conditions for patients treated with PBRT-based CSI. This is consistent with Yock et al.’s [14] findings of processing speed decline in pediatric medulloblastoma patients treated with PBRT-administered CSI.
Taken together, results indicate different patterns of neurocognitive vulnerability depending on the type of PBRT administered. While survivors treated with focal PBRT, as a group, exhibited performance across attention, executive functioning, and processing speed subtests that was consistent with age-expectations, survivors treated with CSI exhibited more areas of relative weakness. These results are consistent with previous XRT research documenting worse intellectual functioning in children who received CSI versus focal XRT [25], studies documenting relationships between higher doses of CSI XRT and worse cognitive outcomes [10,25–29], and research demonstrating associations between CSI XRT and reduced white matter volumes [30]. Still, means for the PBRT CSI group in our sample fell solidly within the average range across all administered tests, with the exception of subtests on the Trail Making Test that approached the clinically significant range. Together, this is suggestive of preserved functioning following PBRT-administered CSI with the exception of specific tasks involving visual scanning, sequencing, and fine motor speed components. The potential neuroprotective benefit of PBRT may seem less likely with CSI since the whole brain is irradiated. Yet, it remains possible that the reduced dose deposited beyond the boundaries of the tumor bed boost with PBRT (vs. XRT) may spare essential structures or regions of tissue from reaching a critical level of RT exposure that would result in clinically significant cognitive decline. Although dosimetry studies are needed to explore this possibility, the benefit of boost volume reduction on IQ has been shown in an XRT sample [27].
Additionally, trend-level associations between neurocognitive performance and medical variables indicated that worse performance on specific executive functioning and processing speed subtests was associated with infratentorial tumor location and history of VP shunt placement. History of hydrocephalus (requiring shunt placement) has been consistently associated with worse intellectual functioning [31–33], academic skills [31], and visual-motor abilities [31] in survivors of pediatric brain tumors. Infratentorial tumor location is another known risk factor for cognitive late effects [34]. Of note, patients with infratentorial tumors were significantly younger at treatment and more likely to receive CSI (versus focal RT) in this sample (not reported), which highlights a subgroup of patients who may be particularly vulnerable due to a multiplicity of cognitive risk factors. Whether these cooccurring risk factors have a synergistic effect on outcome is unknown.
In terms of demographic risk factors, only trends were found between cognitive performance and age at evaluation and age at RT. Older age at evaluation and at RT were associated with worse scores on Verbal Fluency tasks in the CSI group. These findings are inconsistent with past research documenting increased cognitive risk for patients who were younger at diagnosis [24,35–37] and at RT [10,38]. Yet, relevant specifically to the domains studied in this investigation, adolescence is a period of rapid development of executive functions as well as a time when demands at home and school require greater reliance on executive functions. Perhaps, a unique vulnerability exists for older pediatric patients (i.e., those in adolescence) when treatment co-occurs with the development of executive functions. Further investigation is warranted to better understand how the timing of disease and treatment may differentially impact the development of specific cognitive skills.
As with any study, our findings must be considered in light of several limitations. Our sample size was relatively small and diagnostically heterogeneous. Still, given that PBRT has not been widely available, the overall number of survivors that have received this treatment is small. Additionally, the issue of multiple comparisons is a common problem with this rare population that requires consideration of numerous clinical variables. We acknowledge that multiple comparisons present a statistical complication by increasing the likelihood of false positives. Therefore, we used the FDR approach to strike a balance between identifying true associations and recognizing the chance that some significant results could be spurious. Even so, our trend findings were in the direction of expectation based on the available cognitive late effects literature. Further, effect sizes were medium to large for all reported relative weaknesses in cognitive performance suggesting these findings are unlikely to be spurious even though our sample may have been too small to detect other less robust differences. Finally, although our results may be suggestive of potential cognitive sparing from PBRT based on the generally age-typical performance across measures in this sample, we are unable to attribute null findings to treatment without an XRT comparison group and without longitudinal data to demonstrate change in functioning over time as a function of treatment. Prospective data collection is ongoing at our institution so that we may advance our understanding beyond the post-PBRT outcomes available in the present study.
In conclusion, this is the first report on attention, processing speed, and executive functioning in pediatric brain tumor survivors previously treated with PBRT. Results are suggestive of possible sparing, particularly in patients treated with focal PBRT, in these cognitive domains known to be vulnerable to the effects of RT. Yet, specific areas of relative weakness identified in this sample support the continued need for routine neurocognitive monitoring and the need, at least for a subset of survivors, for educational accommodations to address difficulties with processing speed (e.g., extra time to complete assignments and tests through Section 504 Plans). History of CSI appears to be a particularly important clinical risk factor associated with cognitive outcomes in patients treated with PBRT, consistent with findings in the XRT cognitive outcomes literature. As additional data become available from samples of pediatric brain tumor patients treated with PBRT, we will gain a more precise understanding of the relative impact of this treatment modality on cognitive development. It appears, from the results presented here and the few other available cognitive outcome studies examining PBRT samples [13–15], there is currently no evidence that PBRT results in profound cognitive impairment for the majority of treated patients, even in some of the most radiosensitive domains such as those assessed in the current study.
Fig. 2.
Mean scores by PBRT group (CSI vs. Focal) on the D-KEFS Color Word Interference subtests. The solid line indicates the mean of 10.0 for the D-KEFS normative sample. Scores below the dashed line (1.5 standard deviations below the normative mean) are generally considered indicative of clinical impairment. ‡indicates trend association (p < 0.05 and FDR > 0.1). *indicates a statistically significant association (p < 0.001 and FDR < 0.1).
Fig. 3.
Mean scores by PBRT group (CSI vs. Focal) on the D-KEFS Trail Making Test subtests. The solid line indicates the mean of 10.0 for the D-KEFS normative sample. Scores below the dashed line (1.5 standard deviations below the normative mean) are generally considered indicative of clinical impairment. ‡indicates trend association (p < 0.05 and FDR > 0.1). *indicates a statistically significant association (p < 0.001 and FDR < 0.1).
Fig. 4.
Mean scores by PBRT group (CSI vs. Focal) on the D-KEFS Verbal Fluency subtests. The solid line indicates the mean of 10.0 for the D-KEFS normative sample. Scores below the dashed line (1.5 standard deviations below the normative mean) are generally considered indicative of clinical impairment. ‡indicates trend association (p < 0.05 and FDR > 0.1). *indicates a statistically significant association (p < 0.001 and FDR < 0.1).
Acknowledgments
Funding: This work was supported, in part, by the Texas Children’s Hospital Pediatric Pilot Research Fund and by the National Cancer Institute K07CA157923 and R01CA187202 (Principal Investigator: Lisa Kahalley).
Footnotes
Conflicts of interest
There are no known conflicts of interest to disclose.
References
- 1.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10:180–9. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 2.Robinson KE, Pearson MM, Cannistraci CJ, et al. Neuroimaging of executive function in survivors of pediatric brain tumors and healthy controls. Neuropsychology. 2014;28:791–800. doi: 10.1037/neu0000077. [DOI] [PubMed] [Google Scholar]
- 3.Conklin HM, Ashford JM, Di PM, et al. Computerized assessment of cognitive late effects among adolescent brain tumor survivors. J Neurooncol. 2013;113:333–40. doi: 10.1007/s11060-013-1123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahalley LS, Conklin HM, Tyc VL, et al. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology. 2013;22:1979–86. doi: 10.1002/pon.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer SL, Glass JO, Li Y, et al. White matter integrity is associated with cognitive processing in patients treated for a posterior fossa brain tumor. Neuro Oncol. 2012;14:1185–93. doi: 10.1093/neuonc/nos154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–17. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter AL, Conklin HM, Tyc VL, et al. Executive function late effects in survivors of pediatric brain tumors and acute lymphoblastic leukemia. J Clin Exp Neuropsychol. 2014;36:1–13. doi: 10.1080/13803395.2014.943695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyer KH, Willard VW, Gross AM, et al. The impact of attention on social functioning in survivors of pediatric acute lymphoblastic leukemia and brain tumors. Pediatr Blood Cancer. 2012;59:1290–5. doi: 10.1002/pbc.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarsen FK, Paquier PF, Arts WF, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol. 2009;27:3526–32. doi: 10.1200/JCO.2008.19.6303. [DOI] [PubMed] [Google Scholar]
- 10.Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a pediatric oncology group study. J Clin Oncol. 1998;16:1723–8. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–7. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suneja G, Poorvu PD, Hill-Kayser C, Lustig RA. Acute toxicity of proton beam radiation for pediatric central nervous system malignancies. Pediatr Blood Cancer. 2013;60:1431–6. doi: 10.1002/pbc.24554. [DOI] [PubMed] [Google Scholar]
- 13.Kahalley LS, Ris MD, Grosshans DR, et al. Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. J Clin Oncol. 2016;34:1043–9. doi: 10.1200/JCO.2015.62.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17:287–98. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 15.Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 2015;93:400–7. doi: 10.1016/j.ijrobp.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conners CK, Staff M. ™ technical guide and software manual. North Tonawanda, NY: Multi-Health Systems Inc; 2000. Conners’ continuous performance test (CPT II) computer programs for Windows. [Google Scholar]
- 17.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 18.Beery KE, Beery NA. The Beery-Buktenica Developmental Test of Visual-Motor Integration Administration, Scoring, and Teaching Manual. Sixth. Bloomington, MN: Pearson Clinical Assessment; 2010. [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 20.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 21.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–9. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 23.de Ruiter MA, van Mourik R, Schouten-van Meeteren AY, Grootenhuis MA, Oosterlaan J. Neurocognitive consequences of a paediatric brain tumour and its treatment: a meta-analysis. Dev Med Child Neurol. 2013;55:408–17. doi: 10.1111/dmcn.12020. [DOI] [PubMed] [Google Scholar]
- 24.Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31:3494–500. doi: 10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grill J, Kieffer Renaux V, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45:137–45. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 26.Kieffer-Renaux V, Bulteau C, Grill J, Kalifa C, Viguier D, Jambaque I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Dev Med Child Neurol. 2000;42:741–5. doi: 10.1017/s0012162200001377. [DOI] [PubMed] [Google Scholar]
- 27.Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32:1760–8. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- 28.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–8. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 29.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–9. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 30.Reddick WE, Russell JM, Glass JO, et al. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18:787–93. doi: 10.1016/s0730-725x(00)00182-x. [DOI] [PubMed] [Google Scholar]
- 31.Hardy KK, Bonner MJ, Willard VW, Watral MA, Gururangan S. Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psychooncology. 2008;17:1157–61. doi: 10.1002/pon.1349. [DOI] [PubMed] [Google Scholar]
- 32.Reimers TS, Ehrenfels S, Mortensen EL, et al. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003;40:26–34. doi: 10.1002/mpo.10211. [DOI] [PubMed] [Google Scholar]
- 33.Merchant TE, Lee H, Zhu J, et al. The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg. 2004;101:159–68. doi: 10.3171/ped.2004.101.2.0159. [DOI] [PubMed] [Google Scholar]
- 34.Patel SK, Mullins WA, O’Neil SH, Wilson K. Neuropsychological differences between survivors of supratentorial and infratentorial brain tumours. J Intellect Disabil Res. 2011;55:30–40. doi: 10.1111/j.1365-2788.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 35.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–6. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16:1129–36. doi: 10.1093/neuonc/nou006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sands SA, Zhou T, O’Neil SH, et al. Long-term follow-up of children treated for high-grade gliomas: Children’s Oncology Group L991 final study report. J Clin Oncol. 2012;30:943–9. doi: 10.1200/JCO.2011.35.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–9. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]