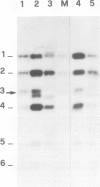
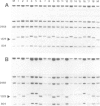
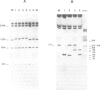
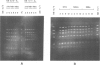
Abstract
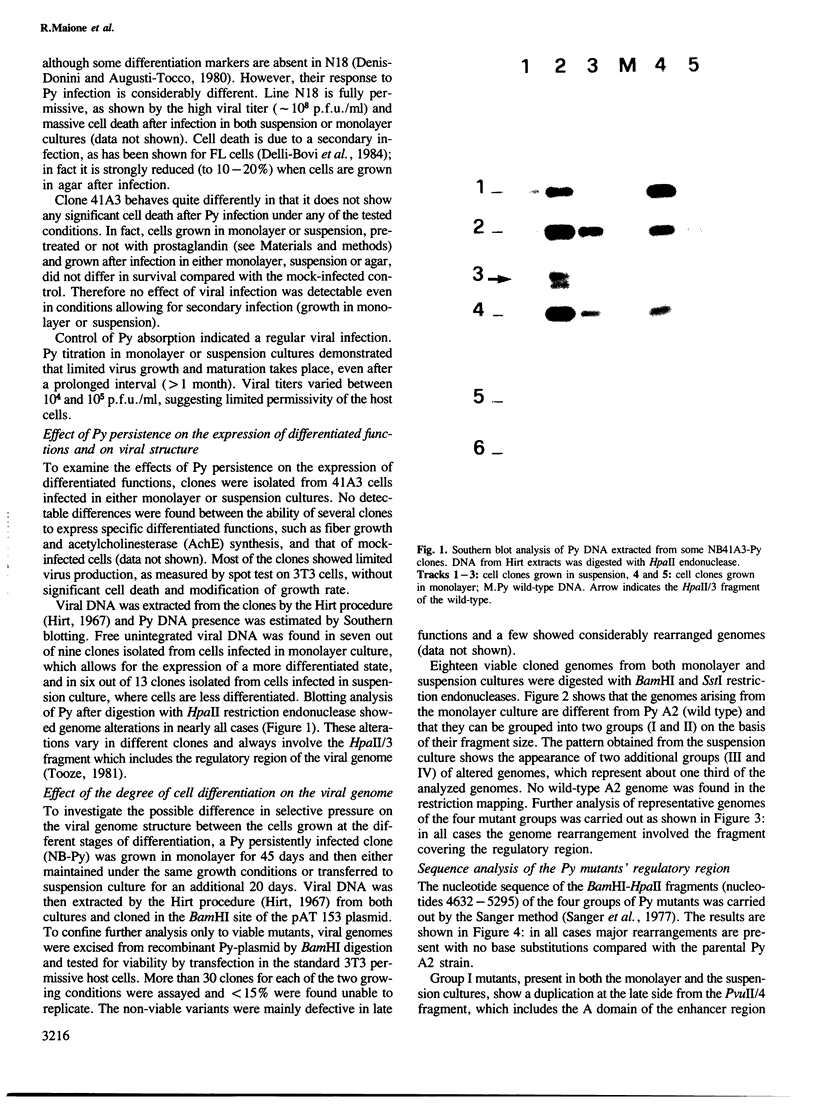
Two mouse neuroblastoma cell lines were analyzed for their permissivity for polyoma virus growth. One (N18) is fully permissive for polyoma replication, the other (41A3) shows limited permissivity and the viral genome persists, without noticeable cell death. Virus persistence does not seem to alter the cells' ability to differentiate in vitro and leads to selection of viral mutants altered in the untranscribed regulatory region of the genome. The mutant types obtained appear to be related to the degree of host cell differentiation. Nucleotide sequence analysis of the restriction fragment covering the regulatory region shows that duplications are present in all mutants, while deletions in the non-duplicated segment are only present in mutants selected from less differentiated cells. These alterations involve both domains of the regulatory region that are considered to be essential for DNA replication and for enhancer activity. Mixed infections with polyoma wild type show that the selected mutants have cis-advantage in replication in neuroblastoma cells and not in 3T6 cells. Mutants carrying the deletion in the non-duplicated segment of the enhancer show a selective advantage in replication over the undeleted one in mixed infection. This advantage is much stronger in neuroblastoma cells in suspension (less-differentiated stage) than in monolayer cells (more-differentiated stage). An interpretation of the overall structure of the regulatory enhancer region, based on the observed differences between the mutants selected at different stages of differentiation in neuroblastoma and previously described mutants selected in undifferentiated cells, is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Casola L., Romano M., Di Matteo G. RNA metabolism in neuroblastoma cultures. I. Ribosomal RNA. Dev Biol. 1974 Dec;41(2):371–379. doi: 10.1016/0012-1606(74)90313-3. [DOI] [PubMed] [Google Scholar]
- De Simone V., La Mantia G., Lania L., Amati P. Polyomavirus mutation that confers a cell-specific cis advantage for viral DNA replication. Mol Cell Biol. 1985 Aug;5(8):2142–2146. doi: 10.1128/mcb.5.8.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., De Simone V., Giordano R., Amati P. Polyomavirus growth and persistence in Friend erythroleukemic cells. J Virol. 1984 Feb;49(2):566–571. doi: 10.1128/jvi.49.2.566-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis-Donini S., Augusti-Tocco A. Molecular and lectin probe analyses of neuronal differentiation. Curr Top Dev Biol. 1980;16:323–348. doi: 10.1016/s0070-2153(08)60161-1. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S., Estenoz M., Augusti-Tocco G. Cell surface modifications in neuronal maturation. Cell Differ. 1978 Aug;7(4):193–201. doi: 10.1016/0045-6039(78)90021-0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- El-Badawi A., Schenk E. A. Histochemical methods for separate, consecutive and simultaneous demonstration of acetylcholinesterase and norepinephrine in cryostat sections. J Histochem Cytochem. 1967 Oct;15(10):580–588. doi: 10.1177/15.10.580. [DOI] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Regulation of adenosine 3',5'-cyclic monophosphate metabolism in cultured neuroblastoma cells. Nature. 1971 Dec 10;234(5328):356–358. doi: 10.1038/234356a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grouse L. D., Schrier B. K., Letendre C. H., Zubairi M. Y., Nelson P. G. Neuroblastoma differentiation involves both the disappearance of old and the appearance of new poly(A)+ messenger RNA sequences in polyribosomes. J Biol Chem. 1980 May 10;255(9):3871–3877. [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Saragosti S., Blangy D., Yaniv M. Fine structure of the origin-proximal DNAase I-hypersensitive region in wild-type and EC mutant polyoma. Cell. 1981 Sep;25(3):651–658. doi: 10.1016/0092-8674(81)90172-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Kelly F., Condamine H. Tumor viruses and early mouse embryos. Biochim Biophys Acta. 1982 Apr 29;651(2-3):105–141. doi: 10.1016/0304-419X(82)90009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman W., Levine A. J. Viruses as probes for development and differentiation. Adv Virus Res. 1981;26:65–116. doi: 10.1016/s0065-3527(08)60421-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyake M. The development of action potential mechanism in a mouse neuronal cell line in vitro. Brain Res. 1978 Mar 24;143(2):349–354. doi: 10.1016/0006-8993(78)90574-7. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Lyon H. D. A microtubule-associated protein specific to differentiated neuroblastoma cells. J Biol Chem. 1981 Apr 10;256(7):3507–3511. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Sequence repeats in a polyoma virus DNA region important for gene expression. J Virol. 1983 Jul;47(1):233–237. doi: 10.1128/jvi.47.1.233-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Jacob F. 5-bromodeoxyuridine-induced differentiation of a neuroblastoma. Proc Natl Acad Sci U S A. 1970 Sep;67(1):247–254. doi: 10.1073/pnas.67.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Chowdhury K., Chang K. S., Israel M., Ito Y. Isolation and characterization of polyoma virus mutants which grow in murine embryonal carcinoma and trophoblast cells. EMBO J. 1982;1(12):1521–1527. doi: 10.1002/j.1460-2075.1982.tb01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Kress C., Montreau N., Blangy D. Isolation and characterization of polyoma virus mutants able to develop in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1068–1072. doi: 10.1073/pnas.77.2.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucco F., Persico M., Felsani A., Metafora S., Augusti-Tocco G. Regulation of protein synthesis at the translational level in neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2289–2293. doi: 10.1073/pnas.72.6.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]