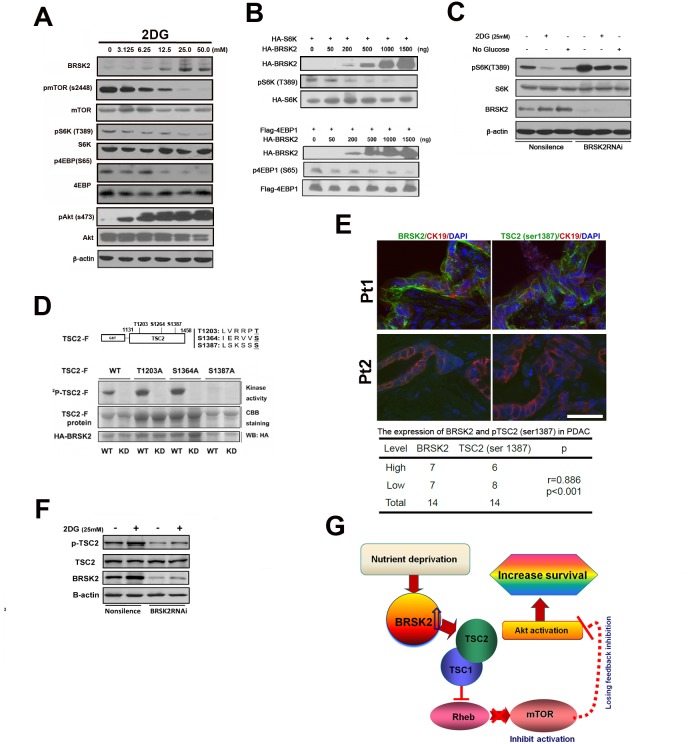
Figure 5. BRSK2 in PDAC cells strengthens Akt activity via TSC2-dependent mTORC1 repression.
(A) Effect of 2-DG treatment on BRSK2 and other components of AKT/mTOR signaling pathway. PANC-1 cells were treated with series concentrations of 2-DG for three hours before analysis. (B) Overexpression of BRSK2 inhibited S6K and 4EBP-1 phosphorylation. PANC-1 cells were transiently co-transfected with pCMV-HA-BRSK2 and pCMV-HA-S6K (or pFLAG-CMV4-4EBP1) constructs. (C) Inhibition of S6K phosphorylation upon energy deprivation was alleviated when BRSK2 was knocked-down. PANC-1 cells were first transiently transfected with BRSK2 specific siRNA fragment or control fragment. 3 days after transfection, cells were treated with regular media plus 2-DG or no glucose media for three hours. (D) BRSK2 phosphorylated TSC2 on Ser1387 in vitro. (E) BRSK2 expression levels are positively correlated with the levels of TSC2 (Ser1387) in human PDAC. We have co-immunostained the consecutive frozen sections of human PDAC samples (n=14) with pTSC2 (Ser1387) or BRSK2 and Cytokeratin. Scale bar: 50 μm. (F) Activation of TSC2 phosphorylation upon energy deprivation was impeded when BRSK2 was knocked-down. PANC-1 cells were first transiently transfected with BRSK2 specific siRNA fragment or control fragment. 3 days after transfection, cells were treated with regular media plus 2-DG for three hours. (G) Scheme of BRSK2's role on Akt activation in PDAC. BRSK2 was significantly upregulated in the nutrient deprivation micro-environment, which inhibited mTOR via phosphorylating TSC2. This repression of mTOR may cause a loss of feedback inhibition on Akt activation. And hyperactivation of Akt possibly would attribute to increased survival in PDAC.