Abstract
Background
We assessed long-term metabolic and endocrine profiles of outpatients with schizophrenia participating in a one-year open-label extension study of monthly aripiprazole lauroxil (AL), a long-acting injectable antipsychotic.
Methods
Patients (N = 478) were enrolled in a 52-week, open-label extension study of AL monotherapy administered by intramuscular injection every 4 weeks. Of these, most (368) received AL 882 mg and the remainder AL 441 mg as their fixed-dose regimen. Among the patients entering the long-term study, 181 (38%) had already received three prior AL injections. The baseline values for this analysis were obtained from the visit before the first AL injection. Patients were followed for the full year of the extension study unless they discontinued early. Changes in metabolic parameters (weight, fasting blood sugar, lipids) and serum prolactin were assessed over the duration of AL exposure, which could extend to a total of 16 AL injections. Data presented are last observation carried forward from baseline to last visit.
Results
Most patients remained for most of the follow-up period, with 409 (86%) remaining at 6 months and 326 (68%) completing the one-year treatment period. The mean (standard deviation) changes from baseline in the overall population were: +1.1 (27.5) mg/dL for glucose, +0.07 (0.6)% for glycated hemoglobin (HbA1c), −3.3 (35.8) mg/dL for total cholesterol and −5.3 (101.9) mg/dL for triglycerides. Prolactin change from baseline was −8.7 ng/mL (14.7) for men and −14.9 (43.4) ng/mL for women. Overall, the mean weight change was +0.8 (5.9) kg. In terms of categorical weight change, 88 patients (18%) gained ≥7% body weight, and 59 (12%) lost ≥7% body weight. Overall, there was no clinically meaningful difference between any of these variables and AL dose.
Conclusion
Long-term treatment with AL in outpatients with schizophrenia was associated with a modest lowering of serum prolactin for both genders and relatively modest changes in average weight, fasting glucose, and HbA1c values. There appeared to be little net change in lipid parameters. This presentation extends a recently published report on the short-term metabolic and endocrine effects of AL over a period of 12 weeks. The present study increased the follow-up period to more than a year and was careful to use the first exposure to AL as the baseline. Limitations include lack of a comparison group and difficulty disentangling effects of medication treatment versus factors. Overall, the metabolic, weight, and endocrine effects reported here are consistent with other long-term effects of oral aripiprazole treatment. This study was funded by Alkermes, Inc.
Keywords: antipsychotic agents, aripiprazole, aripiprazole lauroxil, blood glucose, cholesterol, fasting, prolactin, psychopharmacology, schizophrenia
Background
-
Long-term antipsychotic treatment has been associated with a range of adverse metabolic and endocrine effects, including clinically significant weight gain, dyslipidemia, and elevated prolactin levels1
○ All of these effects can have long-term health consequences and add to the overall burden of treatment associated with schizophrenia
Antipsychotics differ in propensity and magnitude of these metabolic risk factors; therefore, it is important to understand the long-term impact for each antipsychotic
Aripiprazole lauroxil (AL), a long-acting injectable atypical antipsychotic, is approved for the treatment of schizophrenia, with efficacy and safety being established in a phase 3, 12-week, randomized, double-blind, placebo-controlled trial2-4
Here, we report the long-term metabolic and endocrine profiles of outpatients with schizophrenia participating in a one-year, open-label study of monthly AL
Methods
This was an international, 52-week, open-label study (ClinicalTrials.gov Identifier: NCT01626456) to assess the long-term safety and durability of the therapeutic effects of AL monotherapy in patients with stable schizophrenia
-
Eligible patients entered the study in one of two ways:
○ Individuals who had completed the 12-week, double-blind, placebo-controlled study (n = 236)2
○ Those who started AL for clinically stable schizophrenia (Clinical Global Impression–Severity score of ≤3 [mild] at screening) as outpatients and were treated with a first-line oral antipsychotic who had no prior exposure to AL (de novo AL patients; n = 242)
Enrolled patients received active AL by intramuscular gluteal injection (441 mg or 882 mg) every 4 weeks for maintenance treatment of schizophrenia more than one-year (total of 13 injections over 52 weeks)
-
Dose and starting regimen depended on how the patients had entered into the study:
○ Patients who had been assigned to one of the two active AL doses (441 mg or 882 mg) in the prior 12-week study continued on the same blinded dose and received oral placebo for the first 3 weeks
○ Patients who had received placebo in the prior 12-week study were randomized to one of the two AL doses, along with oral aripiprazole (15 mg) for the first 3 weeks
○ All de novo patients received AL 882 mg along with oral aripiprazole (15 mg) for the first 3 weeks
-
Changes in metabolic parameters (weight, fasting blood sugar, lipids) and serum prolactin were assessed over the duration of AL exposure
○ The baseline values for this analysis were obtained from the visit before the first active AL injection (taking into account differences based on study entry)
○ Patients were followed for the full year of the long-term study unless they discontinued early
○ Data presented are last observation carried forward from baseline to last visit
Results
Patient Disposition and Baseline Characteristics
In total, 478 patients participated in the study (236 from the prior AL study and 242 de novo; AL 441 mg, n = 110 and AL 882 mg, n = 368; Figure 1)
-
The majority of the patients remained for most of the follow-up period, with 86% (n = 409) remaining at 6 months and 68% (n = 326) completing the one-year treatment period
○ The most common reason for early termination was withdrawal by patient: 19.1% (n = 21) and 12.5% (n = 46) of patients in the 441 mg and 882 mg groups, respectively
A higher percentage of patients in the 882 mg group discontinued due to AEs compared with patients in the 441 mg group; the majority of patients in the 882 mg group who discontinued were de novo patients (19 of 27 patients) who had not had prior AL exposure before entering this study
The demographics and baseline characteristics presented by pre-enrollment status were comparable across all pre-enrollment groups (Table 1)
The majority of patients were male (58%), with a median age of 39 years, and patients were predominantly white (64%)
Figure 1.
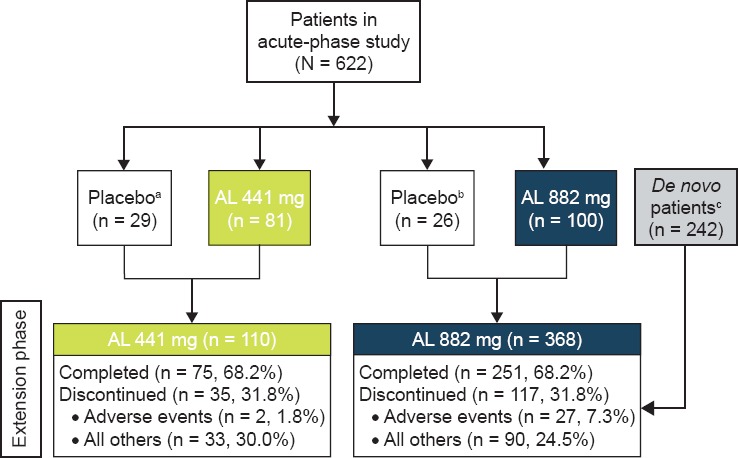
Patient Disposition
Table 1. Demographics and Baseline Characteristics.
| TREATMENT IN ACUTE-PHASE STUDY | |||||
|---|---|---|---|---|---|
| PRE-ENROLLMENT STATUS | PLACEBO (LOW-VOLUME) N = 29 | AL 441 MG N = 81 | PLACEBO (HIGH-VOLUME) N = 26 | AL 882 mg n = 100 | DE NOVO STABLE OUTPATIENT (ORAL ANTIPSYCHOTICS) N = 242 |
| DOSAGE | AL 441 MGa Q4WK (N = 110) | AL 882 MGb Q4WK (N = 368) | |||
| Age, years | 36.3 (11.4) | 38.8 (10.7) | 38.4 (12.4) | 38.9 (11.4) | 40.3 (11.9) |
| Male sex, n (%) | 17 (58.6) | 48 (59.3) | 15 (57.7) | 64 (64.0) | 131 (54.1) |
| BMI, kg/m2 | 26.9 (5.0) | 26.6 (4.8) | 25.6 (4.9) | 26.2 (5.4) | 27.6 (5.4) |
| Weight, kg | 77.7 (17.2) | 74.6 (15.3) | 73.0 (22.0) | 75.8 (19.7) | 80.8 (17.7) |
Notes: aComprising patients on low-volume placebo and AL 441 mg from the acute study assigned to receive AL 441 mg in the long-term study.
bComprising patients on high-volume placebo and AL 882 mg from the acute study, as well as de novo patients, assigned to receive AL 882 mg in the long-term study. All values are expressed as mean (±SD), unless otherwise indicated.
Abbreviations: AL, aripiprazole lauroxil; BMI, body mass index; q4wk, every 4 weeks; SD, standard deviation.
Overall Adverse Events
-
Overall, treatment-emergent AEs (TEAEs) were reported in just greater than 50% (n = 241) of patients, with the majority being mild or moderate in intensity (93% of all TEAEs); incidence of TEAEs was similar in the 441 mg and 882 mg groups
○ The most frequently reported TEAEs in ≥2% of patients were insomnia (8.4%), increased weight (5%), and anxiety (4.4%)
○ TEAEs associated with injection-site reactions were reported in 4.8% of patients (one patient [0.9%] in the 441 mg group and 22 patients [6.0%] in the 882 mg group)
No serious adverse events (SAEs) were reported for AL 441 mg group, while 15 patients (4.1%) reported SAEs in the AL 882 mg group. In the AL 882 mg group, the majority of the SAEs (n = 9) and the majority of SAEs leading to discontinuation (n = 6) were reported in the de novo group, who had not had prior AL exposure before entering the safety study
There were two deaths during the study period: one death was due to cardiopulmonary arrest and the other death was a suicide
Body Weight and Body Mass Index
Overall, the mean (standard deviation [SD]) weight change was +0.8 (5.9) kg; the mean body weight of patients by dose group are presented in Figure 2
A total of 10.9% (n = 52) of patients had a ≥10% increase in body weight from baseline; of these 52 patients, 15 (13.6%) and 37 (10.1%) patients were from the 441 mg and 882 mg groups, respectively
Overall, 6.4% (n = 7) of patients in the 441 mg group and 4.6% (n = 17) patients in the 882 mg group experienced TEAEs of increased weight, whereas 2.7% (n = 3) and 2.4% (n = 9) of patients reported TEAEs of decreased weight, respectively
Among all patients, long-term exposure to AL resulted in a small mean increase in body mass index score (0.3) from baseline to the last post-baseline assessment in the total population
Figure 2.
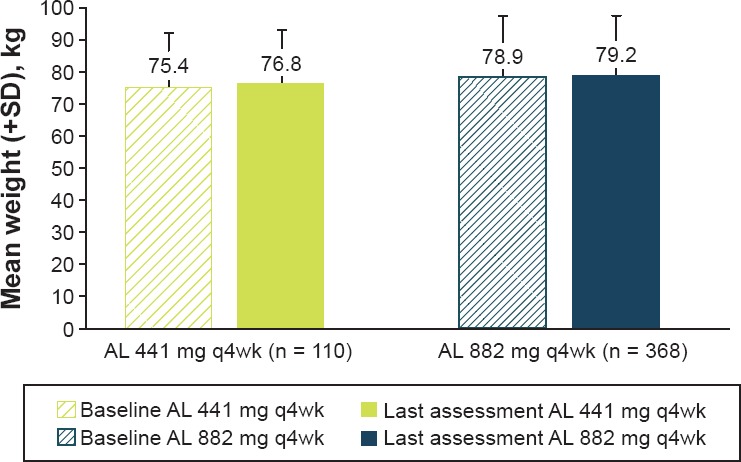
Mean Body Weight at Baseline* and Last Post-Baseline Visit
Metabolic Parameters
The mean (SD) changes from baseline in the overall population were: +1.1 (27.5) mg/dL for glucose, +0.07 (0.6) % for glycated hemoglobin (HbA1c), −3.3 (35.8) mg/dL for total cholesterol and −5.3 (101.9) mg/dL for triglycerides. Changes in metabolic parameters from baseline by dose group are presented in Table 2
TEAEs related to metabolic parameters were reported in four (3.6%) patients in the 441 mg group and 18 (4.9%) patients in the 882 mg group. The most frequently reported TEAE related to metabolic parameters in the 441 mg group was HbA1c increased (n = 3); the most frequently reported TEAEs in the 882 mg group were hyperglycemia (n = 4), increased HbA1c, and diabetes mellitus (n = 3 each)
Table 2. Baseline Values and Changes in Fasting Metabolic Parameters.
| PARAMETER* | AL 441 MG Q4WK (N = 110) | AL 882 MG Q4WK (N = 368) |
|---|---|---|
| Cholesterol, mg/dL | ||
| Baseline | 193.9 (45.8) | 190.8 (40.4) |
| Change† | −3.6 (37.5) | −3.2 (35.4) |
| HDL cholesterol, mg/dL | ||
| Baseline | 49.6 (16.1) | 52.8 (15.3) |
| Change† | 1.3 (11.9) | 0.9 (12.4) |
| LDL cholesterol, mg/dL | ||
| Baseline | 112.0 (36.3) | 111.1 (34.6) |
| Change† | −1.1 (32.5) | −3.1 (29.8) |
| Triglycerides, mg/dL | ||
| Baseline | 156.3 (111.7) | 134.0 (90.9) |
| Change† | −16.1 (89.1) | −2.0 (105.3) |
| Glucose, mg/dL | ||
| Baseline | 91.0 (16.6) | 92.1 (22.3) |
| Change† | 0.3 (21.0) | 1.4 (29.2) |
| HbA1c, % | ||
| Baseline | 5.36 (0.4) | 5.47 (0.5) |
| Change† | 0.05 (0.2) | 0.07 (0.7) |
| BMI, kg/m2 | ||
| Baseline | 26.7 (4.9) | 27.1 (5.4) |
| Change† | 0.5 (2.4) | 0.3 (1.9) |
| Body weight, kg | ||
| Baseline | 75.4 (15.8) | 78.9 (18.7) |
| Change† | 1.3 (6.9) | 0.7 (5.5) |
| Increase of ≥7% at any visit post-baseline, n (%) | 23 (20.9) | 65 (17.7) |
| Decrease of ≥7% at any visit post-baseline, n (%) | 13 (11.8) | 46 (12.5) |
Notes: Values are means of the observed differences between baseline and last study visit of AL. *Values expressed as mean (±SD) unless otherwise noted; †change to last post-baseline value during the treatment period.
Abbreviations: AL, aripiprazole lauroxil; BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Prolactin
Changes in plasma prolactin levels from baseline (Figure 3) were −8.7 ng/mL (14.7) for men and −14.9 (43.4) ng/mL for women
Five (1.4%) patients in the 882 mg group reported TEAEs that were associated with prolactin; none were reported in the 441 mg group. None of the TEAEs were rated as severe; four of the five patients completed the study
Ten (6.6%) male and 21 (19.1%) female patients had potentially clinically significant (PCS) prolactin values at any post-baseline visit that were ≥1 to ≥3 × the upper limit of normal (ULN) (Table 2). Two female patients had PCS values ≥3 × ULN, but both completed the study and neither had any TEAEs associated with prolactin
Figure 3.
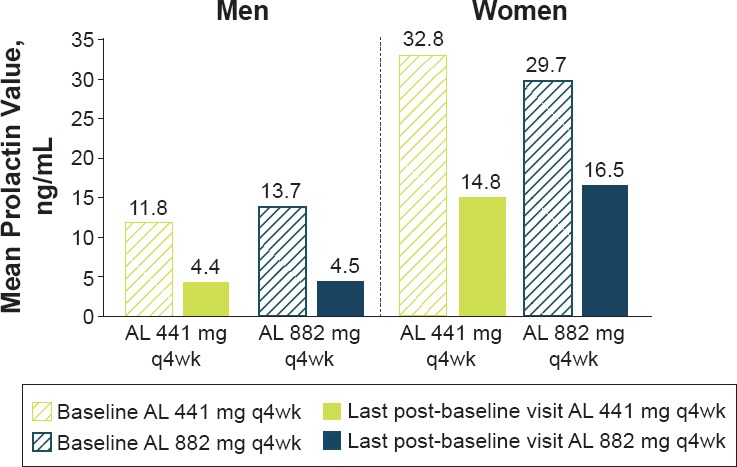
Plasma Prolactin* Levels at Baseline and Last Post-Baseline Visit by Sex
Limitations
The lack of a comparator group
The inclusion of only patients who successfully completed the initial 12-week study may limit the generalizability of the study results. However, inclusion of newly enrolled (de novo) patients allowed for the evaluation of AL in an unselected cohort of patients new to AL therapy
Conclusions
Overall, the incidence of SAEs was low and consistent with the safety profile of aripiprazole
Long-term treatment (for up to 15 months) with AL in outpatients with schizophrenia was associated with a slight lowering of serum prolactin for both sexes and small changes in average weight, fasting glucose, lipids, and HbA1c values
There was no clinically meaningful difference between any of the metabolic or prolactin parameters and AL dose
Overall, the metabolic, weight, and endocrine effects reported here are consistent with long-term effects associated with oral aripiprazole
AL was well tolerated with a low-risk metabolic profile and represents an additional treatment option for the long-term treatment of schizophrenia
Acknowledgments
The authors thank all the patients, investigators, and study coordinators who participated and contributed to this study. The authors would also like to thank ChihChin Liu and Yangchun Du for statistical support. Medical writing and editorial support for the preparation of this poster was provided by Karen Yee, PhD (ApotheCom, London, UK) and supported by Alkermes, Inc.
Footnotes
Presented at the American Psychiatric Association Annual Meeting, May 20–24, 2017, San Diego, CA.
Disclosures
This study was funded by Alkermes, Inc.
References
- 1.Rummel-Kluge C et al. Schizophr Res. 2010;123:225, 233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ARISTADA® USPI. 2017 https://www.aristadahcp.com/downloadables/ARISTADA-PI.pdf. Accessed May 2017. [Google Scholar]
- 3.Meltzer HY et al. J Clin Psychiatry. 2015;76:1085–1090. doi: 10.4088/JCP.14m09741. [DOI] [PubMed] [Google Scholar]
- 4.Nasrallah HA et al. J Clin Psychiatry. 2016;77:1519–1525. doi: 10.4088/JCP.15m10467. [DOI] [PubMed] [Google Scholar]


