Abstract
Background
Valbenazine (VBZ, NBI-98854) is a novel vesicular monoamine transporter 2 (VMAT2) inhibitor approved for the treatment of tardive dyskinesia (TD). The KINECT 3 study (NCT02274558) evaluated the effects of VBZ on TD in subjects with mood disorder or schizophrenia/schizoaffective disorder (SCHZ, presented separately) who received up to 48 weeks of treatment.
Methods
KINECT 3 included: 6-week, double-blind, placebo (PBO)-controlled (DBPC) period (205 completers); 42-week VBZ extension (VE) period (124 completers); 4-week washout period (121 completers). Subjects entering the DBPC were randomized 1:1:1 to once-daily VBZ 80 mg, VBZ 40 mg, or PBO; stable concomitant antipsychotic medication regimens were allowed. Subjects completing the DBPC and entering the VE period were re-randomized (blinded) from PBO to VBZ (80 or 40 mg) or continued VBZ treatment at the same dose. Efficacy assessments included: mean changes from baseline in Abnormal Involuntary Movement Scale (AIMS) total score (items 1–7); mean Clinical Global Impression of Change (CGI-TD) scores; AIMS responders (subjects with ≥50% score reduction from baseline); and CGI-TD responders (subjects with score ≤2 [“much improved” or “very much improved”]). Treatment effect sizes (Cohen’s d) and numbers needed to treat (NNTs) were analyzed for DBPC outcomes.
Results
Efficacy analyses were conducted in 77 subjects (DBPC) and 73 subjects (VE) with a mood disorder. At Week 6 (end of DBPC), AIMS mean score improvements were greater in the VBZ groups (in a dose-related pattern) than in the PBO group (80 mg, -3.6, d = 0.94; 40 mg, -2.4, d = 0.55; PBO, -0.7). AIMS mean score changes at Week 48 (end of VE) showed continued TD improvement during long-term VBZ treatment (80 mg, -5.8; 40 mg, -4.2). By Week 52 (end of washout), AIMS mean scores in both dose groups were returning toward baseline levels, indicating re-emergence of TD. CGI-TD scores showed a similar pattern: Week 6 (80 mg, 2.7, d = 0.64; 40 mg, 2.9, d = 0.39; PBO, 3.2), Week 48 (80 mg, 2.0; 40 mg, 2.2), Week 52 (80 mg, 3.6; 40 mg, 2.8). AIMS responder rates (≥50% score reduction) were greater with VBZ vs PBO at Week 6 (80 mg, 38.5%, NNT = 4; 40 mg, 19.0%, NNT = 9; PBO, 7.7%), were increased at Week 48 (80 mg, 56.0%; 40 mg, 33.3%), and lower after VBZ washout (Week 52 80 mg, 16.7%; 40 mg, 27.8%). CGI-TD responder rates followed a similar pattern: Week 6 (80 mg, 34.6%, NNT = 6; 40 mg, 28.6%, NNT = 8; PBO, 15.4%), Week 48 (80 mg, 80.0%; 40 mg, 61.1%), Week 52 (80 mg, 25.0%; 40 mg, 44.4%).
Conclusion
Sustained TD improvements were found in subjects with a mood disorder who received up to 48 weeks of VBZ, with TD reverting toward baseline severity when assessed 4 weeks after treatment withdrawal. Together with results from SCHZ subjects and the long-term safety profile (presented separately), these results indicate that long-term VBZ can be beneficial for managing TD regardless of psychiatric diagnosis.
Keywords: amines, antipsychotic agents, double-blind method, mood disorders, psychopharmacology, psychotic disorders, schizophrenia, tardive dyskinesia, valbenazine
Introduction
Tardive dyskinesia (TD) is a persistent movement disorder associated with prolonged exposure to dopamine receptor blocking agents (DRBAs), such as antipsychotics1
Antipsychotics are often used as adjunctive therapies in patients with mood disorder, and it is important to evaluate TD treatment in this population
Valbenazine (INGREZZA) is a novel and highly selective inhibitor of vesicular monoamine transporter 2 (VMAT2), which is the first and only FDA-approved product indicated for the treatment of adults with TD
Objective
To evaluate the effects of once-daily valbenazine (40 mg or 80 mg) in participants with TD and a mood disorder (e.g., major depressive disorder, bipolar disorder) who received up to 48 weeks of treatment in the KINECT 3 study (NCT02274558)
Methods
Study Design
-
KINECT 3 included a double-blind, placebo-controlled (DBPC) period (6 weeks),2 followed by a double-blind valbenazine extension (VE) period (42 weeks), and a post-treatment (drug-free) 4-week follow-up period (Figure 1)
○ Participants initially randomized to valbenazine (40 or 80 mg) in the DBPC period continued to receive the same dose during the VE period
○ Participants initially randomized to placebo in the DBPC period were re-randomized (1:1) to valbenazine 40 or 80 mg for the VE period; those re-randomized to valbenazine 80 mg received 40 mg during the first week and 80 mg thereafter
○ All participants, investigators, and central Abnormal Involuntary Movement Scale (AIMS) video raters were blinded to valbenazine dose during the VE period
Figure 1.

Study Design
Participants
-
Key inclusion criteria
○ Adults aged 18–85 years with a Diagnostic and Statistical Manual of Mental Disorders (e.g., DSM-IV) diagnosis of schizophrenia/schizoaffective disorder or mood disorder, and Brief Psychiatric Rating Scale (BPRS) score <50 at screening
○ DSM-IV diagnosis of DRBA-induced TD for ≥3 months prior to screening
○ Moderate or severe TD, as qualitatively assessed by a blinded, external reviewer using a video of the participant’s AIMS assessment at screening
-
Key exclusion criteria
○ Active, clinically significant, and unstable medical condition within 1 month prior to screening
○ Montgomery-Åsberg Depression Rating Scale (MADRS) score >13, and Young Mania Rating Scale (YMRS) score >10, both at screening or baseline
○ Comorbid movement disorder (e.g., parkinsonism, akathisia, truncal dystonia) that is more prominent than TD
○ Significant risk for active suicidal ideation, suicidal behavior, or violent behavior
○ Stable doses of concomitant medications to treat psychiatric disorders were allowed throughout the study
Analyses
Analyses were conducted in the intent-to-treat (ITT) population (i.e., all participants who received study treatment and had ≥1 post-baseline AIMS assessment)
The mood disorder subgroup was analyzed in the ITT population
-
Outcomes included
-
○ AIMS mean score change from baseline and Clinical Global Impression of Change-Tardive Dyskinesia (CGI-TD) mean score (overall ITT population and mood disorder subgroup)
◼ Analyzed by study visit (Weeks 6, 8, 16, 32, 48, 52)
◼ AIMS scoring was based on consensus of 2 central AIMS video raters who were blinded to treatment group and sequence of visits
◼ CGI-TD scoring was conducted by the site investigator who was blinded to treatment group
-
○ Response analyses (mood disorder subgroup):
◼ Analyzed by study visit
◼ AIMS response: ≥50% improvement from baseline in AIMS total score
◼ CGI-TD response: score 1 (very much improved) or 2 (much improved)
-
Results
Participants
205 participants completed the 6-week DBPC period, 198 entered the VE period, 124 completed the VE period, and 121 completed the post-treatment (drug-free) follow-up period
In the participants with mood disorder who received ≤1 dose of treatment during the DBPC, baseline characteristics were generally similar across treatment groups (Table 1)
Table 1. Baseline Demographics and Participant Characteristics in the Mood Disorder Subgroupa.
| CHARACTERISTIC | PLACEBO (N = 26) | VALBENAZINE 40 MG (N = 24) | VALBENAZINE 80 MG (N = 27) |
|---|---|---|---|
| Age, mean years, (SD) | 57.4 (11.6) | 54.7 (9.1) | 54.5 (11.1) |
| Male, n (%) | 8 (30.8) | 11 (45.8) | 10 (37.0) |
| White, n (%) | 23 (88.5) | 18 (75.0) | 17 (63.0) |
| Black, n (%) | 2 (7.7) | 5 (20.8) | 8 (29.6) |
| BMI, kg/m2, mean (SD) | 28.3 (4.7) | 28.9 (5.5) | 28.8 (6.2) |
| Age at TD diagnosis, years, mean (SD) | 51.6 (11.8) | 48.7 (9.3) | 47.6 (10.4) |
| BPRS score at screening, mean (SD) | 24.3 (5.9) | 26.7 (5.8) | 26.6 (6.2) |
| AIMS score, mean(SD) | 11.2 (3.6) | 11.4 (3.5) | 10.9 (3.8) |
Notes: aln the safety population, defined as all participants who received ≥1 dose of assigned study drug; no statistical testing between treatment groups.
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BMI, body mass Index; BPRS, Brief Psychiatric Rating Scale; SD, standard deviation; TD, tardive dyskinesia.
Efficacy
-
AIMS mean score changes in the overall ITT population
○ Week 6, least squares (LS) mean changes from baseline: 80 mg, −3.2 (P < 0.0001, statistically significant primary outcome per fixed-sequence testing procedure2); 40 mg, −1.9 (P < 0.01); placebo, −0.1
○ Week 48, mean changes from baseline: 80 mg, −4.8; 40 mg, −3.0; no statistical testing between dose groups
○ Mean scores increased from Week 48 (80 mg, 6.2; 40 mg, 6.8) to Week 52 (80 mg, 9.8; 40 mg, 8.4), indicating that TD symptoms returned toward baseline levels during the 4-week period following discontinuation of valbenazine
A similar pattern of results was found in the mood disorder subgroup, although AIMS score changes indicated a greater magnitude of TD improvements in these participants relative to the overall ITT population (Figure 2)
-
CGI-TD mean scores in the overall ITT population
○ Week 6, LS mean scores: 80 mg, 2.9; 40 mg, 2.9; placebo, 3.2; no statistically significant difference
○ Week 48, mean scores: 80 mg, 2.1; 40 mg, 2.4; no statistical testing between dose groups
○ Mean scores at Week 52 (80 mg, 3.5; 40 mg, 3.1) were higher than those at Week 48, indicating worsening of TD severity after valbenazine was discontinued
A similar pattern of results was found in the mood disorder subgroup, with a P < 0.05 found at Week 6 in the valbenazine 80 mg group (Figure 3)
-
In the mood disorder subgroup, the percentage of participants with achieving AIMS response (≥50% total score improvement from baseline) remained relatively constant from Weeks 8 to 48 in the 80 mg group and increased over time in the 40 mg group (Figure 4A)
○ At Week 52 (i.e., 4 weeks after valbenazine was discontinued), AIMS response rates dropped to below the response rates at Week 6 (i.e., end of DBPC) with valbenazine 80 mg, but remained higher at Week 6 with valbenazine 40 mg
Similarly, rates of CGI-TD response (score ≤2) in the mood disorder subgroup increased during the VE period for both dose groups but decreased by the end of the drug-free follow-up period (Figure 4B)
Figure 2.
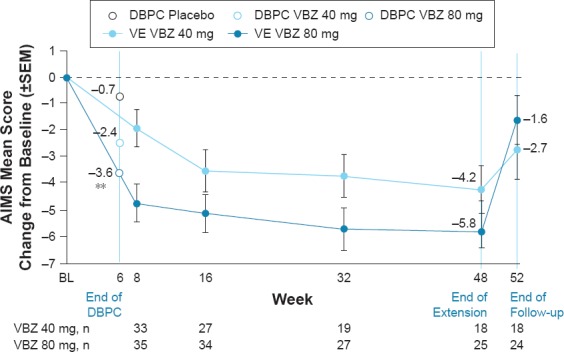
AIMS Mean Score Change from Baseline by Study Visit (Mood Disorder Subgroup)
Figure 3.
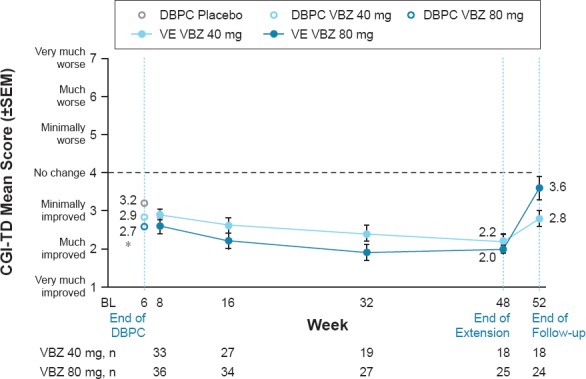
CGI-TD Mean Score by Study Visit (Mood Disorder Subgroup)
Figure 4.
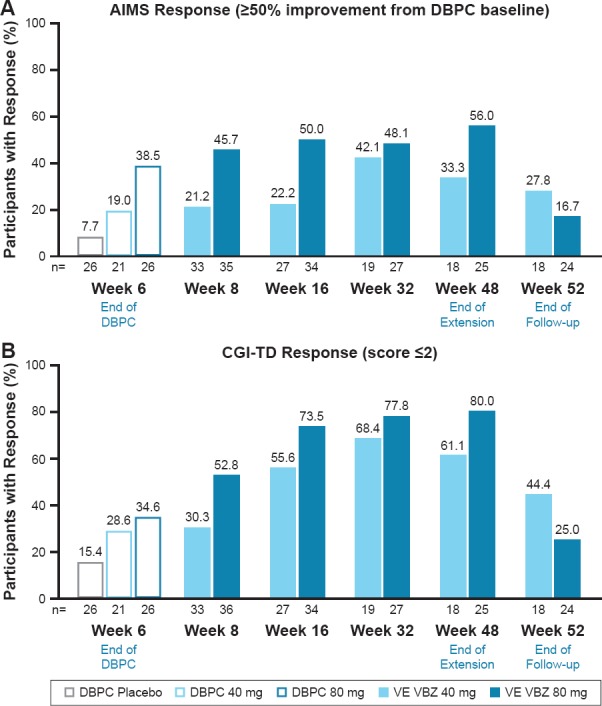
Response Rates by Study Visit (Mood Disorder Subgroup)
Conclusions
-
TD improvements with once-daily valbenazine appeared similar between the overall study population and the subgroup of participants with mood disorder
○ At end of the DBPC period, AIMS mean score changes from baseline were significantly greater with valbenazine as compared with placebo
○ AIMS and CGI-TD results, including response rates, from the VE period indicate sustained TD improvements in participants who received valbenazine for up to 48 weeks
○ After treatment was discontinued, TD severity reverted toward baseline levels and response rates declined
Together with results from participants with schizophrenia/schizoaffective disorder (poster #P5-010), these results indicate that long-term valbenazine may be beneficial for managing TD regardless of psychiatric diagnosis
Footnotes
Presented at the American Psychiatric Association Annual Meeting, May 20–24, 2017, San Diego, CA.
Disclosures
Medical writing and editorial assistance was provided by Prescott Medical Communications Group, Inc., Chicago, IL.
References
- 1.Vijayakumar D, Jankovic J. Drugs. 2016;76:779, 787. doi: 10.1007/s40265-016-0568-1. [DOI] [PubMed] [Google Scholar]
- 2.Hauser RA, Factor SA, Marder SR et al. Am J Psychiatry. 2017;174:476, 484. doi: 10.1176/appi.ajp.2017.16091037. [DOI] [PubMed] [Google Scholar]


