Abstract
Healthcare acquired infections (HAI) pose a great threat in hospital settings and environmental contamination can be attributed to the spread of these. De-contamination and, significantly, prevention of re-contamination of the environment could help in preventing/reducing this threat. Goldshield (GS5) is a novel organosilane biocide marketed as a single application product with residual biocidal activity. We tested the hypothesis that GS5 could provide longer-term residual antimicrobial activity than existing disinfectants once applied to surfaces. Thus, the residual bactericidal properties of GS5, Actichlor and Distel against repeated challenge with Staphylococcus aureus ATCC43300 were tested, and showed that GS5 alone exhibited longer-term bactericidal activity for up to 6 days on 316I stainless steel surfaces. Having established efficacy against S. aureus, we tested GS5 against common healthcare acquired pathogens, and demonstrated that, on average, a 1 log10 bactericidal effect was exhibited by GS5 treated surfaces, although biocidal activity varied depending upon the surface type and the species of bacteria. The ability of GS5 to prevent Pseudomonas aeruginosa biofilm formation was measured in standard microtitre plate assays, where it had no significant effect on either biofilm formation or development. Taken together the data suggests that GS5 treatment of surfaces may be a useful means to reducing bacterial contamination in the context of infection control practices.
Introduction
Healthcare acquired infections (HAIs) are directly and indirectly responsible for increased morbidity and mortality rates in hospitals worldwide. In Europe alone there are >4.5 million cases annually, which result in >37,000 deaths [1]. A further consequence is the financial burden associated with these infections, measured in terms of increased length of patient stay, decreased bed availability as a result and the extra cost of antibiotic therapy to treat the infection. In the USA alone the total annual expenditure on HAI is estimated to be in excess of $9.8 billion (£6–7 billion) [2], while in Europe a figure of over €7 billion (~£5.5 billion) has been proposed [3]. As a consequence, there is increasing interest from industrial, research and development and healthcare sectors in the development of viable and cost-effective alternative methods of reducing HAI.
Common healthcare associated pathogens include Staphylococcus aureus (and predominantly Methicillin resistant Staphylococcus aureus (MRSA)), Vancomycin-resistant Enterococci (VRE), Clostridium difficile, and Pseudomonas aeruginosa. Such microorganisms have been shown to survive on inanimate surfaces for extended periods of time—for example S. aureus has been shown to survive as long as 6 months [4,5] while Enterococci can survive as long as 4 months [6]. Clostridium difficile infections (CDI), the most common HAI type in Europe [7] are attributed in part to the persistence of infectious spores on hospital surfaces for up to 5 months [5]. Bacteria capable of forming biofilms, such as P. aeruginosa and S. aureus, also survive and persist in the environment due to this ability, on top of any intrinsic resistance to antimicrobials [8]. Thus vegetative cells, spores, or biofilms present a threat of infection and indeed a recent report identified biofilm within water taps as the cause of a series of neonatal P. aeruginosa infections [9].
Evidence of a direct correlation between environmental contamination and infection rates exists [5,10,11,12] and microbial contamination of the environment has been shown to act as a source of infection that is directly responsible for transmission of organisms to patients [12]. The most problematic areas tend to be high-touch points such as bed rails, door handles, table top surfaces, bedding (mattress), television controls and staff uniforms [13]. Such contaminated surfaces act as a source of direct to patient, and indirect—via healthcare workers/instruments—spread to patients [5,14]. As long as these organisms persist in a hospital or healthcare facility environment they remain a source of infection and therefore, hospitals have implemented revised and improved infection control practices in order to reduce and ideally eradicate environmental microbial contamination. This is achieved primarily by the use of disinfectants and detergents, although the precise disinfectant used will be dependent on multiple factors. For example, areas of high risk such as operating theatres will require multiple cleans per day, whereas patient waiting rooms may be cleaned only once per day. The choice of disinfectant agent is also multifactorial: body fluid spillages will normally require higher level disinfectants than those used in routine cleaning. As a result, hospitals will use a variety of products including ethyl alcohol in hand rubs and gels, Quaternary ammonium compounds (QACs), chlorine-releasing agents and peroxygen sterilants [15]. Nonetheless, current cleaning methods have in several instances been shown to be ineffective. Work by French et al. [11] showed that 74% of sites in a London hospital were MRSA positive and when these same sites were retested post-cleaning, all were still contaminated [11]. Recurrence of contamination on surfaces, post disinfection, is therefore a significant issue and this is especially true of high-touch surfaces [16]. Given the available evidence for the ineffectiveness of cleaning and rapid recontamination of surfaces, there is currently much interest in alternative approaches to the problem. The development of intrinsically anti-microbial surfaces that incorporate a variety of agents to kill microbes may be considered a useful strategy. Alternatively, the use of specialised agents that are capable of preventing surface contamination, or that exhibit a residual antimicrobial activity post-disinfection, could be employed, and such products have recently been highlighted as of potential utility in the healthcare setting [17].
One such antimicrobial product is Goldshield, distributed by Goldshield Technologies Ltd. [GS hereinafter]. This is a patented, water soluble organosilane, coupled with a quaternary ammonium compound that is designed to coat surfaces with a protective antimicrobial layer to prevent microbial contamination. The product was originally designed at Emory University, USA and is the subject of three US patents (patent nos. US5,959,014, US6,221,944, and US6,632,805). In this paper we report the bactericidal and anti-biofilm of GS5 technology against 11 common healthcare associated pathogens.
Materials and methods
Chemicals, glassware and media
All glassware was sterilised by soaking overnight in 1% Virkon (Antec, UK) and steam sterilised in an autoclave prior to use. All culture media (Oxoid, UK) was prepared as per the manufacturer’s instructions. Phosphate Buffered Saline (Oxoid, UK) was prepared in deionised water and steam sterilised in an autoclave prior to use. Two model surfaces were used. 316l Steel (Aalco, UK) or Formica were cut into 2cm×2cm samples, autoclaved (121°C for 15 min) and stored in a sealed sterile container prior to use.
Microorganisms
Ten bacterial species were obtained from either the American Type Culture Collection (ATCC) or the Leibniz-Institute DSMZ German Collection of Microorganisms and Cell Cultures (DSMZ). Bacteria included Escherichia coli ATCC25922, Klebsiella pneumoniae DSM16358, Mycobacterium smegmatis DSM43469, Pseudomonas aeruginosa DSM3227, Staphylococcus aureus (MRSA) ATCC43300, Staphylococcus aureus (non-MRSA) DSM20231, Staphylococcus epidermidis DSM28319 (all cultured at 37°C using Nutrient broth/agar), Enterococcus faecalis DSM12956 (37°C using Tryptone soya broth/agar), Burkholderia multivorans DSM13243 (28°C using Nutrient broth/agar) and Acinetobacter baumannii DSM30008 (30°C using Nutrient broth and agar). These were chosen as representative organisms of the type causing HAIs commonly seen in hospitals [18] and included Gram positive organisms, Gram negative organisms and Mycobacteria. Mycobacterium smegmatis was used as it is a fasting-growing model Mycobacterium species [19]. Organisms were stored on Cryobeads (Technical Service Consultants Ltd, UK) at -80°C and recovered in suitable media when required.
Disinfectant agents
Three disinfectant agents used (GS5, Actichlor and Distel) are classed bactericidal surface disinfectants. The characteristics of these antimicrobial agents are summarised in Table 1. Agents were acquired as full strength concentrate and working stock concentrations were prepared by dilution with deionised water as per the respective manufacturer’s instructions.
Table 1. Antimicrobial products tested.
| Agent | Type | Active ingredient | Concentration used* |
|---|---|---|---|
| Goldshield5 | Organosilane coupled with Quaternary Ammonium Compound (siQAC) | Trihydroxysilylpropyldimethyloctadecyl ammonium chloride | 1:20 dilution |
| Actichlor | Chlorine-based disinfectant | Sodium dichloroisocyanurate | 1:10 dilution |
| Distel | Quaternary Ammonium Compound | Tertiary alylamine and quaternary ammonium compounds | 1:100 dilution |
* as per manufacturer’s instructions.
Direct bactericidal assessment of GS5
To determine directly the bactericidal activity of GS5, a suspension contact time assay was completed; varying concentrations of GS5 were mixed with S. aureus ATCC43300, followed by recovery and enumeration of viable cells to determine Log10 reduction. 0% (sterile water), 0.25% (v/v), 0.5% (v/v) and 1% (v/v) GS5 dilutions were prepared using sterile water as diluent. A 10 μl aliquot of mid-log S. aureus ATCC43300 was mixed with each of the GS5 concentrations and left to stand at room temperature for 5 min. Bacteria were enumerated by dilution plating 0.1ml aliquots onto Nutrient agar in duplicates and incubating at 37°C for 24 h followed by direct colony counts. Three biologically independent experiments were performed.
Residual surface activity of disinfectants
To investigate the residual activity of surface disinfectants a protocol was developed from the EN13697 standard and the work of Baxa et al. [20]. Staphylococcus aureus ATCC43300 (MRSA) and 316l Steel were used. The 316l Steel surface samples were sprayed with either GS5, Actichlor, Distel or sterile water (no treatment control) using a hand spray. The test surfaces were left to dry in the sterile environment of a category 2 cabinet (Biomat). S. aureus ATCC43300 was grown to mid log phase of growth (OD600 = ~0.48) and diluted 1/100 using sterile PBS (Oxoid, UK). A total of 100 μl of this was added (in 10 μl droplets) to technical triplicate examples of each surface. Bacteria were left on the surfaces for 45 min, and then viable cells recovered in 10 ml of sterile PBS by vortexing for 2 min. Bacteria were enumerated by plating dilution series in duplicate on Nutrient Agar and incubating at 37°C for 24 h followed by direct colony counts [20]. Following recovery of bacteria from the surfaces each surface was individually washed using sterile PBS, air dried and stored in a sterile storage box. These surfaces were then re-challenged with S. aureus ATCC43300 as above. This re-challenge was repeated at 3-day intervals over 15 days. Three biologically independent experiments were performed.
GS5 bactericidal surface testing
A selection of 10 different bacteria, representative of important HAI, were individually tested on 316l Steel and Formica. Testing was performed once to determine the maximum antimicrobial effect for a freshly treated surface. The protocol was as described above, but without re-challenge and only the activity of GS5 was assessed.
Assessment of GS5 efficacy against biofilms
Pseudomonas aeruginosa DSM3227 biofilms were grown in 24-well microtiter plates (4 wells per treatment) and these were stained with 0.1% crystal violet to assess the extent of biofilm growth according to established methods [21,22,23]. To determine efficacy of GS5 against biofilm, Thermo Scientific™ Nunc™ Cell-Culture Treated Multidishes, (Thermo Scientific, UK) were pre-treated with either 5% GS5 or sterile water (untreated): wells were soaked with 1 ml of agent for 10 min following which treatment agents were aspirated and plates left to dry in a sterile environment (Biomat category 2 cabinet). An overnight culture of P. aeruginosa DSM3227 was diluted 1/100 (using sterile nutrient broth) and microtitre plate wells inoculated with a 1 ml aliquot following which the plates were incubated aerobically at 37°C. At defined time points (8 h, 12 h, 24 h, 48 h, 72 h and 96 h) biofilm production was assessed. The medium containing planktonic cells was removed and wells stained with 1.5 ml of 0.1% Crystal Violet (Sigma-Aldrich, UK) for 10 min at room temperature. Unbound crystal violet (Sigma-Aldrich, UK) was removed and stained wells washed twice with 2ml sterile PBS following which bound crystal violet was solubilised using 1.5 ml of 30% Acetic Acid (Thermo Scientific, UK) for 30 min at room temperature. A 1 ml aliquot from each well was transferred to a fresh 24-well microtiter plate and the absorbance of the crystal violet measured at 570nm using a FLUROstar Omega plate reader (BMG LABTECH, Europe). Each experiment was repeated on three separate occasions.
Assessment of GS5 effects on bacterial viability in biofilm
Bacterial viability in biofilms was assessed using the BacLight Live/Dead bacterial viability kit (L-7007; Molecular Probes, Eugene, OR) [24,25]. With Baclight, live cells stain green and dead/damaged cells stain red. A stock solution was prepared by mixing 4 μl of component A (1.67 mM Syto9 plus 1.67 mM propidium iodide), 6 μl of component B (1.67 mM syto9 plus 18.3 mM propidium iodide) and 1ml of sterile water as described by Bauer et al. [25]. P. aeruginosa DSM3227 biofilm was grown in 4-well Nunc™ Lab-Tek™ II Chamber Slide™ Systems (Thermo Scientific, UK) pre-treated with either 5% GS5 or sterile deionised water. Slides were inoculated with 1 ml of a 1/100 dilution of overnight culture of P. aeruginosa and incubated aerobically for 24 h and 48 h at 37°C. At each time point excess media and planktonic cells were removed and the wells washed with sterile PBS followed by staining with 200 μl BacLight mix and 100 μl of sterile water. Stained slides were incubated in the dark at room temperature for 30 min following which the wells were then washed with sterile PBS and viewed using ×100 oil immersion on a Nikon ECLIPSE E400 (Nikon) microscope utilising a dual-band emission filter (450–490 nm/510–560 nm). Images were generated using NIS-Elements BR (Nikon) software version 3.22.09. Image J software was used to generate composite (red/green) images of the baclight stained biofilms.
Statistical analysis
For bactericidal testing, log10 changes in viable bacterial numbers, compared to untreated controls was determined. The equation Log Reduction LR = log10 (Ncontrol)–log10 (Ntreated) was used where Ncontrol is total recovery of untreated bacteria and Ntreated is total recovery of treated bacteria. Data was imported to Graphpad Prism 6.01 and charts constructed. Statistical analysis was completed using SPSS v22.
Results
Direct bactericidal assessment of GS5
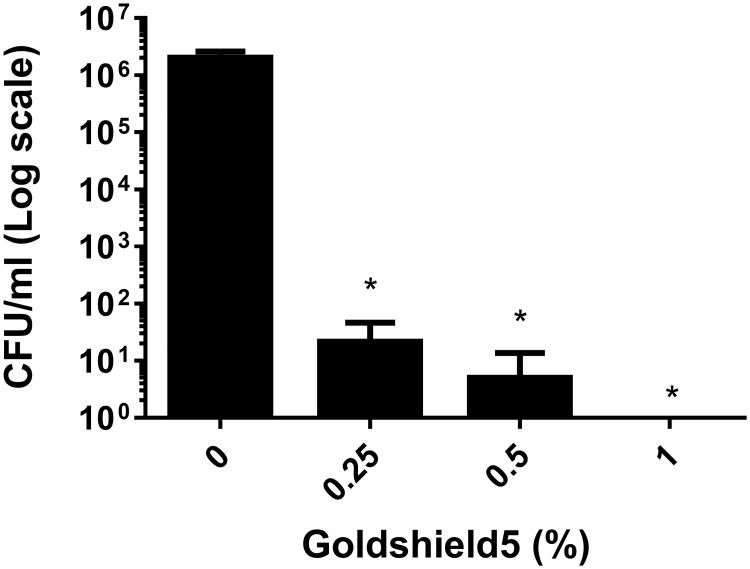
We firstly wished to determine if GS5 was effective against bacteria in solution, prior to surface testing. We hypothesised that a solution of GS5 at working concentration would exhibit a bactericidal effect against a suspension of bacteria. The direct antibacterial effects of GS5 against S. aureus ATCC43300 was assessed using a suspension assay. S. aureus ATCC43300 was challenged with increasing concentrations of GS5 to quantify bactericidal activity. GS5 exhibited bactericidal actions at all concentrations after 5min contact time as shown in Fig 1 (0.25% = 4.96 Log10 reduction; 0.5% = 5.6 Log10 reduction; 1% = 6 Log10 reduction (complete kill). Subsequent testing was completed at 5% as per manufacturer’s instructions.
Fig 1. S. aureus ATCC43300 survival following suspension test using GS5.
~2×106 cfu/ml of S. aureus ATCC43300 was challenged with increasing concentrations of GS5. Data represents mean +/- SD of three independent experiments. Statistical analysis by independent T-tests versus Untreated (0%) controls (* = p<0.05, ** = p<0.005, *** = p<0.001).
Residual activity of surface disinfectants
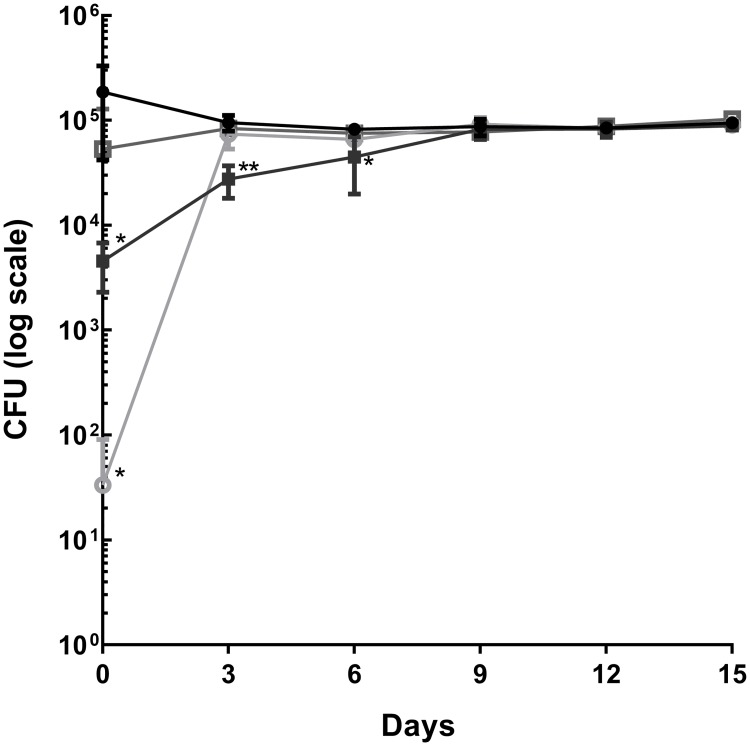
GS5 is reported to form covalent bonds with surfaces, thereby leaving a nanoscale antimicrobial coating which kills microbes that encounter that surface. This, it is claimed, makes GS5 a more effective product due to its residual antimicrobial activity compared to other disinfectants. We designed an experiment to test this hypothesis by determining the residual antimicrobial effect of GS5, Actichlor and Distel. The bactericidal activity of the three surface disinfectant agents was tested against S. aureus ATCC43300 on 316l Steel (Aalco, UK) and residual activity was assessed over 15 days at 3 day intervals. All three products exhibit bactericidal activity on day 0 (Actichlor = 3.75Log10 reduction; Distel = 0.54 Log10reduction; GS5 = 1.16 Log10 reduction). Following subsequent re-challenge of treated surfaces with S. aureus ATCC43300 only GS5 showed significant residual bactericidal activity; this residual activity exerted by GS5 was evident for 6 days (Day 3 GS5 = 0.53 Log10 reduction; Day 6 GS5 = 0.26 Log10 reduction; Fig 2). For subsequent testing of the GS5 product, the maximum effect time point (day 0) was used.
Fig 2. Comparison of residual antimicrobial effects of GS5, Actichlor and Distel on steel surface loaded with Staphylococcus aureus ATCC43300.
GS5 exhibited prolonged antibacterial activity (6 days) whereas Actichlor and Distel showed no antibacterial activity after day 0. Results are representative of three independent experiments (n = 3; mean+/- SD plotted). Statistical analysis using One way ANOVA and Dunnett’s T-test versus Untreated control (* = p<0.05, ** = p<0.005, *** = p<0.001). ▪ = Goldshield; ● = Untreated control; ○ = Actichlor; □ = Distel.
GS5 bactericidal surface testing
Baxa et al. [20] suggested that GS5 exhibited variable effect against different bacterial species. We therefore tested GS5 against a range of healthcare acquired infection microorganisms on 316l Steel or Formica to determine bactericidal effect. As hypothesised, GS5 treated surfaces did indeed exhibit a bactericidal effect against all ten tested microorganisms, and this effect was observed on both Formica and steel. The largest bactericidal effect was observed with Staphylococcus strains where a >1 log10 reduction was observed on 316l Steel (S. aureus ATCC43300 = 1.21 Log10 reduction; S. epidermidis DSM28319 = 1.06 Log10reduction) (Table 2). On Formica, however, the GS5 product exhibited a lower bactericidal effect (<0.5 = Log10reduction) against both Staphylococcus organisms. The average Log10 reduction on steel surfaces for all bacteria tested was 0.6, whereas the average Log10 reduction on Formica was 0.45.
Table 2. Log10 reductions obtained on GS5 treated surfaces challenged with a variety of microbes.
| Organism | Surface | Log10 Untreated ± SD | Log10 Treated ± SD | Log10 change | p-value |
|---|---|---|---|---|---|
| Acinetobacter baumannii DSM30008 | Steel | 4.82 ±0.36 | 4.49 ±0.62 | 0.33* | 0.0138 |
| Formica | 4.25 ±0.04 | 3.67 ±0.29 | 0.58*** | <0.001 | |
| Burkholderia multivorans DSM13243 | Steel | 3.90 ±0.14 | 3.62 ±0.17 | 0.28*** | <0.001 |
| Formica | 3.94 ±0.05 | 3.41 ±0.24 | 0.53** | 0.0011 | |
| Enterococcus faecalis DSM12956 | Steel | 5.27 ±0.3 | 4.8 ±0.08 | 0.47 | 0.0623 |
| Formica | 5.15 ±0.13 | 4.86 ±0.03 | 0.29** | 0.0016 | |
| Escherichia coli ATCC25922 | Steel | 5.57±0.28 | 5.32 ±0.33 | 0.25** | 0.0018 |
| Formica | 5.54 ±0.09 | 5.22 ±0.02 | 0.32*** | <0.001 | |
| Klebsiella pneumoniae DSM16358 | Steel | 4.30±0.27 | 3.54 ±0.33 | 0.76* | 0.0135 |
| Formica | 3.94 ±0.05 | 3.41 ±0.24 | 0.53** | 0.0011 | |
| Mycobacterium smegmatis DSM43469 | Steel | 4.06 ±0.22 | 3.46 ±0.45 | 0.6*** | <0.001 |
| Formica | 5.83 ±0.43 | 5.16 ±0.44 | 0.67** | 0.0026 | |
| Pseudomonas aeruginosa DSM3227 | Steel | 5.09±0.04 | 4.66±0.29 | 0.43** | 0.0017 |
| Formica | 5.15±0.1 | 4.63±0.12 | 0.52*** | <0.001 | |
| Staphylococcus aureus (MRSA) ATCC43300 | Steel | 4.19 ±0.13 | 2.99 ±0.58 | 1.2*** | <0.001 |
| Formica | 5.04 ±0.03 | 4.68 ±0.08 | 0.36*** | <0.001 | |
| Staphylococcus aureus (non-MRSA) DSM20231 | Steel | 4.57±0.22 | 3.48±0.27 | 1.09*** | <0.001 |
| Formica | 5.02±0.23 | 3.94±0.35 | 1.08* | 0.0089 | |
| Staphylococcus epidermidis DSM28319 | Steel | 3.95 ±0.04 | 2.88 ±0.05 | 1.07** | 0.0047 |
| Formica | 5.25 ±0.19 | 4.94 ±0.25 | 0.31*** | <0.001 |
Results are representative of three independent experiments (n = 3; mean+/- SD). p value calculated using paired T-Test (* = p<0.05, ** = p<0.005, *** = p<0.001).
Effect of GS5 on bacterial biofilm formation
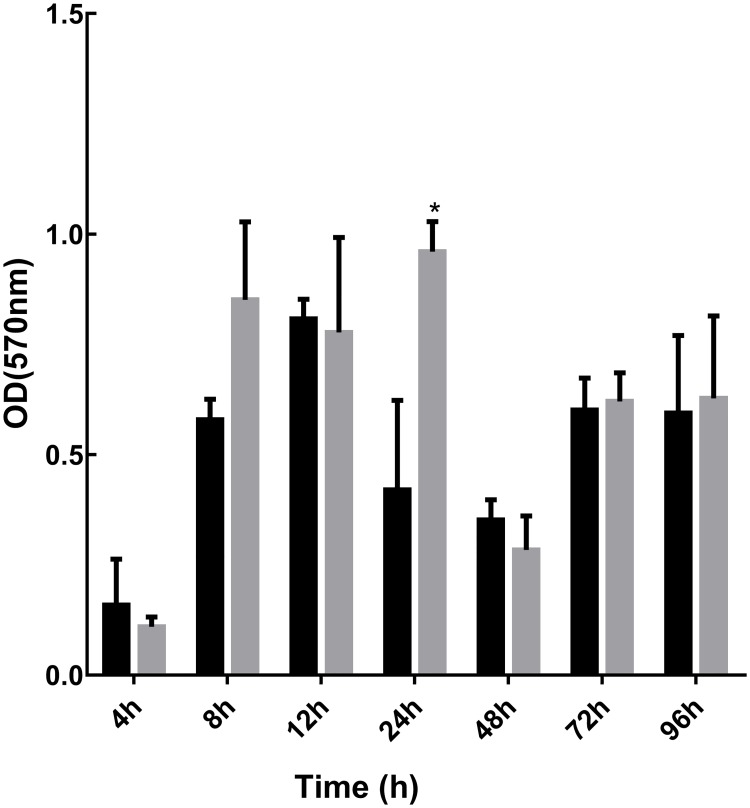
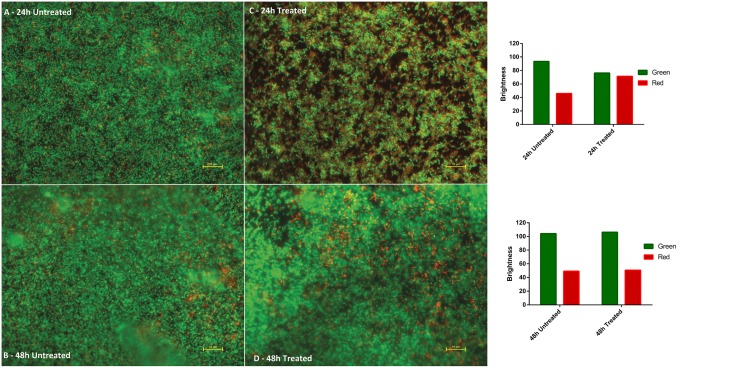
Walker et al. [9] have demonstrated that biofilm contamination can contribute significantly to outbreaks of healthcare acquired infections. Given the efficacy of GS5 against a range of HAI microbes, we hypothesised that a GS5-treated surface would impede the development of bacterial biofilms. P. aeruginosa is a well characterised biofilm former [26], and therefore we pre-treated plastic microtitre plate surfaces with GS5 and assessed the development of P. aeruginosa DSM3227 biofilms. The crystal violet staining method provides a quantitative measure of biofilm development/biomass and somewhat unexpectedly our data revealed that GS5 did not appear to inhibit the development of P. aeruginosa DSM3227 biofilm in plastic microtiter plates (Fig 3). Having observed that P. aeruginosa DSM3227 biofilm development was apparently unaffected, we assessed bacterial viability within the biofilms using the well-established BacLight staining method. This analysis suggested that a proportion of the bacterial cells were damaged or rendered non-viable when grown on GS5 treated surfaces, but that, critically, a sufficient number of viable/undamaged cells remained (Fig 4) which, we hypothesise are responsible for subsequent biofilm development.
Fig 3. Biofilm development following pre-treatment with GS5.
Pseudomonas aeruginosa DSM3227 biofilm biomass was assessed by crystal violet staining at various time points and data presented represents mean +/- SD of three independent experiments. Statistical analysis by independent T-tests versus Untreated controls (* = p<0.05, ** = p<0.005, *** = p<0.001). Grey columns representative of pre-treated samples; black bars representative of untreated controls.
Fig 4. BacLight staining of P. aeruginosa DSM3227 biofilm at 24 h and 48 h.
Live cells appear green and dead/damaged cells appear red. Images A and B show development of extensive biofilm on untreated surfaces. Image C shows biofilm development on GS5 treated surface with a greater proportion of dead/damaged cells. Image D shows GS5 treated surface biofilm at 48 h: biofilm development and cell viability is similar to the untreated control. Images were obtained ×100 magnification (oil immersion) on a Nikon ECLIPSE E400 (Nikon) microscope utilising a dual-band emission filter (450–490 nm/510–560 nm) and NIS-Elements BR (Nikon) software; composite (red/green) images generated using Image J software. Scale bar = 10 μm. Brightness values were generated for each panel (fig 4 a/b/c/d) using ‘imageJ colour histogram analysis’ software which converts RBG pixels to brightness values (V = (R+G+B)/3). These red/green brightness values are presented as bar charts to the right of the micrographs.
Discussion
Only a single published report exists which details the effects of GS5 used as a surface biocide. GS5 is reported to exert its antimicrobial effect via bonding of the silane end of the molecule to surfaces, following which microbes are drawn onto the hydrocarbon chain. The resultant puncturing of cell membranes and denaturation of proteins is proposed as the cause of cell death [20]. As a covalent bond is formed with the surface it is hypothesised that this mode of action is prolonged creating a ‘bactericidal surface’.
When we tested the prolonged activity GS5 exhibited bactericidal activity for 6 days (0.26 log10 reduction) whereas the other surface disinfectants tested showed no activity beyond day 0 (Fig 1). In comparison with previous residual testing of the GS5 product by Baxa et al. [20], which was completed on fabric swatches rather than on hard surfaces, we observed that residual antimicrobial activity of GS5 was lower (6 days rather than 14 days) [20]. However, the residual antibacterial effect decreased over time to a <1 log10 reduction in bacterial numbers, suggesting that GS5 would need regular reapplication and would not be sufficient as a surface disinfectant alone.
GS5 treated surfaces exhibited bactericidal activity which varied in effectiveness between surface type and bacterial species (Table 1). Thus, bacterial species challenged, in addition to surface type/properties, appears to have a significant influence on the performance of the GS5 product. Surface hydrophobicity, charge and roughness have all been reported as important with respect to performance of biocides [12]. Indeed, variations in the response of bacterial species to disinfectants is evident in the literature with disparate log10 reductions and widely varying minimum inhibitory concentrations (MICs); biocidal resistance is also evident [20,27]. GS5 is said to not induce resistance in microorganisms as a result of its physical mode of action, reported as membrane disruption and protein denaturation. We noted differences between the results of our current work and data reported by Baxa et al. [20] who also tested S. aureus, E. coli and P. aeruginosa on steel and Formica. The work of Baxa et al. [20] suggested that GS5 had greater efficacy against E. coli and P. aeruginosa, however this observation could be a result of differing surface properties across different types of Steel and Formica used. However, like Baxa et al. [20], we have shown that the performance of GS5 against different bacterial species varies considerably, which indicates that the specific type of microbial contaminant will be of greater influence on the effectiveness of GS5, than the actual surface on which it is used.
The ability of HCAI pathogens to adhere, via specific surface proteins to a range of substrates likely to be found in healthcare settings, including polystyrene, has been reported [28]. While biofilms that develop on medical devices such as catheters, chest tubes, prosthetic joints etc. are of concern [29], such medical devices were not the focus of our work. Beyond medical devices, on which biofilms most certainly develop, the contamination of any surface with bacteria in a matrix containing nutrients, will potentially enable development of biofilm. Hospital water systems, from storage to taps, allow biofilm formation and such contamination has been directly linked to adverse health outcomes [9].
Experiments in which plastic surfaces were pre-treated for 10 min with GS5 showed that there was no significant inhibitory effect against P. aeruginosa biofilm formation (Fig 3). It is well documented that biofilms exhibit increased resistance to antimicrobials and disinfectants, mainly due to the inability of these molecules to penetrate the biofilm [27]. Given that the GS5-treated plate surfaces would be expected to possess antimicrobial activity, we then considered the viability of cells within developing biofilms. Using BacLight, we observed an initial apparent bactericidal effect on P. aeruginosa DSM3227 cells (Fig 4c) as evidenced by a reduction in biofilm coverage and increased numbers of red stained, damaged, cells at 24 h. This did not translate however, into reduced biofilm formation as measured by crystal violet staining, and indeed later 48 h samples (Fig 4D) showed a well-developed biofilm containing viable cells, similar to that observed in the untreated control (Fig 4B) It is likely, therefore, that residual viable cells maintain the ability to form biofilm and we hypothesise that the cells that are initially damaged by GS5 could actually promote biofilm formation: it has been suggested that dead bacterial cell constituents could comprise a key component of the biofilm or indeed even enhance adhesion and stability of cells, thereby allowing biofilm development [30]. Our data, taken together suggest that GS5 treatment will not significantly inhibit biofilm formation.
Conclusion
Current NHS Infection control practices require that when choosing disinfectants, a 4–5 Log10 reduction is required in viable vegetative bacterial cells within a contact/drying time of 10 min, in addition to a spore reduction of 3 Log10 within the same period. When tested directly on a suspension of bacterial cells, GS5 achieved a more than 4 Log10 reduction with a 5 min contact time however the residual surface active antimicrobial activity of GS5 was much less, at approximately 1 Log10 reduction in bacterial numbers. The surface protective effect of GS5 remained for a further 3–6 days without reapplication of the product, however we noted a diminution of the measured Log10 reductions over time to a level which was much lower than that required for use in infection control.
Bacteria can form biofilm on surfaces allowing prolonged survival and increased resistance to biocides. Considering the GS5 mode of action we hypothesised a regime where GS5 could be utilised to prevent biofilm formation on surfaces subsequently reducing risk of infection. However GS5 has been shown to possess limited anti-biofilm properties as biofilm production is not impeded on GS5 coated surfaces.
Within the NHS, certain disinfectants (for example, DifficilS) routinely achieve 4 Log10 reductions in both vegetative cell and spore numbers within 3–5 min however control of infection is only achievable in practice by using these products in intensive cleaning up to twice daily in a rolling programme of disinfection. Thus, on the basis of the data generated in this work, it appears unlikely, despite modest reductions in bacterial cell viability and evidence for a short lived residual effect, that GS5 would replace current infection control products such as DifficilS or Actichlor in reducing the transmission of HAI pathogens within hospitals and care settings.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JM was supported by a postgraduate research studentship from the Department of Employment and Learning (N Ireland).
References
- 1.Zingg W, Holmes A, Dettenkofer M, Goetting T, Secci F, Clack L, et al. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet. Infect. Dis. 2015;15:212–224 doi: 10.1016/S1473-3099(14)70854-0 [DOI] [PubMed] [Google Scholar]
- 2.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. doi: 10.1001/jamainternmed.2013.9763 [DOI] [PubMed] [Google Scholar]
- 3.van Kleef E, Robotham J V, Jit M, Deeny SR, Edmunds WJ. Modelling the transmission of healthcare associated infections: a systematic review. BMC Infect. Dis. 2013;13:294 doi: 10.1186/1471-2334-13-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagenvoort JH, Sluijsmans W, Penders RJ. Better environmental survival of outbreak vs. sporadic MRSA isolates. J. Hosp. Infect. 2000;45:231–234 doi: 10.1053/jhin.2000.0757 [DOI] [PubMed] [Google Scholar]
- 5.Otter JA, Yezli S, Salkeld JAG, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control. 2013;41:S6–11 doi: 10.1016/j.ajic.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Wagenvoort JHT, De Brauwer EIGB, Penders RJR, Willems RJ, Top J, Bonten MJ. Environmental survival of vancomycin-resistant Enterococcus faecium. J. Hosp. Infect. 2011;77:282–283 doi: 10.1016/j.jhin.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 7.Thomas E, Bémer P, Eckert C, Guillouzouic A, Orain J, Corvec S, et al. Clostridium difficile infections: analysis of recurrence in an area with low prevalence of 027 strain. J. Hosp. Infect. 2016. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah M, Benoliel C, Drider D, Dhulster P, Chihib N-E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol., vol. 196, no. 7, pp. 453–472, July 2014. [DOI] [PubMed] [Google Scholar]
- 9.Walker JT, Jhutty A, Parks S, Willis C, Copley V, Turton JF, et al. Investigation of healthcare-acquired infections associated with Pseudomonas aeruginosa biofilms in taps in neonatal units in Northern Ireland. J. Hosp. Infect. 2014;86:16–23 doi: 10.1016/j.jhin.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 2004;39:1182–1189 doi: 10.1086/424667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French GL, Otter JA, Shannon KP, Adams NMT, Watling D, Parks MJ. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2004;57:31–33 doi: 10.1016/j.jhin.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Beggs C, Knibbs LD, Johnson GR, Morawska L. Environmental contamination and hospital-acquired infection: factors that are easily overlooked. Indoor Air. 2015;25:462–474 doi: 10.1111/ina.12170 [DOI] [PubMed] [Google Scholar]
- 13.Ramphal L, Suzuki S, McCracken IM, Addai A. Improving hospital staff compliance with environmental cleaning behavior. Proc. (Bayl. Univ. Med. Cent). 2014;27:88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy KJ, Oppenheim BA, Gossain S, Gao F, Hawkey PM. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect. Control Hosp. Epidemiol. 2006;27:127–132 doi: 10.1086/500622 [DOI] [PubMed] [Google Scholar]
- 15.Abreu AC, Tavares RR, Borges A, Mergulhão F, Simões M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013;68:2718–2732 doi: 10.1093/jac/dkt281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldeyab MA, Mcelnay JC, Elshibly SM, Hughes CM, Mcdowell DA, McMahon MAS, et al. Evaluation of the Efficacy of a Conventional Cleaning Regimen in Removing Methicillin—Resistant Staphylococcus aureus From Contaminated Surfaces in an Intensive Care Unit. Infect Control Hosp Epidemiol. 2009;30(3):304–306. doi: 10.1086/595964 [DOI] [PubMed] [Google Scholar]
- 17.Muller MP, MacDougall C, Lim M. Antimicrobial surfaces to prevent healthcare-associated infections: a systematic review. J. Hosp. Infect. 2015;92:7–13 doi: 10.1016/j.jhin.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 18.Diseases and Organisms in Healthcare Settings. U.S. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HAI/organisms/organisms.html. Published 2014. Accessed 08 March 2016.
- 19.Altaf M, Miller CH, Bellows DS, O’Toole R. Evaluation of the Mycobacterium smegmatis and BCG models for the discovery of Mycobacterium tuberculosis inhibitors. Tuberculosis (Edinb). 2010;90:333–337 [DOI] [PubMed] [Google Scholar]
- 20.Baxa D, Shetron-Rama L, Golembieski M, Golembieski M, Jain S, Gordon M, et al. In vitro evaluation of a novel process for reducing bacterial contamination of environmental surfaces. Am. J. Infect. Control. 2011;39:483–487 doi: 10.1016/j.ajic.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 21.Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter Plate Assay for Assessment of Listeria monocytogenes Biofilm Formation. Appl. Environ. Microbiol. 2002;68:2950–2958 doi: 10.1128/AEM.68.6.2950-2958.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Toole GA. Microtitre Dish Biofilm Formation Assay. JoVE. 2011;47:2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y, Köller T, Kreikemeyer B, Nelson DC. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J. Antimicrob. Chemother. 2013;68:1818–1824 doi: 10.1093/jac/dkt104 [DOI] [PubMed] [Google Scholar]
- 24.Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, et al. Cell Death in Pseudomonas aeruginosa Biofilm Development. J. Bacteriol. 2003;185:4585–4592 doi: 10.1128/JB.185.15.4585-4592.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer J, Siala W, Tulkens PM, Van Bambeke F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2013;57:2726–2737 doi: 10.1128/AAC.00181-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA Required for Bacterial Biofilm Formation. Science. 2002;295(5559):1487 doi: 10.1126/science.295.5559.1487 [DOI] [PubMed] [Google Scholar]
- 27.Otter JA, Vickery K, Walker JT, deLancey Pulcini E, Stoodley P, Goldenberg SD, et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect. 2015;89(1):16–27. doi: 10.1016/j.jhin.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 28.Percival SL, Suleman L, Vuotto C, Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol. 2015;64(4):323–334. [DOI] [PubMed] [Google Scholar]
- 29.Devlin-Mullin A, Todd NM, Golrokhi Z, Geng H, Konerding MA, Ternan NG, et al. Atomic Layer Deposition of a Silver Nanolayer on Advanced Titanium Orthopedic Implants Inhibits Bacterial Colonization and Supports Vascularized de Novo Bone Ingrowth. Adv Healthc Mater. 2017;6(11). [DOI] [PubMed] [Google Scholar]
- 30.Bayles KW. The biological role of death and lysis in biofilm development. Nature Rev. Microbiol. 2007;5:721–726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.