Abstract
BACKGROUND & AIMS
Primary sclerosing cholangitis (PSC) is an orphan hepatobiliary disorder associated with inflammatory bowel disease (IBD). We aimed to estimate the risk of disease progression based on distinct clinical phenotypes in a large international cohort of patients with PSC.
METHODS
We performed a retrospective outcome analysis of patients diagnosed with PSC from 1980 through 2010 at 37 centers in Europe, North America, and Australia. For each patient, we collected data on sex, clinician-reported age at and date of PSC and IBD diagnoses, phenotypes of IBD and PSC, and date and indication of IBD-related surgeries. The primary and secondary endpoints were liver transplantation or death (LTD) and hepatopancreatobiliary malignancy, respectively. Cox proportional hazards models were applied to determine the effects of individual covariates on rates of clinical events, with time-to-event analysis ascertained through Kaplan-Meier estimates.
RESULTS
Of the 7121 patients in the cohort, 2616 met the primary endpoint (median time to event of 14.5 years) and 721 developed hepatopancreatobiliary malignancy. The most common malignancy was cholangiocarcinoma (n = 594); patients of advanced age at diagnosis had an increased incidence compared with younger patients (incidence rate: 1.2 per 100 patient-years for patients younger than 20 years old, 6.0 per 100 patient-years for patients 21–30 years old, 9.0 per 100 patient-years for patients 31–40 years old, 14.0 per 100 patient-years for patients 41–50 years old, 15.2 per 100 patient-years for patients 51–60 years old, and 21.0 per 100 patient-years for patients older than 60 years). Of all patients with PSC studied, 65.5% were men, 89.8% had classical or large-duct disease, and 70.0% developed IBD at some point. Assessing the development of IBD as a time-dependent covariate, Crohn’s disease and no IBD (both vs ulcerative colitis) were associated with a lower risk of LTD (unadjusted hazard ratio [HR], 0.62; P =.001 and HR, 0.90; P = .03, respectively) and malignancy (HR, 0.68; P = .008 and HR, 0.77; P = .004, respectively). Small-duct PSC was associated with a lower risk of LTD or malignancy compared with classic PSC (HR, 0.30 and HR, 0.15, respectively; both P < .001). Female sex was also associated with a lower risk of LTD or malignancy (HR, 0.88; P = .002 and HR, 0.68; P < .001, respectively). In multivariable analyses assessing the primary endpoint, small-duct PSC characterized a low-risk phenotype in both sexes (adjusted HR for men, 0.23; P < .001 and adjusted HR for women, 0.48; P = .003). Conversely, patients with ulcerative colitis had an increased risk of liver disease progression compared with patients with Crohn’s disease (HR, 1.56; P < .001) or no IBD (HR, 1.15; P = .002).
CONCLUSIONS
In an analysis of data from individual patients with PSC worldwide, we found significant variation in clinical course associated with age at diagnosis, sex, and ductal and IBD subtypes. The survival estimates provided might be used to estimate risk levels for patients with PSC and select patients for clinical trials.
Keywords: Risk Stratification, Immune-Mediated Liver Disease, Autoimmune Liver Disease, Cholestasis
Primary sclerosing cholangitis (PSC) is a chronic immune-mediated liver disorder strongly associated with inflammatory bowel disease (IBD).1 Although rare, PSC carries an ongoing and disproportionate clinical need, with clinical outcomes being determined by the development of end-stage biliary cirrhosis and an independent risk of hepatopancreatobiliary (HPB) malignancy. To date, medical therapies have not been effective,8 and liver transplantation (LT) remains the only proven life-extending intervention, with 10%–15% of all transplant activity in Europe now being performed for PSC.5–7
Accurately reporting the natural history of disease remains a critical challenge not only for clinicians, but also for industry and regulatory agencies who collectively recognize the need for new therapies and equally appreciate the risks and obstacles in demonstrating patient benefit against the background of an orphan disease with a relatively variable, often slow clinical course.9 Moreover, patients seek reassurance and guidance as to their own prognosis, whereas clinicians wish to confidently recognize those at highest risk of poor outcomes as equally as they strive to reassure individuals with a more favorable prognosis.
To expand on single-center and single-country descriptors, the International PSC Study Group (IPSCSG) sponsored a multi-center outcome study to model the natural history of the disease. Our primary aim was to evaluate and report the clinical course from a large internationally representative PSC cohort, which included 7121 patients seen at 37 centers across 17 countries, and encompassing >30 years of clinical observation, 1696 LTs, 920 deaths, and 721 incidents of HPB malignancy. In so doing, we not only validate the presence of key phenotypic descriptors, but also determine the extent of their interaction and how they may impact the clinical course that patients may experience.
Patients and Methods
Study Setting and Design
We collected and analyzed data from well-characterized patients diagnosed with PSC between January 1, 1980 and December 31, 2010, having previously attended or under current clinical follow-up until study completion (June 30, 2014). Any individual with an established diagnosis of PSC (including small-duct disease; sdPSC) in accordance with European or American recommendations10–12 was considered eligible for inclusion. When biochemical, serologic, and/or histologic features of autoimmune hepatitis (AIH) were evident concurrently or sequentially,13 the diagnosis of a PSC phenotype with AIH features (PSC/AIH variant) was made according to discretion of the participating center. IBD phenotypes were determined according to local expertise,14–16 and classified as ulcerative colitis (UC), Crohn’s disease (CD), or indeterminate colitis, in keeping with consensus guidelines.17,18
Data Collection
Identification of study participants was performed at a local level, either through a pre-existing and prospectively collected local PSC database, or in a retrospective manner via review of medical records by a named site investigator at a given institution. All individual center data were captured onto a multiparametric standardized case record form formulated by the IPSCSG and amalgamated into a common ‘master’ database for downstream analysis on study completion. Individual clinical characteristics pertained to patient sex, clinician-reported age at and date of diagnosis of PSC, sub-phenotype and IBD phenotype, date and indication of IBD-related surgical resections, date of LT, date of death, and date and type of first HPB malignancy. Patients with sclerosing cholangitis suspected because of alternate etiologies (eg, IgG4-related disease, acquired immunodeficiency syndromes, confirmed biliary transporter defects) were excluded from the analysis, as were those with inadequate/unknown follow-up duration. On completion of data capture, all patient datasets were checked for plausibility and validity, and duplicated patient entries were removed before analysis.
Data Interpretation and Analysis
All patients were identified at the time of diagnosis or during subsequent follow-up. ‘Time zero’ was set from the point of diagnosis of first PSC phenotype, with the primary endpoint being the incidence rate (and associated risk) of LT, or death in non-transplanted patients. Any individual not experiencing a clinical event in this regard was censored at the date of last known follow-up. A secondary endpoint of HPB malignancy was also studied, and in this instance the date of first liver transplantation or death (LTD), or last date of event-free follow-up comprised our censor points. Diagnosis of HPB malignancy was made according to clinical, radiologic and/or histologic findings as dictated by center-specific protocols.
Categorical variables are expressed as numbers (n), with percentages in parenthesis, and continuous data as mean ± standard deviation unless otherwise indicated. Statistical comparisons between groups were performed using Pearson’s χ2 test. Differences in the means and proportions between individual groups of continuous data were assessed using the independent samples t test, following Levene’s test for equality of variances.19 A P value <.05 was considered statistically significant.
Univariate and multivariable Cox proportional hazards models were fit to assess the impact of individual covariates on the instantaneous rate of clinical events, with time-to-event analysis ascertained through Kaplan-Meier estimates. Given that the development of IBD does not parallel that of PSC, the independent prognostic impact of IBD-phenotype was assessed separately as a time-fixed as well as a time-dependent covariate. All individual covariates were assessed for statistically significant interaction terms, including patient demographic features (age and sex) and individual phenotypic descriptors for PSC and IBD subtypes separately. All analyses were stratified by geographic region (Australia, North America, Northern Europe, Central Europe, Western Europe, or Southern Europe) and adjusted for year of PSC diagnosis. Incidence rates were calculated by the life tables’ method. Statistical analyses were performed with IBM SPSS Statistics 22.0 (SPSS Inc, Chicago, IL).
Ethical Approval
This study was conducted in accordance with the protocol and principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the local institutional ethical boards of all participating centers.
Results
Study Population
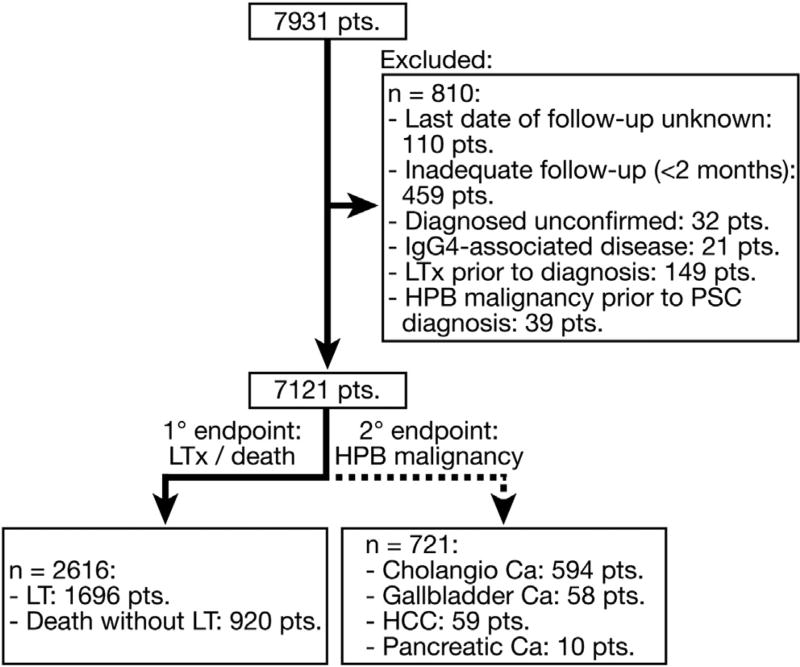
We accrued clinical data pertaining to 7931 patients (53,983 patient-years); however, those with inadequate follow-up or indeterminate diagnosis of PSC were exempted from further analysis (Figure 1). The final patient cohort consisted of 7121 patients, either having PSC in its classical form (89.8%), as small-duct disease (3.6%), or the PSC/AIH variant (6.6%) (Table 1). Observing the cohort in its entirety, the majority of patients were men (65.5%), with a mean age at diagnosis of 37 years vs 40 years in women (P < .001). Seventy percent of all patients developed concomitant IBD before, at, or following PSC diagnosis, which under most circumstances was morphologically consistent with UC. However, the development of UC was less common in women than men (48.1% vs 61.0%, respectively; P < .001), and in those with variant PSC sub-phenotypes relative to classical PSC (frequency of UC in patients with classical PSC: 58.1% vs 33.5% in sdPSC, and vs 47.7% in PSC/AIH; P < .001 for both pairwise comparisons) (Supplementary Tables 1, 2, and 3).
Figure 1.
Study cohort: At the time of analysis data were available for 7931 patients. However, following exclusion of groups with an alternate diagnose or inadequate follow-up, the final study group consisted of 7121 patients, of which 2616 underwent LT or died, with a total of 721 developing primary HPB malignancy.
Table 1.
Summary of Patient Characteristics
| No. of patients | 7121 |
| No. of men | 4661 (65.5%) |
| Age at diagnosis, y: | |
| Mean | 38.5 (SD: 15.5) |
| ≤20 | 940 (13.2%) |
| 21–30 | 1508 (21.2%) |
| 31–40 | 1617 (22.7%) |
| 41–50 | 1435 (20.2%) |
| 51–60 | 953 (13.4%) |
| > 60 | 665 (9.3%) |
| unknown | 3 (0.04%) |
| PSC sub-phenotype: | |
| classical PSC | 6397 (89.8%) |
| small duct PSC | 254 (3.6%) |
| PSC/AIH variant | 470 (6.6%) |
| Diagnosis year: | |
| 1980–1984 | 217 (3.0%) |
| 1985–1989 | 424 (6.0%) |
| 1990–1994 | 773 (10.9%) |
| 1995–1999 | 1414 (19.9%) |
| 2000–2004 | 1802 (25.3%) |
| 2005–2010 | 2491 (35.0%) |
| IBD phenotype at baseline: | |
| Ulcerative colitis | 2761 (38.8%) |
| Crohn’s disease | 595 (8.4%) |
| Indeterminate colitis | 113 (1.6%) |
| No IBD | 3082 (43.3%) |
| Unknown timing | 503 (7.1%) |
| Unknown IBD status | 67 (0.9%) |
| IBD phenotype at end of follow-up: | |
| Ulcerative colitis | 3989 (56.0%) |
| Crohn’s disease | 786 (11.0%) |
| Indeterminate colitis | 210 (2.9%) |
| No IBD | 2069 (29.1%) |
| Unknown IBD status | 67 (0.9%) |
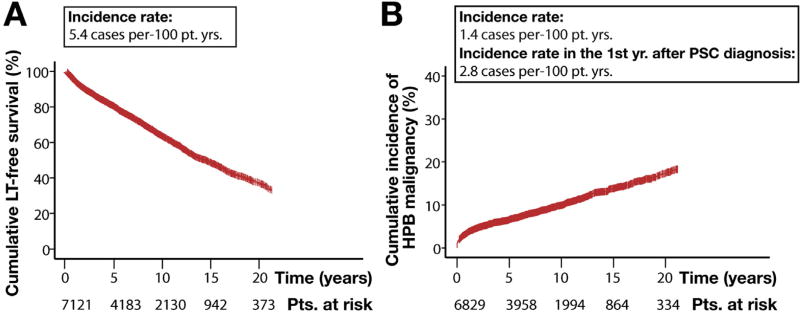
During the defined observation period, 20.2%, 37.0%, 52.3%, and 63.6% of patients underwent LT or died at 5, 10, 15, and 20 years, respectively (Figure 1), yielding a median transplant-free survival time of 14.5 years (95% confidence interval [CI]: 13.6–15.2 years; Figure 2A). With regard to our secondary endpoint, 7.1%, 10.9%, 16.0%, and 21.6% of the patient population developed a HPB malignancy at the aforementioned time points (Figure 2B) (overall n = 721).
Figure 2.
Cumulative incidence of clinical events. Kaplan-Meier estimates of (A) LT-free survival rate across the patient population and (B) incidence of all HPB malignancies. Notably, 37.8% (n = 272) of all HPB malignancies occurred in the first year of PSC diagnosis, with the vast majority being CCA during this time (incidence rate in the first year after PSC diagnosis: 2.6 cases per 100 patient-years). Patients with unknown transplantation, mortality, or malignancy status at the time of study completion were excluded from respective analysis.
The majority of HPB malignancy events were cholangiocarcinoma (CCA) (n = 594), and over one third of all malignancies were detected in the first year following PSC diagnosis. The incidence of CCA increased with advancing age at PSC diagnosis (Supplementary Figure 1); whilst hepatocellular carcinoma (n = 59) or gallbladder carcinoma (n = 58) were less frequent. Only 10 patients across 7 centers were diagnosed with pancreatic carcinoma. HPB malignancy developed most often in association with classical PSC, with only a small number of such events occurring in patients with sdPSC (1 CCA, 2 hepatocellular carcinoma, 1 pancreatic carcinoma) or PSC/AIH variants (12 CCA, 1 gallbladder carcinoma, 1 hepatocellular carcinoma). Overall, the development of HPB malignancy at any point during the clinical course was associated with a significantly increased risk of patient mortality (hazard ratio): 15.7; 95% CI: 14.12–17.34; P < .001).
Clinical Stratifiers for LTD and HPB Malignancy
The incidence rates of clinical events according to baseline phenotypic descriptors are provided in Supplementary Tables 4 and 5. By univariate analysis, older age at diagnosis was associated with significantly poorer transplant-free survival; whereas female sex, CD (relative to UC), and sdPSC (relative to classical PSC) were identified as being protective (Supplementary Table 6). No significant difference in transplant-free survival was observed between the PSC/AIH variant vs the classical PSC sub-phenotype (Supplementary Figure 2A), although patients with the former were at a low risk of developing HPB malignancy (Supplementary Figure 2B and Supplementary Table 6).
The number of patients with IBD increased during our observation period (from 3469 patients at baseline to 4985 patients by the end of our study). Given that intestinal disease onset did not necessarily parallel that in the liver, the impact of IBD was subsequently determined as a time-dependent covariate. In this context, both CD and an absence of IBD carried stratification properties of a lower risk PSC phenotype; whereas patients developing UC were at highest risk for disease progression, or future development of HPB malignancy (Supplementary Table 6).
Patient Sex Modifies the Risk of Liver Disease Progression in Classical PSC
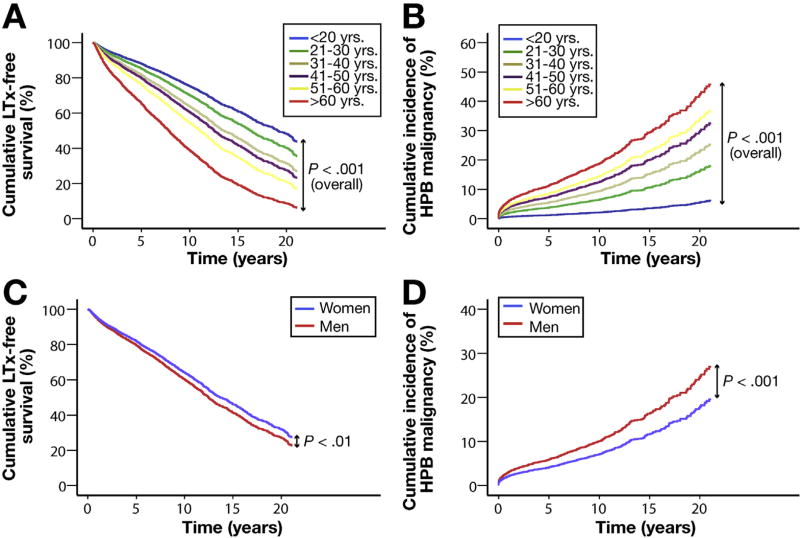
To verify the relative independence of predictive phenotypic features, a comparative multivariable evaluation was performed. Through multivariable Cox regression analysis, the prognostic impact of advancing age at diagnosis, as well as protective influences of female sex, having small duct disease, or CD at time of PSC diagnosis, all retained statistical significance in terms of stratifying risk of liver disease progression (Figures 3 and 4).
Figure 3.
Impact of patient age and gender on clinical outcome. Cox plots with regard to LT or HPB malignancy. All data are stratified by geographic region of referring center and year of diagnosis, presented according to patient age at diagnosis and weighted for patient gender, IBD phenotype at baseline, and PSC sub-phenotype (A and B); or patient gender weighted for patient age at diagnosis, IBD phenotype at baseline, and PSC sub-phenotype (C and D).
Figure 4.
Impact of variant PSC sub-phenotypes and IBD phenotypes on clinical outcome. Cox plots with regard to LT or HPB malignancy. All data are stratified by geographic region of referring center and year of diagnosis, presented according to PSC sub-phenotype weighted for patient age at PSC diagnosis, gender, and IBD phenotype at baseline (A and B); or patient IBD phenotype at baseline weighted for age at PSC diagnosis, gender, and PSC sub-phenotype (C and D).
Despite both factors being proven as independent risk-predictors, there was a statistically significant interaction (P = .013) between patient sex and PSC sub-phenotypes when evaluating LTD as an endpoint. To this effect, patients with sdPSC demonstrated significantly improved transplant-free survival relative to same-sex counterparts with classical PSC and PSC/AIH when matched for their age at PSC diagnosis as well as baseline IBD phenotype (Figure 4A). These differences were retained when adjusting for the latter as a time-dependent covariate in our multivariable analysis (Table 2). Although women more commonly exhibited non-classical PSC sub-phenotypes than men, statistically significant differences in the risk of LTD between the sexes were retained when restricting our analyses to only those patients with classical PSC (Table 2).
Table 2.
Risk Stratification of LT/Death by Disease Phenotype
| Reference phenotype | Adjusted hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|
| PSC phenotype | Male | |||
| Small-duct PSC | vs Classical PSC | 0.23 (0.13–0.40) | <.001 | |
| PSC/AIH variant | vs Classical PSC | 0.73 (0.56–0.94) | .015 | |
| PSC/AIH variant | vs Small-duct PSC | 3.18 (1.71–5.92) | <.001 | |
| Female | ||||
| Small-duct PSC | vs Classical PSC | 0.48 (0.29–0.77) | .003 | |
| PSC/AIH variant | vs Classical PSC | 1.19 (0.91–1.54) | .20 | |
| PSC/AIH variant | vs Small-duct PSC | 2.49 (1.45–4.27) | .001 | |
| Sex | Classical PSC | |||
| Female | vs Male | 0.84 (0.77–0.92) | .022 | |
| Small-duct PSC | ||||
| Female | vs Male | 1.76 (0.84–3.69) | .13 | |
| PSC/AIH variant | ||||
| Female | vs Male | 1.38 (0.97–1.97) | .075 | |
| IBD phenotype | Crohn’s disease | vs Ulcerative colitis | 0.64 (0.54–0.75) | <.001 |
| Indeterminate colitis | vs Ulcerative colitis | 0.94 (0.71–1.26) | .69 | |
| No IBD | vs Ulcerative colitis | 0.87 (0.79–0.95) | .002 | |
| Crohn’s disease | vs no IBD | 0.73 (0.62–0.87) | <.001 | |
| Indeterminate colitis | vs no IBD | 1.10 (0.83–1.48) | .51 | |
| Indeterminate colitis | vs Crohn’s disease | 1.50 (1.09–2.07) | .013 |
NOTE. All analyses are stratified by geographical region of diagnosis; adjusted for calendar year and age at diagnosis. Inflammatory bowel disease phenotype is defined as a time-dependent covariate. Hazard ratios for PSC sub-phenotypes are presented separately for men and women, and hazard ratios for female versus male are presented separately for each PSC sub-phenotype, given the presence of a significant interaction term between gender and PSC sub-phenotype (P = .005).
Unlike our primary endpoint, no statistically significant interactions were evident between patient sex and PSC sub-phenotypes when determining future HPB risk; wherein being female continued to exert a small, yet independent protective effect (but not an additive one) to that provided by small-duct disease (Figures 3 and 4; Table 3).
Table 3.
Stratification of Hepatopancreatobiliary Malignancy Risk by Disease Phenotype
| Reference phenotype | Adjusted hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|
| PSC phenotype | Small-duct PSC | vs Classical PSC | 0.19 (0.07–0.51) | .001 |
| PSC/AIH variant | vs Classical PSC | 0.31 (0.17–0.55) | <.001 | |
| PSC/AIH variant | vs Small-duct PSC | 1.62 (0.52–5.04) | .41 | |
| Sex | Female | vs Male | 0.68 (0.57–0.82) | .001 |
| IBD phenotype | Crohn’s disease | vs Ulcerative colitis | 0.69 (0.52–0.92) | .01 |
| Indeterminate colitis | vs Ulcerative colitis | 1.03 (0.52–1.75) | .931 | |
| No IBD | vs Ulcerative colitis | 0.73 (0.61–0.87) | <.001 | |
| Crohn’s disease | vs no IBD | 0.96 (0.71–1.29) | .77 | |
| Indeterminate colitis | vs no IBD | 1.41 (0.82–2.44) | .22 | |
| Indeterminate colitis | vs Crohn’s disease | 1.48 (0.82–2.67) | .20 |
NOTE. All analyses stratified by geographic region of diagnosis; adjusted for calendar year and age at diagnosis. Inflammatory bowel disease phenotype is defined as a time-dependent covariate.
IBD Phenotype as an Independent Predictor of Clinical Outcome in PSC
CD (at time of PSC diagnosis) relative to UC continued to exert a protective influence with respect to transplant-free survival and the development of HPB malignancy, irrespective of the effect exerted by sex and PSC sub-phenotype. Such impact was not demonstrated in the group without IBD at baseline (Figure 4). However, when addressing the impact of IBD as a time-dependent covariate, both CD and IBD absence retained independent stratifying properties of a lower-risk PSC population (Tables 2 and 3). No statistically significant interactions existed between the different IBD phenotypes and either PSC sub-phenotype or patient sex.
Reciprocally, development of UC before, or that which manifest during the clinical course of PSC, significantly increased the risk of LTD by 56% and 15% relative to CD or IBD absence, respectively (Table 2), and of HPB malignancy by approximately 45% and 37%, respectively (Table 3). Of all patients with UC, 18.0% (n = 718) underwent colectomy before reaching a primary or secondary endpoint; however, no significant difference in outcome was evident in such individuals relative to those retaining an intact colon (hazard ratio for colectomy in terms of LTD and HPB malignancy: 0.90 [95% CI: 0.78–1.05; P = .187] and 0.81 [95% CI: 0.61–1.07; P = .14], respectively).
IBD Phenotype Overrides the Prognostic Impact of Patient Sex
The prognostic impact of IBD phenotype when assessed as a time-dependent variable negated the marginal protective influence of female sex. This means that although sex was an independent risk factor of both clinical endpoints statistically, there were no demonstrable differences in either primary or secondary outcomes between men and women when matched for IBD phenotype as a time-dependent variable (data not shown). Moreover, the lower prevalence of UC in women (Supplementary Table 1) may account partially for differences in liver disease progression between the sexes.
Discussion
PSC is a disease with significant clinical and societal burden, and in recognition of the hurdles involved in developing effective new therapies for patients, it is essential that robust descriptions of disease course are generated.2–4 In this study, we validate the critical importance of specific phenotypic variants (ie, the more favorable prognosis that limited small-duct variants offers patients), the negative prognostic impact of UC on liver-related outcomes, and the high incidence of CCA in the first year following PSC diagnosis.2,20–22 In addition, it is shown that patients with PSC and overlapping AIH features carry a similar risk of liver disease progression to those with a more classical PSC phenotype; although development of HPB malignancy appears to be a rare event in PSC/AIH variants, and also for patients with a young presenting age at PSC diagnosis. Furthermore, we were able to address the prognostic impact of IBD development as a time-dependent covariate, recognizing that development of UC is a key stratifier of adverse hepatobiliary consequences in PSC. Conversely, IBD absence, and CD in particular, confer prognostic favor independent of the other phenotypic risk factors described.
To date, sex-specific variations in clinical phenotype and correlations with patient outcomes in PSC have lacked robust definition. Large-scale studies have demonstrated the negative prognostic impact of male sex in patients with related disorders, such as primary biliary cholangitis; specifically an association with treatment non-response and a higher incidence of HPB malignancy.23,24 As an immune-mediated disease PSC is somewhat atypical, with a propensity for ‘most’ patients being younger men. However, the sex distribution of PSC appears more balanced if cholangiographic screening is applied to all IBD patients, irrespective of biochemical abnormalities or symptomatology.25 In any event, utilizing the large size of the IPSCSG cohort, men with classical PSC are seen to carry a slight, albeit statistically significant, increased risk of disease progression compared with women of matched phenotype.
Our analysis also demonstrates that women with PSC have a much lower prevalence of UC than men. This is important because IBD phenotype, particularly when determined as a time-dependent covariate, proves to be an independent risk factor for disease progression and may explain the observed differences in outcome between sexes. Conversely, patients without IBD or those having CD are at a comparatively lower risk of developing adverse events; a finding suggested previously in 2 single-center studies, which we now validate convincingly.14,16 Of note, the IPSCSG has recently demonstrated genetic distinctions between patients with PSC and IBD vs those with IBD alone.26–28 Notwithstanding efforts to better understand clinical outcomes, our study further supports the need to improve IBD classification in PSC, particularly as the intestinal phenotype is often distinct compared with classical colitis descriptors,15 and more so given that genetic signals in PSC/CD may be disparate to those with PSC/UC.28,29 Of note, our study does not capture details pertaining to the precise distribution of intestinal inflammation; however, prior evidence suggests that CD in PSC is invariably localized to the colon, with isolated ileal disease being a seldom-reported finding.14,16
No significant outcome differences are apparent between men and women with the variant PSC sub-phenotypes, and consequently patients with sdPSC (irrespective of gender) experience a relatively sedentary clinical course compared with classical PSC. Perhaps more striking, however, is the highly similar transplant-free survival rate seen for patients with classical PSC and those with the PSC/AIH variant. Accepting the caveat that PSC/AIH lacks a codified diagnostic criteria,30 these observations challenge the view of PSC/AIH variants imparting a lesser disease burden.31 Instead, our findings indicate that once overt sclerosing cholangitis has manifest, liver disease may progress at a similar rate irrespective of the initial mode of disease presentation.
We also show how development of HPB malignancy (mainly CCA) manifests as a critical event in the clinical course of patients, particularly with advancing age at PSC diagnosis, and associated with significantly diminished patient survival. It is plausible that the reason for one third of CCA being identified within the first year following PSC diagnosis is because of a delay in the latter’s detection (length-time bias), and not being manifest until CCA is clinically overt. This observation highlights the need for improving CCA screening and surveillance, especially in high-risk PSC patients with coexisting UC. If better noninvasive surveillance methods for CCA surveillance became available it could support the rationale for systematic screening for PSC in UC patients.25 On the contrary, as previously described, patients with small duct disease, perhaps indicative of PSC in an earlier form or of shorter duration, carry a lower risk of developing malignancy.2,22 While this observation was somewhat expected, patients with the PSC/AIH variant are also noted to develop HPB malignancy infrequently. This could possibly be a result of a lower UC burden2,20,32,33 that, as our data suggests, is itself an independent hazard for future carcinoma development. Furthermore, with only 10 cases during 51,500 patient-years of follow-up we could not validate previous reports37 of a significant increased incidence of pancreatic carcinomas, albeit accepting the clinical challenges that exist in differentiating distal CCAs from primary pancreatic lesions.
The natural history of PSC has previously been studied by some of the participating centers comprising the IPSCSG (Supplementary Table 7), although these cohorts are estimated to constitute, at most, <50% of our current patient population. Whilst certain patient characteristics that we describe mirror those in population-based registries,2 ours is highly representative of a specialist-center PSC experience. In light of our prolonged study period, transplant center ‘designation’ and organ allocation policies have evolved significantly across institutions over time. Thus, it is not possible to accurately discriminate clinical outcomes based solely on the division between transplant vs non-transplant centers, as conducted in other settings.2 Admittedly, we do not present a population-based epidemiologic study, and because more than 95% of included patients derived from centers with contemporary LT activity, a degree of referral bias cannot be discounted. This may also explain the relatively low prevalence of sdPSC in our cohort.
Given the retrospective nature of our study, the interval frequency of repeated cholangiography varied between centers. Therefore, exhaustive surveillance imaging may not have been performed to exclude progression of all small duct cases to classical PSC. Similarly, there is no universally accepted guideline for repeated screening colonoscopy in those without IBD, hence we cannot discount that subclinical colitis may have developed in a subset of patients classified as having no IBD. Of note, our reported colectomy rate was 18% in patients with UC, which mirrors the incidence reported in single-center studies, but is lower than that observed in population-based cohorts and prospective multi-center registries of UC alone.34–36
Our analyses were intentionally restricted to addressing the prognostic impact of well-defined patient phenotypes. Consequently, data pertaining to laboratory variables, extent of strictures, intervals of surveillance imaging, or specific pharmacological interventions (eg, ursodeoxycholic acid and/or immunosuppression) fell outside of the current study’s remit. Further large-scale investigation of therapeutic impact is of critical importance, given the inconsistently reported effects of these agents on disease progression and malignancy risk in PSC.8 Additionally, because a systematic autopsy review was not performed from all mortality cases it is plausible that the incidence of HPB malignancy may in fact be higher than actually reported,37 particularly as CCA cannot always be discriminated from more benign changes in PSC.38 We are also unable to classify all causes of death in our retrospective patient cohort, although previous studies indicate that mortality in PSC is invariably caused by liver disease or a complication of coexisting IBD.2,39 A further restriction caused by the retrospective nature and prolonged follow-up period (since 1980) is the fact that serum IgG4 levels were not determined systematically in all patients. Therefore, it is not possible to conclusively exclude IgG4-associated cholangiopathy within a subset of our population.
The IPSCSG study confirms significant phenotypic diversity across the global PSC patient population. The estimates provided for transplant-free survival and the lifetime risk of HPB malignancy would facilitate appropriate patient counselling and also aid in the future evaluation of potential new approaches to malignancy screening. In a drive to limit heterogeneity in clinical trials, which currently group together individuals at a high risk of disease progression (classical PSC and UC) together with patients at intermediate risk (CD or IBD-absence) and low risk (sdPSC), our data underpins a collaborative effort to better appraise future therapeutic ventures for this orphan disease. As a clear consequence of our findings, future clinical trials may now be able to stratify entry according to a combination of precise phenotypic risk factors, limit the heterogeneity within studied cohorts, and provide a more objective evaluation of therapeutic efficacy in specific patient groups.
Supplementary Material
EDITOR’S NOTES.
BACKGROUND AND CONTEXT
Existing data on the natural history and risks for disease progression of PSC are mostly monocentric or regional.
NEW FINDINGS
Incidence of hepatopancreatobiliary (HPB) cancer increased with age at PSC diagnosis. Patients with features of autoimmune hepatitis compared with classical PSC showed a similar transplant-free survival but reduced incidence of HPB malignancy. PSC patients could be stratified into low-risk, intermediate-risk, and high-risk categories regarding transplant-free survival and HPB cancer.
LIMITATIONS
The results are based on a cohort of mostly retrospectively interrogated clinical data from mainly tertiary centers.
IMPACT
These results may be used to provide an individualized risk assessment for patient’s disease course including HPB malignancy, and to stratify patients for clinical trials.
Acknowledgments
The following collaborators are acknowledged for their contributions with data acquisition: Benoît Almer, MD (University of Lund).
The International PSC Study Group would also like to extend thanks to all patients who contributed to data in the study, as well as the patient support groups (PSC partners and PSC support).
Funding
T.J.W. was supported by the German Federal Ministry of Education and Research through the Integrated Research and Treatment Center Transplantation (reference no. 01EO0802) at Hannover Medical School. P.J.T. has received funding from the Wellcome Trust. P.J.T. and G.M.H. also receive funding and salary support from the NIHR Birmingham Biomedical Research Centre (BRC) (the views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health, UK). S.P.P. was supported by the NIHR University College London Hospitals Biomedical Research Centre. K.N.L. was supported by NIH grant no. R01 DK84960. T.M. was supported by the German Research Community grant nos. MU 2864/1-1 and MU 2864/1-3. C.S. was supported by the German Research Community (DFG, SFB 841), by the YAEL-Foundation, and the Helmut and Hannelore Greve Foundation.
Abbreviations used in this paper
- AIH
autoimmune hepatitis
- CCA
cholangiocarcinoma
- CD
Crohn’s disease
- CI
confidence interval
- IBD
inflammatory bowel disease
- IPSCSG
International PSC Study Group
- LT
liver transplantation
- LTD
liver transplantation or death
- PSC
primary sclerosing cholangitis
- sdPSC
small-duct primary sclerosing cholangitis
- UC
ulcerative colitis.
Footnotes
Note: To access the Supplementary Material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2017.02.038.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382:1587–1599. doi: 10.1016/S0140-6736(13)60096-3. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–2055. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Lindkvist B, Benito de Valle M, Gullberg B, Björnsson E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010;52:571–577. doi: 10.1002/hep.23678. [DOI] [PubMed] [Google Scholar]
- 5.Patkowski W, Skalski M, Zieniewicz K, et al. Orthotopic liver transplantation for cholestatic diseases. Hepatogastroenterology. 2010;57:605–610. [PubMed] [Google Scholar]
- 6.Charman S, Copley L, Tovikkai C, et al. UK liver transplant audit (NHS Blood and Transplant) [Accessed on May 7, 2017];2012 Available at: http://www.odt.nhs.uk/pdf/organ_specific_report_liver_2016.pdf.
- 7.Fosby B, Melum E, Bjøro K, et al. Liver transplantation in the Nordic countries - An intention to treat and post-transplant analysis from The Nordic Liver Transplant Registry 1982–2013. Scand J Gastroenterol. 2015;50:797–808. doi: 10.3109/00365521.2015.1036359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindor KD, Kowdley KV, Luketic VAC, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: Opportunities for clinicians and trialists. Hepatology. 2016;63:644–659. doi: 10.1002/hep.28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 12.Lindor KD, Kowdley KV, Harrison ME. ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015;110:646–659. doi: 10.1038/ajg.2015.112. [DOI] [PubMed] [Google Scholar]
- 13.Boberg KM, Chapman RW, Hirschfield GM, et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374–385. doi: 10.1016/j.jhep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Halliday JS, Djordjevic J, Lust M, et al. A unique clinical phenotype of primary sclerosing cholangitis associated with Crohn’s disease. J Crohns Colitis. 2012;6:174–181. doi: 10.1016/j.crohns.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Boonstra K, van Erpecum KJ, van Nieuwkerk KMJ, et al. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270–2276. doi: 10.1002/ibd.22938. [DOI] [PubMed] [Google Scholar]
- 16.Fevery J, Van Steenbergen W, Van Pelt J, et al. Patients with large-duct primary sclerosing cholangitis and Crohn’s disease have a better outcome than those with ulcerative colitis, or without IBD. Aliment Pharmacol Ther. 2016;43:612–620. doi: 10.1111/apt.13516. [DOI] [PubMed] [Google Scholar]
- 17.Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis. Part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Levene H. Contributions to probability and statistics: essays in honor of Harold Hotelling. Stanford University Press; 1960. [Google Scholar]
- 20.Boberg KM, Bergquist A, Mitchell S, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205–1211. doi: 10.1080/003655202760373434. [DOI] [PubMed] [Google Scholar]
- 21.Singal AK, Stanca CM, Clark V, et al. Natural history of small duct primary sclerosing cholangitis: a case series with review of the literature. Hepatol Int. 2011;3:808–813. doi: 10.1007/s12072-011-9260-4. [DOI] [PubMed] [Google Scholar]
- 22.Björnsson E, Olsson R, Bergquist A, et al. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology. 2008;134:975–980. doi: 10.1053/j.gastro.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Carbone M, Mells GF, Pells G, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560–569. e7. doi: 10.1053/j.gastro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi PJ, Lammers WJ, van Buuren HR, et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut. 2016;65:321–329. doi: 10.1136/gutjnl-2014-308351. [DOI] [PubMed] [Google Scholar]
- 25.Lunder AK, Hov JR, Borthne A, et al. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology. 2016;151:660–669. e4. doi: 10.1053/j.gastro.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Ellinghaus D, Folseraas T, Holm K, et al. Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology. 2013;58:1074–1083. doi: 10.1002/hep.25977. [DOI] [PubMed] [Google Scholar]
- 27.Liu JZ, Hov JR, Folseraas T, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670–675. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji S-G, Juran BD, Mucha S, et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49:269–273. doi: 10.1038/ng.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maroni L, van de Graaf SFJ, Hohenester SD, et al. Fucosyltransferase 2: a genetic risk factor for primary sclerosing cholangitis and Crohn’sdisease—a comprehensive review. Clin Rev Allergy Immunol. 2015;48:182–191. doi: 10.1007/s12016-014-8423-1. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther. 2012;36:517–533. doi: 10.1111/j.1365-2036.2012.05223.x. [DOI] [PubMed] [Google Scholar]
- 31.Floreani A, Rizzotto ER, Ferrara F, et al. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516–1522. doi: 10.1111/j.1572-0241.2005.41841.x. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph G, Gotthardt D, Kloeters-Plachky P, et al. In PSC with dominant bile duct stenosis, IBD is associated with an increase of carcinomas and reduced survival. J Hepatol. 2010;53:313–317. doi: 10.1016/j.jhep.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Gulamhusein AF, Eaton JE, Tabibian JH, et al. Duration of inflammatory bowel disease is associated with increased risk of cholangiocarcinoma in patients with primary sclerosing cholangitis and IBD. Am J Gastroenterol. 2016;111:705–711. doi: 10.1038/ajg.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manetti N, Bagnoli S, Rogai F, et al. Disease course and colectomy rate of ulcerative colitis: a follow-up cohort study of a referral center in Tuscany. Inflamm Bowel Dis. 2016;22:1945–1953. doi: 10.1097/MIB.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 35.Samuel S, Ingle SB, Dhillon S, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis. 2013;19:1858–1866. doi: 10.1097/MIB.0b013e31828c84c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 37.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 38.Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 39.Claessen MMH, Vleggaar FP, Tytgat KMAJ, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158–164. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.