Abstract
Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) is a serious pest of stored agricultural products and one of the most common insects found in grain storage and food processing facilities. Heat treatment has been revisited to control stored-product insects as a potential alternative to methyl bromide for disinfesting mills and food-processing facilities. The influence of acclimation of T. castaneum adults, pupae, larvae, and eggs to sublethal temperatures of 36, and 42°C on their subsequent susceptibility to lethal temperature of 50°C was respectively investigated. The acclimation of T. castaneum eggs, larvae, pupae, and adults to 36, and 42°C significantly decreased their subsequent susceptibility to lethal high temperature of 50°C. The influence of acclimation to 42°C was significantly greater than that of acclimation to 36°C. The most influential acclimation times at 42°C for mortality of T. castaneum eggs, larvae, pupae, and adults were 15, 5, 5, and 5 h, respectively, and their corresponding mortality were 41.24, 5.59, 20.19, and 4.48%, compared to 100% mortality of T. castaneum eggs, larvae, pupae, and adults without acclimation when exposed to 50°C for 35 min, respectively. The present results have important implications for developing successful heat treatment protocols to control T. castaneum, improving disinfestation effectiveness of heat treatment and understanding insect response to high temperatures.
Introduction
The red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) ranks as one of the most destructive insects associated with food-processing facilities in the world. Fumigating food processing facilities with phosphine or methyl bromide has been a very effective method for controlling T. castaneum population for decades [1,2]. However, methyl bromide has been thoroughly phased out due to its ozone depleting potential according to the Montreal Protocol worldwide [3]. Meanwhile, intensive and repeated use of phosphine for control of T. castaneum population has resulted in extensive concerns regarding insecticide resistance, environmental contamination, pesticide residues, lethal effects on non-target organisms and so on [4–7]. Exploration and implementation of alternative control strategy and integrated pest management system have recently been considered to be the only solution to combat the increasing insecticide-resistant insect.
Heat treatment, as a potential alternative to methyl bromide for disinfesting mills and food-processing facilities, has been revisited to control stored-product insects [1, 8, 9]. Heat treatment, as an environment-friendly and convenient method, has been widely evaluated and implemented to effectively disinfest mills and food-processing facilities [10–13]. During the heat treatment, the target mill or food-processing facility usually is heated to 50–60°C which are then maintained for 24–36 h to completely eradicate stored product insects [14–17]. Naturally, the stored product insects inevitably experience acclimation to sublethal high temperature during gradually elevating temperature of the target mill or food-processing facility from ambient temperature to at least 50°C, which is the minimum effective temperature for disinfestation [17].
Although most of the researches have investigated the effect of high temperatures on mortality of stored grain insects [15,16,18–23], the influence of acclimation to sublethal temperatures on the mortality of T. castaneum is little known so far, which is very useful for developing successful heat treatment protocols for controlling T. castaneum, understanding insect response to high temperatures and the evolution of the reaction norms [24–27]. Here, our aim was to investigate if short-term acclimation to sublethal temperature affects heat tolerance of T. castaneum adults, pupae, larvae, and eggs when subsequently exposed to lethal high temperature of 50°C.
Materials and methods
Insects
Cultures of the T. castaneum were maintained in a controlled temperature and humidity chamber at 27±2°C, 75±5% r.h. and a 12:12 light:dark photoperiod without exposure to any pesticide at the Institute of Stored Product Insects of Henan University of Technology, Zhengzhou, China. The food media used were wheat flour and rolled oats (6:1, w/w). One-week-old adults, 1-d-old pupae, 18-d-old larvae, and 1-d-old eggs were randomly chosen for bioassays [16].
Experimental protocol of acclimation to sublethal temperature
Twenty T. castaneum adults, pupae, larvae, and eggs were randomly selected and respectively put into empty plastic vials with a few of small holes for heat quick distribution, and then exposed to 36°C or 42°C [28, 29] in a temperature chamber (Thermocenter TN/GDW-010B, Tainuo Experiment Instrument Factory, Wuxi, Jiangsu, China) for 0 (control), 1, 5, 10, and 15 h as different acclimation periods, respectively. Subsequently, the acclimated T. castaneum adults, pupae, larvae, and eggs were respectively exposed to 50°C for 0, 10, 15, 20, 25, 30, and 35 min, and then the plastic vials holding adults or larvae were immediately opened. The treated adults or larvae in one plastic vial were gently brushed into a petri dish for determining their mortality. The adults or larvae were considered dead if no movement was observed when prodded with a camel’s hair brush. The plastic vials holding T. castaneum pupae were then maintained in insect culture environment, and the number of pupae developing into adults was recorded everyday for the following 7 days. The pupae that could not develop into adults were considered dead. The treated eggs in one plastic vial were gently brushed into a petri dish, and then maintained in insect culture environment. The number of eggs hatching into larvae was recorded everyday for the following 7 days. Three replicates were conducted. The acclimated T. castaneum adults, pupae, larvae, and eggs were respectively exposed to 50°C because it is the minimum effective temperature for facility heat treatment [17].
RNA sequencing and quality control
Twenty T. castaneum larvae, pupae, and adults with acclimation to 42°C for 0 (control) and 10 h were flash-frozen in liquid N2 and stored at -80°C. Total RNA was isolated using Trizol Reagent (Invierogen, USA) according to the manufacturer’s instructions. An Agilent Bioanalyzer 2100 (Agilent Technologies) was used to assess the integrity/quality of the mRNA. Total RNA from the each treatment of T. castaneum larvae, pupae, and adults were respectively pooled for mRNA purification, and Illumina sequencing analysis was performed using a HiSeq 2500 with Beijing Biomarker Technologies CO., LTD (Beijing, China).
As a fundamental quality control measure, the Quality Score was used to evaluate base quality of the raw data. Clean reads were obtained by filtering the containing adapter or ploy-N and the low quality reads from raw data. The T. castaneum genome sequence was obtained from NCBI. Clean reads were aligned to the T. castaneum reference using TopHat 2.0 software. The relative expression levels of hsp70, trpA1, painless and pyrexia genes of T. castaneum larvae, pupae, and adults were determined using the fragments per kilobase of transcript per million mapped fragments method.
Statistical analysis
The acclimated T. castaneum mortality after exposure to 50°C for different time intervals was calculated as a percentage. Mean ± SE mortality of the control T. castaneum adults, pupae, larvae, and eggs in all combinations of exposure temperature and exposure time was 0.00 ± 0.00, 1.11±1.11, 1.15±1.15, and 14.57±1.75%, respectively. Therefore, treatment mortality data in T. castaneum adults, pupae, and larvae were not corrected for control mortality, and treatment mortality data in T. castaneum eggs were corrected for control mortality [30]. Treatment percentage mortality was transformed to arcsine square-root value before subjecting to two-way analysis of variance (ANOVA) with insect mortality as response variable, and acclimation time, and exposure time as fixed effects. The mean mortality was compared and separated by Scheffe’s test at p = 0.05 level. These analyses were performed using SPSS Version 16.0 software [27].
Results
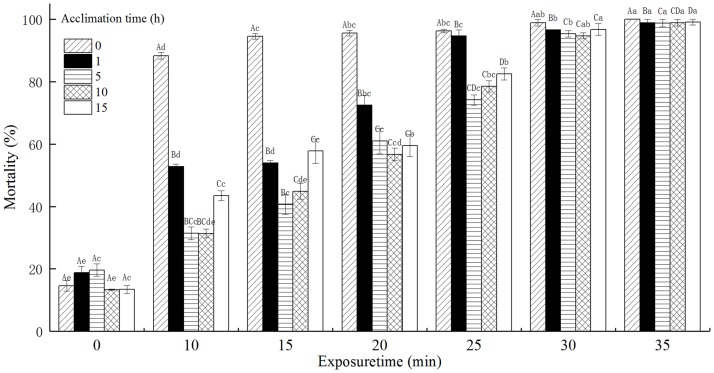
The mortality of T. castaneum eggs with acclimation to 36°C
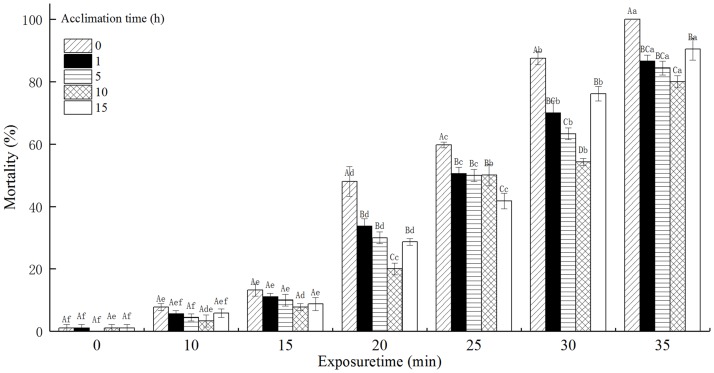
The mortality of T. castaneum eggs with acclimation to 36°C significantly increased with increasing exposure time when subsequently exposed to 50°C (Fig 1). Compared with the mortality of T. castaneum eggs without acclimation to 36°C (control), the mortality of acclimated T. castaneum eggs was significantly reduced when exposed to 50°C for the range of 0 to 25 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum eggs at p < 0.05 level.
Fig 1. The effect of acclimation to 36°C on mortality (%) of T. castaneum eggs exposed to 50°C.
Note: Different lowercase letters indicate significant differences at different exposure times for mortality (%) of T. castaneum with the same acclimation time, and different capital letters indicate significant differences at the same exposure time for mortality (%) of T. castaneum with different acclimation times (p<0.05). The same as below.
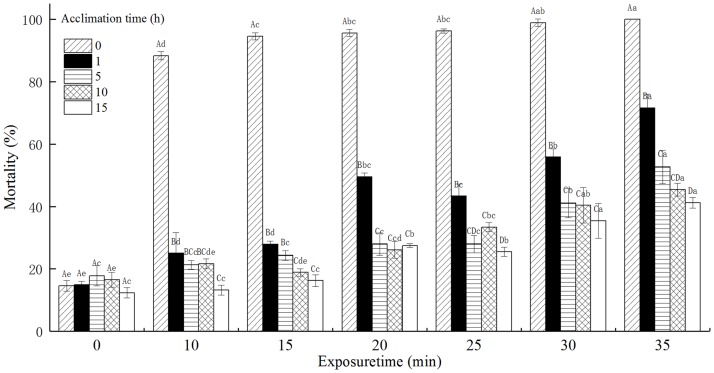
The mortality of T. castaneum eggs with acclimation to 42°C
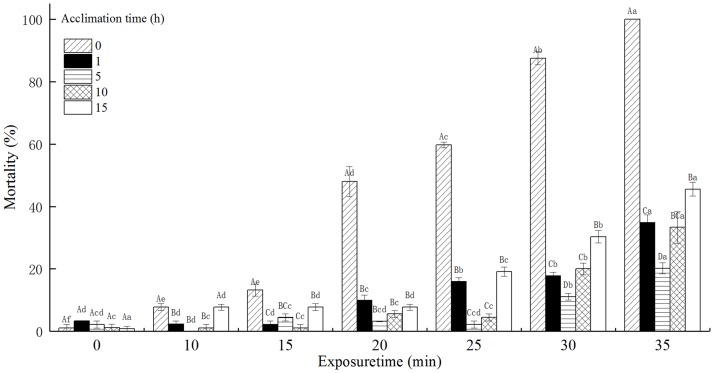
The mortality of T. castaneum eggs with acclimation to 42°C significantly increased with increasing exposure time when subsequently exposed to 50°C. The mortality of T. castaneum eggs significantly decreased with increasing acclimation time (Fig 2). Especially, the mortality of T. castaneum eggs without acclimation to 42°C (control) reached 100%, while the mortality of T. castaneum eggs with 15 h of acclimation to 42°C was only 41.24% when exposed to 50°C for 35 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum eggs at p < 0.05 level.
Fig 2. The effect of acclimation to 42°C on mortality (%) of T. castaneum eggs exposed to 50°C.
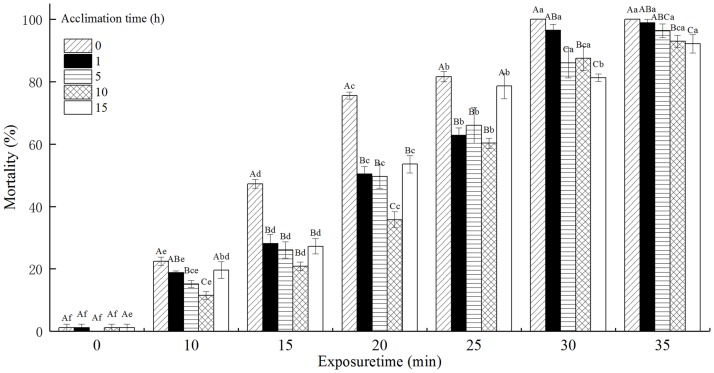
The mortality of T. castaneum larvae with acclimation to 36°C
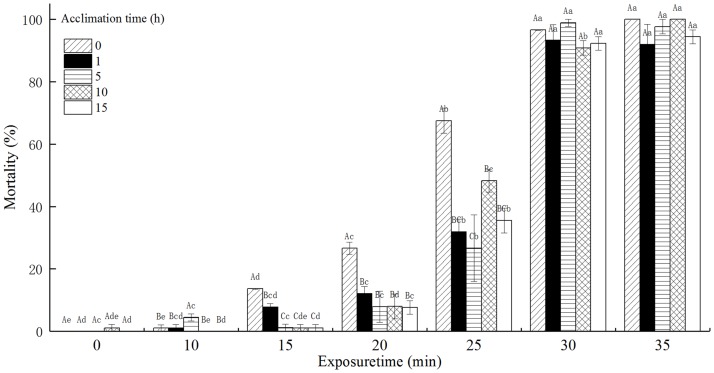
The mortality of T. castaneum larvae with acclimation to 36°C significantly increased with increasing exposure time when subsequently exposed to 50°C, and decreased with increasing acclimation time (Fig 3). Especially, the mortality of T. castaneum larvae without acclimation to 36°C (control) reached 100%, while the mortality of T. castaneum larvae with 15 h of acclimation to 36°C reached only 81.30% when exposed to 50°C for 30 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum larvae at p < 0.05 level.
Fig 3. The effect of acclimation to 36°C on mortality (%) of T. castaneum larvae exposed to 50°C.
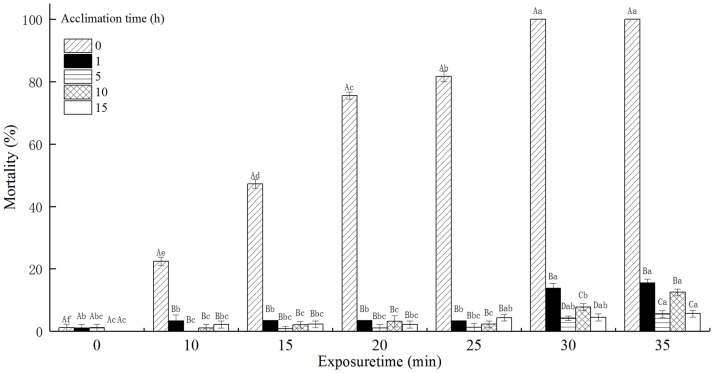
The mortality of T. castaneum larvae with acclimation to 42°C
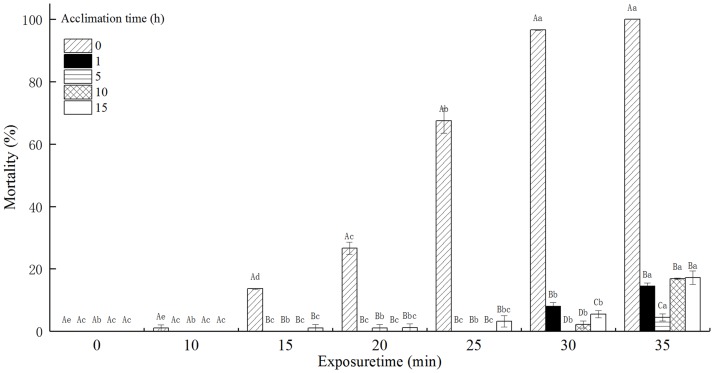
The mortality of T. castaneum larvae with acclimation to 42°C significantly increased with increasing exposure time when subsequently exposed to 50°C, and decreased with increasing acclimation time (Fig 4). Especially, the mortality of T. castaneum larvae without acclimation to 42°C (control) reached 100%, while the mortality of T. castaneum larvae with 1, 5, 10, and 15 h of acclimation to 42°C respectively reached only 13.85, 4.21, 7.78, and 4.44% when exposed to 50°C for 30 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum larvae at p < 0.05 level.
Fig 4. The effect of acclimation to 42°C on mortality (%) of T. castaneum larvae exposed to 50°C.
The mortality of T. castaneum pupae with acclimation to 36°C
The mortality of T. castaneum pupae with acclimation to 36°C significantly increased with increasing exposure time when subsequently exposed to 50°C, and decreased with increasing acclimation time (Fig 5). Especially, the mortality of T. castaneum pupae without acclimation to 36°C (control) reached 100%, while the mortality of T. castaneum pupae with 10 h of acclimation to 36°C reached only 80.00% when exposed to 50°C for 35 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum pupae at p < 0.05 level.
Fig 5. The effect of acclimation to 36°C on mortality (%) of T. castaneum pupae exposed to 50°C.
The mortality of T. castaneum pupae with acclimation to 42°C
The mortality of T. castaneum pupae with acclimation to 42°C significantly increased with increasing exposure time when subsequently exposed to 50°C, and decreased with increasing acclimation time (Fig 6). Especially, the mortality of T. castaneum pupae without acclimation to 42°C (control) reached 100%, while the mortality of T. castaneum pupae with 1, 5, 10, and 15 h of acclimation to 42°C respectively reached only 34.87, 20.19, 33.33, and 45.56% when exposed to 50°C for 35 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum pupae at p < 0.05 level.
Fig 6. The effect of acclimation to 42°C on mortality (%) of T. castaneum pupae exposed to 50°C.
The mortality of T. castaneum adults with acclimation to 36°C
The mortality of T. castaneum adults with acclimation to 36°C significantly increased with increasing exposure time, and decreased with increasing acclimation time, especially when subsequently exposed to 50°C from 0 to 25 min (Fig 7). Particularly, the mortality of T. castaneum adults without acclimation to 36°C (control) reached 67.56%, while the mortality of T. castaneum adults with 5 h of acclimation to 36°C reached only 26.67% when exposed to 50°C for 25 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum adults at p < 0.05 level.
Fig 7. The effect of acclimation to 36°C on mortality (%) of T. castaneum adults exposed to 50°C.
The mortality of T. castaneum adults with acclimation to 42°C
The mortality of T. castaneum adults with acclimation to 42°C significantly increased with increasing exposure time when subsequently exposed to 50°C, and decreased with increasing acclimation time (Fig 8). Especially, the mortality of T. castaneum adults without acclimation to 42°C (control) reached 100%, while the mortality of T. castaneum adults with 1, 5, 10, and 15 h of acclimation to 42°C respectively reached only 14.44, 4.48, 16.86, and 17.20% when exposed to 50°C for 35 min. The acclimation time, exposure time, and the interaction between the acclimation time and exposure time significantly affected the mortality of T. castaneum adults at p < 0.05 level.
Fig 8. The effect of acclimation to 42°C on mortality (%) of T. castaneum adults exposed to 50°C.
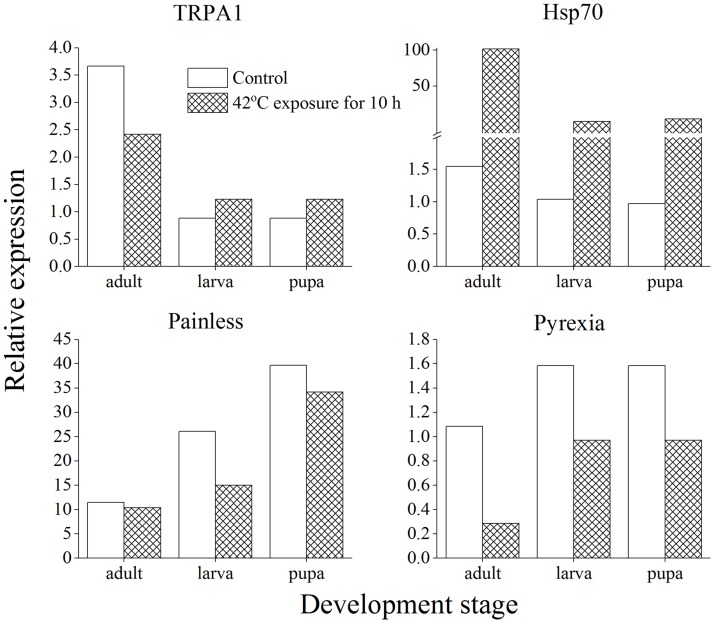
The relative expression levels of hsp70, trpA1, painless and pyrexia genes of T. castaneum
The Fig 9 shows that relative expression of hsp70, trpA1, painless and pyrexia genes of T. castaneum with acclimation to 42°C for 10 h. Compared to the controls, hsp70 gene of T. castaneum larvae, pupae, and adults was significantly up-regulated after acclimation to 42°C for 10 h, the trpA1 gene of T. castaneum larvae and pupae was also up-regulated, and the painless and pyrexia genes of T. castaneum was down-regulated.
Fig 9. The relative expression of hsp70, trpA1, painless and pyrexia genes of T. castaneum with acclimation to 42°C for 10 h.
Discussion
The susceptibility of insects to lethal high temperatures was usually influenced by a variety of treatment factors, including insect strain, developmental stage, acclimation time, acclimation temperature, temperature-time combination, heating rate and so on [31, 32]. The present study results indicated that prior short-term acclimation to sublethal high temperatures of 36, and 42°C could significantly enhance the survival of T. castaneum eggs, larvae, pupae, and adults subsequently exposed to lethal high temperature of 50°C, and reduce their mortality. The mortality of T. castaneum eggs, larvae, pupae, and adults significantly increased with increasing exposure time at lethal high temperature of 50°C. Therefore, acclimation to sublethal high temperatures greatly enhanced the heat tolerance level of T. castaneum eggs, larvae, pupae, and adults, and significantly reduced their subsequent susceptibility to lethal high temperature.
Mahroof et al. (2003b) investigated time-mortality relationships for different life stages of T. castaneum exposed to constant temperatures of the range of 42 to 60°C [16]. Mortality of each life stage increased with increasing temperature and exposure time, and young larvae are the most heat-tolerant stage exposed to 50°C. The present study also gave the similar results. In addition, the present study results are also similar to Arthur (2006) [33] research results, which show that the mortality of late-instar larvae, pupae, and adults of T. castaneum and Tribolium confusum in gradually increasing temperature conditions was lower than that in suddenly increasing temperature conditions. The difference is that the T. castaneum was acclimated at constant temperatures in the current study, whereas the T. castaneum is exposed in gradually increasing temperature conditions in Arthur (2006) [33] research. The present results as well as the previous research results are very helpful to understand insect response to different high temperature treatments, in favor of designing effective heat treatment protocols for controlling T. castaneum in practice.
In the current study, the influence of short term acclimation of T. castaneum eggs, larvae, pupae, and adults to sublethal temperature of 42°C on their subsequent susceptibility to lethal high temperature of 50°C was apparently greater than that of acclimation to 36°C. The most influential acclimation times to 42°C for mortality of T. castaneum eggs, larvae, pupae, and adults were 15, 5, 5, and 5 h, respectively, and the most influential acclimation times to 36°C for mortality of T. castaneum eggs, larvae, pupae, and adults were 5, 10, 10, and 15 h, respectively. The influence of acclimation time and developmental stage on the heat tolerance level of T. castaneum was variable. This may be related to the ability of different developmental stage insects to deal with different high temperature stress, which deserves to be further investigated.
The ability of insects to cope with high temperature stress can be achieved by physiological and biochemical mechanisms [34–36]. Emekci et al. (2002) [37] showed that respiration of T. castaneum young larvae is significantly higher than other developmental stages, which may result in higher metabolic rates often associated with a response to stress and may enhance adaption to unfavorable conditions. Insects also can alter their sensitivity to heat stress through short-term acclimation or long-term evolutionary adaptation [38]. Short-term heat acclimation in laboratory may involve physiological and biochemical changes in insects, and then change their responses to the ambient variable environmental temperatures [39–41]. Three different hsp70 genes, tchsp70 I, tchsp70 II, and tchsp70 III, respectively encode a heat-inducible HSP, a constitutively expressed HSP, and a developmentally regulated HSP in T. castaneum young larvae which are responsible for enhancing heat tolerance level [42]. The trpA1 is closely associated with high temperature sensing and also increasing high-temperature tolerance, the painless is responsible for rapid acclimation to high temperature, and the pyrexia plays an important role in protecting T. castaneum adults from acute heat stress. RNAi bioassay results also showed that relatively short exposure to high temperature (1 min at 52°C or 10 min at 42°C) was enough to give rise to thermal acclimation [29]. Heat shock increases three heat shock protein (hsp) genes hsps—Szhsp70, Szhsc70, and Szhsp90 expression in S. zeamais with the highest upregulation at 40°C, and the intensity of upregulation is ranked as follows: Szhsp70 > Szhsp90 > Szhsc70 [43]. The present results indicated that hsp70 and trpA1 genes of T. castaneum were up-regulated after acclimation to 42°C for 10 h. Therefore, the hsps as well as some other physiological and biochemical adaptation mechanisms, were involved in the heat tolerance of T. castaneum with acclimation to sublethel high temperatures for varying periods. Detailed genetic expression variation and metabolic pathways associated with acclimation of T. castaneum eggs, larvae, pupae, and adults to sublethel high temperatures deserve to be further investigated, which are beneficial to understand insects responses to thermal stress and their adaptation evolution mechanisms in response to ongoing climate warming [44].
Usually, the temperatures don’t increase at the same rate, and not evenly distribute at different portions of the whole facility during heat treatment, which inevitably makes the stored product insects experience the acclimation to sublethal temperatures. The present research simulated discrete heating condition in some portions by exposing the insects to sublethal temperatures for a short period. The results clearly indicated that short-term acclimation to sublethal temperatures enhanced their ability of adaption to the high temperature stress environment, and significantly reduced subsequent heat susceptibility of T. castaneum exposed to 50°C. Therefore, the temperatures of the different portions in the whole target facility should be increased to more than 50°C at the quickest rate, which will avoid the acclimation of stored product insects to sublethal temperatures to the maximum extent during implementing heat treatment, and enhance efficacy of heat treatment in practice.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by National Natural Science Foundation of China (No. U1604115), National Program on Key R & D Project (No. 2016YFD0401005), Key Technologies R & D Program of the Education Department of Henan Province (No. 16A210017) and the Collaborative Innovation Center of Grain Storage and Security in Henan Province to JL.
References
- 1.Dowdy AK Mortality of red four beetle, Tribolium castaneum (Coleoptera: Tenebrionidae) exposed to high temperature and diatomaceous earth combinations. J. Stored Prod. Res. 1999; 35: 175–182. https://doi.org/10.1016/S0022-474X(98)00043-5 [Google Scholar]
- 2.Tilley DR, Casada ME, Arthur FH. Heat treatment for disinfestation of empty grain storage bins. J. Stored Prod. Res. 2007; 43(3): 221–228. https://doi.org/10.1016/j.jspr.2006.04.005 [Google Scholar]
- 3.Fields PG, White ND. Alternatives to methyl bromide treatments for stored—product and quarantine insects. Annu. Rev. Entomol. 2002; 47(1): 331–359. doi: 10.1146/annurev.ento.47.091201.145217 [DOI] [PubMed] [Google Scholar]
- 4.Rajendran S, Narasimhan KS. Phosphine resistance in the cigarette beetle Lasioderma serricorne (Coleoptera: Anobiidae) and overcoming control failures during fumigation of stored tobacco. Int. J. Pest Manage. 1994; 40: 207–210. [Google Scholar]
- 5.Jembere B, Obeng-Ofori D, Hassanali A, Nyamasyo GNN. Products derived from the leaves of Ocimum kilimanndscharicum (Labiatae) as post-harvest grain protectants against the infestation of three major stored product insect pests. Bull. Entomol. Res. 1995; 85: 361–367. [Google Scholar]
- 6.Liu ZL, Ho SH. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst), J. Stored Prod. Res. 1999; 35: 317–328. https://doi.org/10.1016/S0022-474X(99)00015-6 [Google Scholar]
- 7.Jovanović Z, Kostić M, Popović Z. Grain-protective properties of herbal extracts against the bean weevil Acanthoscelides obtectus Say, Ind. Crop. Prod. 2007; 26: 100–104. https://doi.org/10.1016/j.indcrop.2007.01.010 [Google Scholar]
- 8.Wijayaratne LKW, Fields PG. Effect of methoprene on the heat tolerance and cold tolerance of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2010; 36(1): 1–8. https://doi.org/10.1016/j.jspr.2010.04.001 [Google Scholar]
- 9.Frederick JL. Heat Treatment of Empty Storage Bins and Grain-Processing Facilities: Factors Influencing Efficacy against Adults of the Red Flour Beetle, Tribolium castaneum (Herbst). Ph.D thesis, Kansas State University; 2016.
- 10.Shellie KC, Mangan RL. Postharvest quality of Valencia orange after exposure to hot, moist, forced air for fruit fly disinfestation. HortScience. 1994; 29:1524–1527. [Google Scholar]
- 11.Roesli R, Subramanyam B, Fairchild F, Behnke K. Trap catches of stored-product insects before and after heat treatment of a pilot feed mill. J. Stored Prod. Res. 2003; 39: 521–540. https://doi.org/10.1016/S0022-474X(02)00058-9 [Google Scholar]
- 12.Jin Z. Innovation concept and achievements of China grain storage science and technology. Sci. Technol. Cereals, Oils Foods. 2011; 19:1–5. [Google Scholar]
- 13.Opit GP, Arthur FH, Bonjour EL, Jones CL, Phillips TW. Efficacy of heat treatment for disinfestation of concrete grain silos. J. Econ. Entomol. 2011; 104(4):1415–1422. https://doi.org/10.1603/EC11104 [DOI] [PubMed] [Google Scholar]
- 14.Fields PG. The control of stored-product insects and mites with extreme temperatures. J. Stored Prod. Res. 1992; 28: 89–118. https://doi.org/10.1016/0022-474X(92)90018-L [Google Scholar]
- 15.Mahroof R, Subramanyam B, Eustace D. Temperature and relative humidity profiles during heat treatment of mills and its efficacy against Tribolium castaneum (Herbst) life stages. J. Stored Prod. Res. 2003a; 39: 555–569. https://doi.org/10.1016/S0022-474X(02)00062-0 [Google Scholar]
- 16.Mahroof R, Subramanyam B, Throne JE. Menon A. Time-mortality relationships for Tribolium castaneum (Coleoptera: Tenebrionidae) life stages exposed to elevated temperatures. J. Econ. Entomol. 2003b; 96: 1345–1351. https://doi.org/10.1093/jee/96.4.1345 [DOI] [PubMed] [Google Scholar]
- 17.Mahroof R, Subramanyam B. Flinn P. Reproductive performance of Tribolium castaneum (Coleoptera: Tenebrionidae) exposed to the minimum heat treatment temperature as pupae and adults. J. Econ. Entomol. 2005; 98(2):626–633. https://doi.org/10.1093/jee/98.2.626 [DOI] [PubMed] [Google Scholar]
- 18.Gonen M. Susceptibility of Sitophilus granarius and S. oryzae (Coleoptera: Curculionidae) to high temperature after exposure to supra‐optimal temperature. Entomol. Exp. Appl. 1977; 21(3): 243–248. doi: 10.1111/j.1570-7458.1977.tb02678.x [Google Scholar]
- 19.Evans DE. The influence of some biological and physical factors on the heat tolerance relationships for Rhyzopertha dominica (F.) and Sitophilus oryzae (L.) (Coleoptera: Bostrychidae and Curculionidae). J. Stored Prod. Res. 1981; 17(2): 65–72. https://doi.org/10.1016/0022-474X(81)90018-7 [Google Scholar]
- 20.Beckett SJ, Fields PG, Subramanyam B. Disinfestation of stored products and associated structures using heat In: Tang J, Mitcham E, Wang S, Lurie S (eds). Heat treatments for postharvest pest control: theory and practice. CAB International, Oxon, United Kingdom, 2007;182–236. [Google Scholar]
- 21.Beckett SJ, Morton R. Darby JA. The mortality of Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) and Sitophilus oryzae (L.) (Coleoptera: Curculionidae) at moderate temperatures. J. Stored Prod. Res. 1998; 34(4): 363–376. [Google Scholar]
- 22.Wang S, Johnson JA, Tang J. Yin X. Heating condition effects on thermal resistance of fifth-instar Amyelois transitella (Walker) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2005; 41(4): 469–478. https://doi.org/10.1016/j.jspr.2004.07.003 [Google Scholar]
- 23.Yan R, Huang Z, Zhu H, Johnson JA, Wang S. Thermal death kinetics of adult Sitophilus oryzae and effects of heating rate on thermotolerance. J. Stored Prod. Res. 2014; 59: 231–236. https://doi.org/10.1016/j.jspr.2014.03.006 [Google Scholar]
- 24.Neven LG. Physiological responses of insects to heat. Postharvest Biol. Tech. 2000; 21(1): 103–111. https://doi.org/10.1016/S0925-5214(00)00169-1 [Google Scholar]
- 25.Johnson JA, Valero KA, Wang S, Tang J. Thermal death kinetics of red flour beetle (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2004: 96(7): 1868–1873. https://doi.org/10.1093/jee/97.6.1868 [DOI] [PubMed] [Google Scholar]
- 26.Lachenicht MW, Clusella-Trullas S, Boardman L, LeRoux C, Terblanche JS. Effects of acclimation temperature on thermal tolerance, locomotion performance and respiratory metabolism in Acheta domesticus L. (Orthoptera: Gryllidae). J. Insect Physiol. 2010; 56: 822–830. https://doi.org/10.1016/j.jinsphys.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 27.Lü J, Zhang H. The effect of acclimation to sublethal temperature on subsequent susceptibility of Sitophilus zeamais Mostchulsky (Coleoptera: Curculionidae) to high temperatures. PLoS ONE 2016; 11(7): e0159400 doi: 10.1371/journal.pone.0159400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma G, Ma CS. Effect of acclimation on heat-escape temperatures of two aphid species: implications for estimating behavioral response of insects to climate warming. J. Insect Physiol. 2012; 58: 303–309. https://doi.org/10.1016/j.jinsphys.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 29.Kim HG, Margolies D, Park Y. The roles of thermal transient receptor potential channels in thermotactic behavior and in thermal acclimation in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 2015; 76: 47–55. https://doi.org/10.1016/j.jinsphys.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 30.Abbott WS. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18: 265–267. [Google Scholar]
- 31.Piyaphongkul J, Pritchard J, Bale J. Effects of acclimation on the thermal tolerance of the brown planthopper Nilaparvata lugens (Stål). Agr. Forest Entomol. 2014; 16: 174–183. [Google Scholar]
- 32.Li W, Wang K, Chen L, Johnson J A, Wang S J. Tolerance of Sitophilus zeamais (Coleoptera: Curculionidae) to heated controlled atmosphere treatments. J. Stored Prod. Res. 2015, 62: 52–57. https://doi.org/10.1016/j.jspr.2015.04.001 [Google Scholar]
- 33.Arthur FH. Initial and delayed mortality of late-instar larvae, pupae, and adults of Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae) exposed at variable temperatures and time intervals. J. Stored Prod. Res. 2006; 42(1): 1–7. https://doi.org/10.1016/j.jspr.2004.10.003 [Google Scholar]
- 34.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann. Rev. Physiol. 1999; 61: 243–282. [DOI] [PubMed] [Google Scholar]
- 35.Chown SL, Nicolson SW. Insect Physiological Ecology: Mechanisms and Patterns. Oxford University Press, UK; 2004. [Google Scholar]
- 36.Angilletta MJ, Huey RB, Frazier MR. Thermodynamic effects on organismal performance: is hotter better? Physiol. Biochem. Zool. 2010; 83: 197–206. doi: 10.1086/648567 [DOI] [PubMed] [Google Scholar]
- 37.Emekci M, Navarro S, Donahaye E, Rindner M, Azrieli A. Respiration of Tribolium castaneum (Herbst) at reduced oxygen concentrations. J. Stored Prod. Res. 2002; 38: 413–425. https://doi.org/10.1016/S0022-474X(01)00045-5 [Google Scholar]
- 38.Huey RB. Evolutionary physiology of insect thermal adaptation to cold environments In: Denlinger DL, Lee RE, editors. Low Temperature Biology of Insects. Cambridge University Press, UK; 2010. pp. 223–241. [Google Scholar]
- 39.Bennett AF, Lenski RE. Evolutionary adaptation to temperature. 6. Phenotypic acclimation and its evolution in Escherichia coli. Evolution. 1997; 51: 36–44. doi: 10.1111/j.1558-5646.1997.tb02386.x [DOI] [PubMed] [Google Scholar]
- 40.Addo-Bediako A, Chown SL, Gaston KJ. Metabolic cold adaptation in insects: a large-scale perspective. Funct. Ecol. 2002; 16: 332–338. doi: 10.1046/j.1365-2435.2002.00634.x [Google Scholar]
- 41.Hazell SP, Pedersen BP, Worland RW, Blackburn TM, Bale JS. A method for the rapid measurement of thermal tolerance traits in studies of small insects. Physiol. Entomol. 2008; 33: 389–394. doi: 10.1111/j.1365-3032.2008.00637.x [Google Scholar]
- 42.Mahroof R. Elevated Temperatures for Insect Management in Mills: Biological, Biochemical, and Molecular Responses of Tribolium castaneum (Herbst). Ph.D thesis, Kansas State University; 2004.
- 43.Tungjitwitayakul J, Tatun N, Vajarasathira B, Sakurai S. Expression of heat shock protein genes in different developmental stages and after temperature stress in the maize weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2015, 108(3): 1313–1323. https://doi.org/10.1093/jee/tov051 [DOI] [PubMed] [Google Scholar]
- 44.Ju R T, Gao L, Zhou X H, Li B. Tolerance to high temperature extremes in an invasive lace bug, Corythucha ciliata (Hemiptera: Tingidae), in subtropical China. PLoS ONE 2013; 8(1): e54372 https://doi.org/10.1371/journal.pone.0054372 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.