Abstract
Background:
While medication adherence in chronic disease has been evaluated in the general population, limited data are available among Medicaid recipients, especially within federally qualified health centers (FQHCs). This study determined baseline medication adherence for Medicaid recipients receiving care in an FQHC for first-line medications used in hypertension, hyperlipidemia, and diabetes. Secondary outcomes included baseline adherence for individual patient factors.
Methods:
Patients from the Salud Family Health Centers, an FQHC with a large percentage of both Spanish-speaking patients and providers, were included in this study. Using retrospective prescription claims reports from 1 January 2015 to 1 October 2015, medication possession ratios (MPRs) and proportion of days covered (PDC) were calculated for each medication group. Patients with adherence ⩾0.80 were considered adherent.
Results:
From 1034 individual patients, 1788 medications were evaluated. Using MPRs, adherence rates were highest among medications for hypertension (67.2% adherent), followed by hyperlipidemia (67.0%), and lastly diabetes (58.0%); p < 0.001. Likewise, using PDC, adherence rates were highest for medications for hypertension (56.6%), followed by hyperlipidemia (52.2%), and lastly diabetes (45.0%); p = 0.010. Lower rates of adherence were seen among men, patients age 18–29 years old, African Americans, and patients with English documented as their preferred language.
Conclusions:
Although overall medication adherence rates within our FQHC patients were comparable with those in previous literature, differences seen among medication groups and patient individual factors suggest that there is still much to be learned in improving adherence. Future efforts will require a multifaceted approach, tailored to patient-specific needs.
Keywords: community health centers, diabetes, hyperlipidemia, hypertension, Medicaid, medication adherence, primary health care
Introduction
In 2003, the World Health Organization (WHO) reported that approximately 50% of patients with chronic illnesses in developed countries do not take medications as prescribed, ultimately leading to increased morbidity, mortality, and costs.1 In the United States, 33–69% of all medication-related hospitalizations are linked to poor medication adherence, resulting in approximately 125,000 deaths per year and an estimated $100 billion annually.2 This estimated burden includes both direct costs, such as those incurred by the treatment of disease, as well as indirect costs, such as lost work productivity.3
Despite a general awareness of the harms associated with medication nonadherence, improving medication adherence with chronic diseases has been historically challenging, due to its complex and patient-specific nature. Among commercially insured patients, patients taking both antihypertensive and lipid-lowering medications exhibit <50% medication adherence within the first 3 months and <40% by 12 months.4 Even in well-controlled clinical trials for the treatment of chronic conditions, medication adherence has ranged from 43–78%.5–7 Results from the National Health and Nutrition Examination Survey revealed an increase in the percentage of adults in the United States (US) using any prescription drugs, from 51% to 59% between 1990–2000 and 2011–2012.6 Similarly, the prevalence of polypharmacy, defined as the use of ⩾5 prescription drugs, increased from an estimated 8.2% to 15%.8 As the use of medications rises, there is a growing need to understand and improve medication adherence.8
Previous literature has repeatedly shown the inverse relationship between multiple chronic stressors, such as financial hardship, and medication adherence.9 Within the US, there has been increased provision of primary care to communities with low-income and uninsured individuals through publicly funded health centers, known as federally qualified health centers (FQHCs).10 These serve as primary medical homes for >24 million Americans, a number that continues to grow.11 Despite the abundance of evidence demonstrating the ability of medications such as antihypertensive therapy to reduce the risk of stroke and coronary heart disease, many of these agents remain well underutilized due to poor adherence.12 Patients receiving care from an FQHC often experience overlapping vulnerabilities from various social disadvantages, the requirements of complex clinical needs, and challenges specific to ethnic minorities/immigrants.9 Medication adherence is often thought of exclusively as the responsibility of the patient. However, this view neglects the responsibilities that healthcare providers have in support of patient success. While research has continued to assess various intervention strategies such as reminder tools and patient education services to improve adherence to chronic disease medications in the US, many of these have shown statistically insignificant results and have been difficult to generalize to larger patient populations due to the heterogeneity of the studies.3,13 Ultimately, reasons for nonadherence are complex, multifactorial, and largely patient specific. Thus, it is important that healthcare providers develop patient-specific, effective strategies that help assess and promote medication adherence.
There are limited data evaluating medication adherence within the Medicaid recipient population and even less in the setting of an FQHC.14 Thus, this study was conducted to evaluate medication adherence in Medicaid recipients receiving care within an FQHC.
Methods
This study was approved by the Colorado Multiple Institutional Review Board, CO, USA.
Study design, setting, and participants
This retrospective, descriptive study was conducted at Salud Family Health Centers (Salud), a comprehensive primary health care system comprised of 12 FQHCs spread across 9 communities in northeast Colorado, USA with a large percentage of both Spanish-speaking patients and providers. Patients receiving care from two primary care clinic locations, Brighton and Commerce City, were included in this study.
The target population for this study included all patients enrolled in the Colorado Access Medicaid Regional Care Collaborative Organization (RCCO) receiving care from Salud in Brighton and Commerce City. Prescription claims analyzed ranged from 1 January 2015 to 1 October 2015. With the passing of the Affordable Care Act, Colorado elected to expand Medicaid. The study occurred after Colorado’s participation in Medicaid expansion through the Affordable Care Act. This increased the number of Coloradans receiving Medicaid coverage and improved innovative models of managed care for Medicaid recipients. Colorado Access RCCOs help Colorado Medicaid members manage their medical and/or behavioral health needs through community and social services and through care management support. Colorado Access funds two full-time clinical pharmacists and one PGY2 Ambulatory Care clinical pharmacy resident at Salud.
Chronic disease medications included in this study were predetermined and limited to three medication groups: hypertension medications, hyperlipidemia medications, and diabetes medications (Table 1). These medications were the most commonly prescribed, first-line agents within Salud. Patients and all their associated medications were excluded if they were <18 years old or >89 years old. They were also excluded if any of the patients’ prescribed medications possessed an overall day supply of <30 days; if the medication was prescribed <30 days from the end of the study period; and if the medication was discontinued by a provider before the end of the study period. These parameters were required in order to perform an accurate calculation of medication adherence.
Table 1.
Medication groups.
| Hypertension | Hyperlipidemia | Diabetes |
|---|---|---|
| Lisinopril Losartan Lisinopril/hydrochlorothiazide Losartan/hydrochlorothiazide Amlodipine Amlodpine/benazepril Hydrochlorothiazide Chlorthalidone |
Simvastatin Atorvastatin Pravastatin Rosuvastatin |
Metformin Metformin extended-release |
Data source
Age, sex, race, clinic location, medication name, medication group, paid quantity, and prescription fill information were all obtained from Colorado Access prescription claims reports. Patients’ preferred language, prescribed daily dose and subsequently, prescribed day supply were obtained by performing manual chart reviews within the Salud electronic medical record, eClinicalWorks (eClinicalWorks, LLC; Westborough, MA, USA).
Outcome and assessments
The primary outcome was the calculated baseline adherence for the three medication groups: hypertension, hyperlipidemia, and diabetes. Adherence was calculated using two measures: medication possession ratio (MPR) and proportion of days covered (PDC).15 MPR is defined as the number of days a medication is supplied within the refill interval divided by the number days in the refill interval. PDC is defined as the number of days the medication is supplied during the study period divided by the number of days in the study period. These two measures are widely used in health research and supported by the International Society for Pharmaceutical Outcomes Research.16,17 These computed measures of adherence were classified into binary outcomes, where patients were either nonadherent (MPR or PDC < 0.80) or adherent (MPR or PDC ⩾ 0.80).18–23 Medication adherence was calculated for each individual medication, classified as nonadherent or adherent, then reported based on their respective medication groups. Secondary outcomes were the calculated baseline adherence rates for individual patient factors including age, sex, clinic location, race, and a patient’s preferred language.
Statistical analysis
Descriptive statistics were used to summarize findings with mean and standard deviation values reported for continuous variables, and frequencies and percentages for categorical variables. Binary outcomes were compared using the Chi-square test and standardized residuals to determine significance and the existence of group differences. Odds ratios with 95% confidence intervals were calculated and reported to assess the degree of differences. Statistical significance was considered to be p < 0.05. All data were analyzed using SPSS Statistics 23 (IBM; Armonk, NY, USA).
Results
A total of 3384 eligible prescriptions were reviewed for inclusion, and 1788 prescriptions among 1038 individual patients met study eligibility. Table 2 represents the baseline characteristics of the study population. Patients had a mean age of 52 years old and were predominately female (59.2%), Hispanic (56.9%), and English-speaking (70.5%). The number of patients at each of the two clinic locations was comparable.
Table 2.
Prescription and patient demographics.
| Characteristics | Patient prescriptions (n = 1788) |
|---|---|
| Mean ± SD age, years | 52 ± 10 |
| Female, n (%) | 1058 (59.2) |
| Clinic location (Commerce City), n (%) | 946 (52.9) |
| Race, n (%) | |
| Hispanic | 1017 (56.9) |
| White | 639 (35.6) |
| African American | 61 (3.4) |
| Other | 71 (4.1) |
| Preferred Language, n (%) | |
| English | 1260 (70.5) |
| Spanish | 509 (28.5) |
| Other | 19 (1.0) |
| Medication Group, n (%) | |
| Hypertension | 949 (53.1) |
| Hyperlipidemia | 452 (25.3) |
| Diabetes | 387 (21.6) |
Figure 1 depicts adherence rates within each medication group. There was a relationship between adherence rates and medication group. Using MPR, adherence rates were highest among medications for hypertension (67.2%), followed by hyperlipidemia (67.0%), and lastly diabetes (58.0%). Similarly, using PDC, adherence rates were highest among medications for hypertension (56.6%), followed by hyperlipidemia (52.2%), and lastly diabetes (45.0%). Adherence rates for diabetes medications were significantly lower than those for hypertension and hyperlipidemia using both MPR and PDC adherence measures (MPR: p < 0.001; PDC: p = 0.010). Table 3 describes these results in detail.
Figure 1.
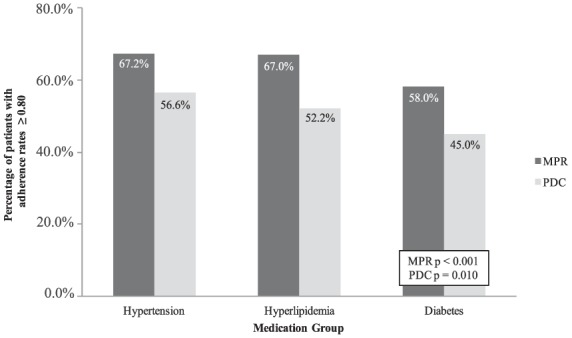
Primary outcome: baseline adherence rates among medication groups.
MPR, medication possession ratio; PDC, proportion of days covered.
Table 3.
Primary outcome: baseline adherence among medication groups.
| Medication group | MPR n (%) |
PDC n (%) |
||
|---|---|---|---|---|
| <0.80 | ⩾0.80 | <0.80 | ⩾0.80 | |
| Hypertension | 258 (32.8) | 528 (67.2) | 412 (43.4) | 537 (56.6) |
| Hyperlipidemia | 119 (33.0) | 242 (67.0) | 216 (47.8) | 236 (52.2) |
| Diabetesa | 130 (42.0) | 179 (58.0) | 213 (55.0) | 174 (45.0) |
| Total | 507 (34.8) | 949 (65.2) | 841 (47.0) | 947 (53.0) |
MPR: p < 0.001; PDC: p = 0.010.
MPR, medication possession ratio; PDC, proportion of days covered.
Table 4 describes the proportion of nonadherent and adherent recipients based on individual patient factors. Age was categorized by 10-year intervals and patients 18–29 years old were significantly less adherent than other age groups: 40% via MPR, p < 0.001; 29.2% via PDC, p < 0.001. African Americans had lower rates of adherence than White and Hispanic patients: 46.5% via MPR, p = 0.034; 37.7% via PDC, MPR p = 0.036. There was no statistical difference in adherence rates between men and women using MPR: 63.3% versus 66.4%, p = 0.223. However, men were less adherent than women when using PDC: 49.6% versus 55.3%, p = 0.018. Likewise, patients with English as their preferred language had lower adherence rates than patients with Spanish as their preferred language when using PDC alone: 51.5% versus 57.2%, p = 0.031. There was no statistical difference in medication adherence between the two clinic populations.
Table 4.
Secondary outcomes: baseline adherence based on individual patient factors.
| Individual patient factors | MPR n (%) |
PDC n (%) |
||
|---|---|---|---|---|
| <0.80 | ⩾0.80 | <0.80 | ⩾0.80 | |
| Age (years) | ||||
| 18–29a | 18 (60.0) | 12 (40.0) | 34 (70.8) | 14 (29.2) |
| 30–39 | 52 (47.3) | 58 (52.7) | 98 (62.8) | 58 (37.2) |
| 40–49 | 123 (37.2) | 208 (62.8) | 197 (48.8) | 206 (51.1) |
| 50–59 | 204 (33.1) | 412 (66.9) | 334 (44.5) | 417 (55.5) |
| 60–69 | 105 (31.2) | 232 (68.8) | 164 (41.9) | 227 (58.1) |
| 70–79 | 2 (8.3) | 22 (91.7) | 3 (11.5) | 23 (88.5) |
| 80–89 | 3 (37.5) | 5 (62.5) | 11 (84.6) | 2 (15.4) |
| Sex | ||||
| Maleb | 210 (36.7) | 362 (63.3) | 368 (50.4) | 362 (49.6) |
| Female | 297 (33.6) | 587 (66.4) | 473 (44.7) | 585 (55.3) |
| Clinic locationc | ||||
| Brighton | 249 (35.8) | 447 (64.2) | 392 (46.6) | 450 (53.4) |
| Commerce City | 258 (33.9) | 502 (66.1) | 449 (47.5) | 497 (52.5) |
| Race | ||||
| African Americand | 23 (53.5) | 20 (46.5) | 38 (62.3) | 23 (37.7) |
| Asian | 7 (24.1) | 22 (75.9) | 15 (44.1) | 19 (55.9) |
| White | 174 (32.2) | 366 (67.8) | 278 (43.5) | 361 (56.5) |
| Hispanic | 290 (35.8) | 521 (64.2) | 494 (48.6) | 523 (51.4) |
| Other | 13 (39.4) | 20 (60.6) | 16 (43.2) | 21 (56.8) |
| Preferred language | ||||
| Englishe | 374 (36.2) | 658 (63.8) | 611 (48.5) | 649 (51.5) |
| Spanish | 128 (31.2) | 282 (68.8) | 218 (42.8) | 291 (57.2) |
| Other | 5 (35.7) | 9 (64.3) | 12 (63.2) | 7 (36.8) |
| Total | 507 (34.8) | 949 (65.2) | 841 (47.0) | 947 (53.0) |
Note: Individual patient factors with attached footnotes represent statistically significant differences in MPR or PDC compared with other patient factors in their respective categories. Individual patient factors with no attached footnotes were not statistically significant.
CI, confidence interval; MPR, medication possession ratio; OR, odds ratio; PDC, proportion of days covered.
MPR: p < 0.001; PDC: p < 0.001.
MPR: OR 1.15 (95% CI 0.92–1.43, p = 0.223); PDC: OR 1.26 (95% CI 1.04–1.51, p = 0.018).
MPR: OR 1.08 (95% CI 0.87 – 1.35, p = 0.465); PDC: OR 0.96 (95% CI 0.80–1.15, p = 0.701).
MPR: p = 0.034; PDC: p = 0.036.
MPR: OR 1.25 (95% CI 0.98–1.60, p = 0.071); PDC: OR 1.26 (95% CI 1.02–1.55, p = 0.031).
Discussion
To the best of our knowledge, this is the largest medication adherence study using prescription claims data in Medicaid recipients receiving care in an FQHC. By focusing on medication adherence specific to this patient population, we aimed to provide data for a vulnerable population that is not currently well described in literature. This is particularly needed among the Colorado population. Subsequent to the passing of the Affordable Care Act, Colorado has undergone significant expansion of Medicaid, which has resulted in many previously uninsured patients now being Medicaid recipients.24 Colorado has a successful model of cost reduction through Medicaid Accountable Care Organizations, and a recent study has shown decreased costs and improved quality for Medicaid enrollees who receive a majority of their care in FQHCs.25,26 Even so, any steps that can be taken to improve patient outcomes though better management of chronic diseases should continually be assessed. This includes assessing patient characteristics associated with nonadherence, and targeting interventions to improve adherence in those populations. Improving adherence may lead to improved outcomes for diabetes and reduction in heart disease and stroke. Although adherence rates differed slightly between the various medication groups and individual patient factors, overall adherence rates were similar to adherence rates published in previous literature.
There are a few plausible explanations to why adherence rates to diabetes medications may have been lower than the other two medication groups. One possible explanation is that the drug prescribed, metformin, has the inherent potential to cause adverse effects such as gastrointestinal discomfort. However, this does not explain why medications such as statins, that have increased potential for myalgia-like symptoms, possessed a higher adherence rate. In fact, our results conflict with a previous study that reported slightly greater adherence in patients taking medications for diabetes than those for hypercholesterolemia.20 Of note, however, this previous study measured adherence using a nonvalidated adherence scoring tool. Another reason for this result would be that in many cases, metformin requires twice daily dosing versus once daily dosing seen with most antihypertensives and statins.
In regards to the secondary outcomes, many studies in the past utilized self-reporting questionnaires and surveys to measure adherence.14, 27–30 Thus, it is difficult to make direct comparisons with the previous literature when assessing individual patient factors associated with adherence. Even in the available studies using MPR or PDC as measures of adherence, there have been conflicting results of whether men or women are less adherent to their medications.2,18,22,23 Consistent with previous findings, however, younger patients have repeatedly been shown to be less adherent compared with older patients.18,22,27,29 This is especially evident in Table 4, where patients 70–79 years old possess the highest adherence rates. Perhaps this can be explained by younger patients who were less familiar with their disease and therefore, more likely to be nonadherent with their medications. One interesting finding in this study was that patients with English as their preferred language were slightly less adherent than patients with Spanish as their preferred language. This finding is not commonly observed in adherence studies. In fact, the inverse is usually observed.14,22 We conjecture that having a majority of Spanish-speaking providers within our clinics may have promoted adherence among our Spanish-speaking patients.
One main aspect of our approach that strengthens the validity of our study is the use of both MPR and PDC measures. Previous studies have focused on the use of a single measure of adherence.18,19,22,23 However, neither of these measures are perfect. While MPR measures the accuracy of prescription fills within a refill interval, otherwise known as ‘compliance’, PDC is a better measure of ‘persistence’, which reflects the duration of time a patient continues a medication as prescribed. This is the reason that lower MPR values were able to be calculated as compared with PDC. By the definition of MPR, prescriptions that are filled once but never refilled cannot generate an MPR value, while PDC calculations would yield a relatively low value. By reporting both MPR and PDC in our study, we account for both compliance and persistence, eliminating biases that may produce drastically different results within the two measures. Nevertheless, in our study, results for the two measures were generally consistent with one another. One noticeable difference, however, was found in patient sex. As aforementioned, while there was no positive association between nonadherence rates and sex when using MPR, men were less adherent than women when using PDC. This finding suggests that there may be a larger problem of medication persistence among men than among women.
Limitations
There are some limitations to our study. First, this study analyzed adherence for each individual medication rather than overall patient adherence. That is, patients who demonstrated positive adherence to multiple medications contributed multiple positive data points. As a result, we may have potentially overestimated adherence. However, we felt that this was necessary, as the primary outcome of this study was to assess differences in adherence among the various medication groups. Second, the medications evaluated in this study were not cross-referenced to WHO’s ICD-10 codes (10th revision of the International Statistical Classification of Diseases and Related Health Problems). It is probable that medications could have been used for disorders others than those listed. For example, angiotensin-converting enzyme inhibitors could have been used for heart failure as opposed to hypertension. Thus, as reported, medication adherence should be interpreted based on medication group and not necessarily on the diagnosis most often associated. Third, this study was dependent upon the accuracy of documentation within the electronic medical record. All medications included were presumably active for the entirety of the study period. However, this is only as accurate as the electronic documentation. Fourth, the adherence measures used in this study were based on prescription claims, which are surrogate markers for the actual consumption of medications. It is also possible that some patients may have used medications outside of their Medicaid benefit (e.g. samples, discount drug programs) that would not have been captured in our claims database. Fifth, patients in this study included both incident and prevalent cases, which theoretically could influence the likelihood of adherence. As a result, depending on the distribution of these patients, adherence rates could have been potentially skewed. However, we felt the composite of these patients represents an accurate cross-section of real-life clinic populations. Lastly, as Medicaid recipient demographics and prescription benefits vary from state to state, the results of this study may not be generalizable to other state Medicaid programs.
Conclusion
Medication adherence is a significant barrier to optimal patient care for chronic diseases. Our analyses demonstrated similar overall adherence rates within our FQHC Medicaid recipients compared with previous literature. The lower rates of adherence with diabetes medications, among younger patients, and among our English-speaking patients suggest that additional adherence barriers should be further explored. These data will be used to develop future targeted interventions for our patients. With the many different challenges and barriers that each patient faces, improving medication adherence requires a multifaceted and patient-specific approach. We speculate that a majority of Spanish-speaking providers may have potentially promoted adherence among our Spanish-speaking patients. Future interventions will seek to employ adherence improvement strategies that are tailored to patient-specific needs such as reinforced medication counseling with our patients with diabetes and younger patients, personalized medication calendar or pillbox appointments, increased pharmacist-operated disease management clinics, and greater utilization of technology systems.
Acknowledgments
At the time this project was conducted, Dr Oung was a PGY2 Ambulatory Care Pharmacy Resident at the University of Colorado, CO, USA.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Alvin B. Oung, School of Pharmacy, University of Wyoming, Laramie, WY, USA
Emily Kosirog, University of Colorado Anschutz Medical Campus, 12850 E. Montview Blvd. (C238), Aurora, CO 80045, USA.
Benjamin Chavez, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Clinical Pharmacy, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Jason Brunner, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Clinical Pharmacy, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Joseph J. Saseen, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Clinical Pharmacy, University of Colorado Anschutz Medical Campus, Aurora, CO, USA School of Medicine, Department of Family Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
References
- 1. Sabaté E. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 2. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 3. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J 2011; 162: 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med 2005; 165: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 5. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value Health 2003; 6: 566–573. [DOI] [PubMed] [Google Scholar]
- 6. Waeber B, Leonetti G, Kolloch R, et al. Compliance with aspirin or placebo in the Hypertension Optimal Treatment (HOT) study. J Hypertens 1999; 17: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 7. Claxton AJ, Cramer J, Pierce C. A systematic review of associations between dose regimens and medication compliance. Clin Ther 2001; 23: 1296–1310. [DOI] [PubMed] [Google Scholar]
- 8. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314: 1818–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osborn CY, Mayberry LS, Wagner JA, et al. Stressors may compromise medication adherence among adults with diabetes and low socioeconomic status. West J Nurs Res 2014; 36: 1091–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Money EB. Federally qualified health center expansion through the Affordable Care Act. N C Med J 2013; 74: 325–326. [PubMed] [Google Scholar]
- 11. National Association of Community Health Centers. About our health centers, http://nachc.org/about-our-health-centers/ (2017, accessed 22 May 2017).
- 12. Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess 2003; 7: 1–94. [DOI] [PubMed] [Google Scholar]
- 13. Viswanathan M, Golin CE, Jones CD, et al. Medication adherence interventions: comparative effectiveness. Closing the quality gap: revising the state of the science. AHRQ Evidence Report No. 208. Rockville, MD: Agency for Healthcare Research and Quality, 2012. [PMC free article] [PubMed] [Google Scholar]
- 14. Alton S, March AL, Mallary L, et al. Medication adherence in a nurse practitioner managed clinic for indigent patients. J Am Assoc Nurse Pract 2015; 27: 433–440. [DOI] [PubMed] [Google Scholar]
- 15. Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007; l0: 3–12. [DOI] [PubMed] [Google Scholar]
- 16. Cramer JA, Roy A, Burrell A. Medication compliance and persistence: terminology and definitions. Value Health 2008; 11: 44–47. [DOI] [PubMed] [Google Scholar]
- 17. Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006; 40: 1280–1288. [DOI] [PubMed] [Google Scholar]
- 18. Calderón-Larrañaga A, Diaz E, Poblador-Plou B, et al. Non-adherence to antihypertensive medication: the role of mental and physical comorbidity. Int J Cardiol 2016; 207: 310–316. [DOI] [PubMed] [Google Scholar]
- 19. Hedegaard U, Kjeldsen LJ, Pottegård A, et al. Improving medication adherence in patients with hypertension: a randomized trial. Am J Med 2015; 128: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 20. Khanna R, Pace PF, Mahabaleshwarkar R, et al. Medication adherence among recipients with chronic diseases enrolled in a state Medicaid program. Popul Health Manag 2012; 15: 253–260. [DOI] [PubMed] [Google Scholar]
- 21. Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 2009; 120: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 22. Rolnick SJ, Pawloski PA, Hedblom BD, et al. Patient characteristics associated with medication adherence. Clin Med Res 2013; 11: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong MC, Tam WW, Cheung CS, et al. Medication adherence to first-line antihypertensive drug class in a large Chinese population. Int J Cardiol 2013; 167: 1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson E, Clark B, Downs A, et al. Colorado Health Institute. Medicaid expansion in Colorado, http://www.coloradohealthinstitute.org/sites/default/files/file_attachments/MK_Expansion_Report_0.pdf (2016, accessed 3 April 2017).
- 25. McConnell KJ, Renfro S, Chan BKS, et al. Early performance in Medicaid Accountable Care Organizations-a comparison of Oregon and Colorado. JAMA Intern Med 2017; 177: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nocon RS, Lee SM, Shama R, et al. Health care use and spending for Medicaid enrollees in federally qualified health centers versus other primary care settings. AJPH 2016; 106: 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmad NS, Ramli A, Islahudin F, et al. Medication adherence in patients with type 2 diabetes mellitus treated at primary health clinics in Malaysia. Patient Prefer Adherence 2013; 7: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chew BH, Hassan NH, Sherina MS. Determinants of medication adherence among adults with type 2 diabetes mellitus in three Malaysian public health clinics: a cross-sectional study. Patient Prefer Adherence 2015; 9: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langley CA, Bush J. The Aston Medication Adherence Study: mapping the adherence patterns of an inner-city population. Int J Clin Pharm 2014; 36: 202–211. [DOI] [PubMed] [Google Scholar]
- 30. Lyons I, Barber N, Raynor DK, et al. The Medicines Advice Service Evaluation (MASE): a randomised controlled trial of a pharmacist-led telephone based intervention designed to improve medication adherence. BMJ Qual Saf 2016; 25: 759–769. [DOI] [PubMed] [Google Scholar]


