Abstract
Background
Preclinical experimental studies revealed an acute alteration of pituitary adenylate cyclase-activating polypeptide in response to a single activation of the trigeminovascular system, which suggests a potential role of pituitary adenylate cyclase-activating polypeptide in the pathogenesis of migraine. However, changes in pituitary adenylate cyclase-activating polypeptide after repeated migraine-like attacks in chronic migraine are not clear. Therefore, the present study investigated chronic changes in pituitary adenylate cyclase-activating polypeptide and related receptors in response to repeated chemical dural stimulations in the rat.
Methods
A rat model of chronic migraine was established by repeated chemical dural stimulations using an inflammatory soup for a different numbers of days. The pituitary adenylate cyclase-activating polypeptide levels were quantified in plasma, the trigeminal ganglia, and the trigeminal nucleus caudalis using radioimmunoassay and Western blotting in trigeminal ganglia and trigeminal nucleus caudalis tissues. Western blot analysis and real-time polymerase chain reaction were used to measure the protein and mRNA expression of pituitary adenylate cyclase-activating polypeptide-related receptors (PAC1, VPAC1, and VPAC2) in the trigeminal ganglia and trigeminal nucleus caudalis to identify changes associated with repetitive applications of chemical dural stimulations.
Results
All rats exhibited significantly decreased periorbital nociceptive thresholds to repeated inflammatory soup stimulations. Radioimmunoassay and Western blot analysis demonstrated significantly decreased pituitary adenylate cyclase-activating polypeptide levels in plasma and trigeminal ganglia after repetitive chronic inflammatory soup stimulation. Protein and mRNA analyses of pituitary adenylate cyclase-activating polypeptide-related receptors demonstrated significantly increased PAC1 receptor protein and mRNA expression in the trigeminal ganglia, but not in the trigeminal nucleus caudalis, and no significant differences were found in the expression of the VPAC1 and VPAC2 receptors.
Conclusions
This study demonstrated the chronic alteration of pituitary adenylate cyclase-activating polypeptide and related receptors in response to repeated chemical dural stimulation in the rat, which suggests the crucial involvement of pituitary adenylate cyclase-activating polypeptide in the development of migraine. The selective increase in pituitary adenylate cyclase-activating polypeptide-related receptors suggests that the PAC1 receptor pathway is a novel target for the treatment of migraine.
Keywords: Pituitary adenylate cyclase-activating polypeptide, PAC1 receptor, VPAC1 receptor, VPAC2 receptor, migraine (need to expand)
Introduction
The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) is released from the sensory neurons in the trigeminal, and it plays a possible role in trigeminovascular system activation and the central sensitization involved in migraine. PACAP was discovered in ovine hypothalamus as a potent stimulant of cyclic adenosine monophosphate production in the pituitary gland.1 PACAP belongs to the secretin/glucagon/vasoactive intestinal peptide (VIP) superfamily, and it exists in 27(PACAP27) and 38(PACAP38) amino acid forms. The latter form is the predominant peptide in mammalian tissues.2 VIP and PACAP act via three classes of shared G-protein-coupled receptors, PAC1, VPAC1, and VPAC2, which initiate multiple signaling pathways.2 PACAP and its related receptor are widely distributed in the central nervous system and peripheral tissues,3 including several important levels of the migraine-related pain transmission pathways,4–6 such as the trigeminal ganglia (TG) and trigeminal nucleus caudalis (TNC).7
Human studies suggest a possible role for PACAP in the pathogenesis of migraine, and preclinical experimental studies revealed that PACAP plays a crucial role in activation of the trigeminovascular system. Human studies demonstrated that PACAP levels in the peripheral blood increased during spontaneous migraine attacks compared to the interictal period, and lower interictal plasma PACAP levels were observed in migraine patients compared to patients with tension-type headache and healthy controls.8,9 The interictal plasma levels of PACAP in chronic migraine (CM) patients are lower than episodic migraine patients, and a negative correlation was found between interictal PACAP levels and migraine duration in the CM group, but not the episodic migraine group.9
Activation of the trigeminovascular system and sensitization process plays important roles in the pathophysiology of migraine, while central sensitization participation is the main mechanism of chronic migraine. A previous rat study demonstrated increased concentrations of PACAP in the TNC after a single nitroglycerol injection or electrical stimulation to the TG, which suggests an important role of PACAP in central sensitization in migraine-like headache.10 Given migraine is a chronic disease that is characterized by recurrent headache attacks, this single activation of the trigeminovascular system does not adequately model the chronic process of migraine. However, chronic changes in PACAP and related receptors in the rat model of recurrent headache are still not clear.
Repeated application of inflammatory soup (IS) to the dura surrounding the superior sagittal sinus (SSS) is one of the most widely used CM animal models.11,12 In the previous studies, central sensitization has been confirmed in the IS-induced migraine model.13,14 As an important marker of the central sensitization formation, the analysis of periorbital nociceptive threshold has been firstly applied to authenticate the rat model. Then, the present study investigated chronic changes in PACAP and its related receptors (PAC1, VPAC1, and VPAC2) in response to repeated chemical dural stimulation using IS in the rat.
Materials and methods
Animals and habituation period
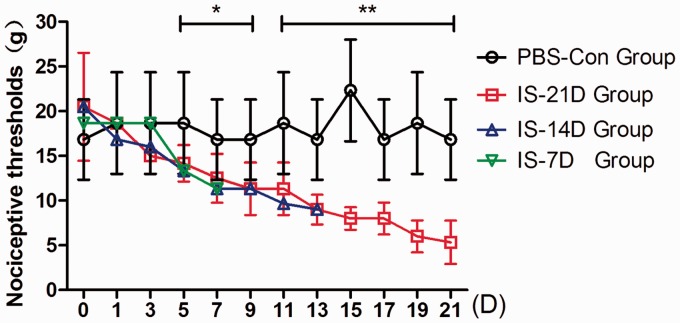
Male adult Sprague-Dawley rats (body weight 180–200 g) were obtained from the Laboratory Animal Center of the Chinese PLA 302 Hospital. All animals were used in accordance with the ethical standards of the committee for Research of the International Association for the Study of Pain in conscious animals (1983).15 The studies were approved by the Ethics Committee of the Chinese PLA General Hospital. The animals were bred and maintained in controlled laboratory conditions at a constant room temperature of 23℃ ± 2℃ and 50% ± 10% humidity under standard lighting conditions on a 12-h dark 12-h light cycle. All rats were given unrestricted access to food and water and housed individually to avoid loss of the implanted cannula. Habituation consisted of a seven-day period before surgery. Each rat was placed in the transparent glass cage daily for observation for 15 min. Researchers held the animal and touched its periorbital region several times using von Frey monofilaments to acclimate the rats to the testing apparatus and measured their baseline periorbital sensory thresholds (26 g or 15 g). These baseline thresholds were denoted by D0 in Figure 1.
Figure 1.
Comparison of periorbital nociceptive thresholds between groups. Nociceptive thresholds were measured using the perpendicular application of von Frey monofilaments to the periorbital region of rats. The periorbital nociceptive thresholds in the IS-7D, IS-14D, and IS-21D groups gradually dropped after the repeated IS infusion, while the changes in PBS-Con group were not obvious. After five days of administration, the thresholds in three IS groups were significantly lower than the PBS-Con group, and these differences increased over time.*p < 0.05, **p < 0.01, IS-21D group vs. the PBS-Con group.
Surgery
Rats were generally anesthetized using 10% chloral hydrate (0.50 ml/100 g, i.p.), and absence of a corneal reflex confirmed the level of anesthesia. The rat was affixed in a stereotactic apparatus, and the scalp covering the dorsal surface was incised to expose the skull. Bupivacaine (5 mg/1 ml) was applied under the skin during surgery as a local preventive anesthesia to prevent pain as a result of the surgical incision. A 1-mm diameter craniotomy was performed in the left frontal bone (1.5 mm lateral to midline and 1.5 mm posterior to the bregma) using a burr drill to expose the dura mater adjacent to the SSS, taking care not to burn or damage the dura. A plastic cannula (RWD Life Science Co., Ltd., Shenzhen, China) made of a biocompatible material was implanted for the dural delivery of IS or phosphate-buffered saline (PBS) through the cranial window. A plastic cap with a stainless steel inner cannula (No. 62001 from RWD) was oriented into the drilled hole without touching the dura mater. Sterile dental cement was used to surround the cannula to protect and fix it to the skull with the help of two small screws. The cannula was sealed with a matched obturator cap (No. 62101 from RWD) that had a tip just farther than the total length of the cannula, which prevented scar tissue from forming over the hole of the inner cannula. The skin was sutured with 4-0 nylon, and only the obturator cap remained outside of the skin. We applied topical penicillin after surgery to prevent infection of the surgical area. The rat was returned to a clean individual cage when it was fully awake and mobile. All rats were allowed to recover for three days before the experimental began, and all rats received prophylactic antibiotic injections (penicillin, 0.1 million IU/100 g) for at least two days. Periorbital sensory thresholds were measured during the recovery period to ensure that they returned to the pre-surgery baseline.
Treatment groups and repeated chemical stimulation
Rats were randomly divided into the following four groups to receive repeated daily applications of PBS or IS to the dura for different numbers of days (n = 6 for each experimental group): (a) IS stimulation for seven days (IS-7D group), (b) IS stimulation for 14 days (IS-14D group), (c) IS stimulation for 21 days (IS-21D group), and (d) PBS control for 21 days (PBS-Con group). The IS, a mixture of inflammatory mediators, consisted of 1 mM histamine, 1 mM serotonin, 1 mM bradykinin, and 0.1 mM prostaglandin E2 (PGE2) in PBS at pH 7.4. Rats in the IS-7D, IS-14D, and IS-21D groups received an infusion of 10 µl IS once daily for 7 days, 14 days, and 21 days, respectively. Rats in the PBS-Con group received an infusion of 10 µl PBS once daily for 21 days. We infused the IS/PBS over 5 min while the rat was freely moving to allow for diffusion into the tissue surrounding the dura. A visual inspection of the skin was performed to ensure there was no irritation due to IS leakage onto the skin. The stimulation procedures were conducted at 9 a.m., and rats were placed in the transparent glass cage for observation and stimulation for 15 minutes before the IS or PBS infusion. Rats were transported to the observation room in their cages after receiving the dural infusion.
Periorbital nociceptive threshold testing
Rats were placed in a cage that was 40 cm in diameter and 17.5 cm in height, which is a convenient size for the measurement of nociceptive thresholds. The tests were conducted every other day between 8 a.m. and 9 a.m. prior to the infusion of IS or PBS. Researchers restrained each rat by hand to prevent it from walking away. The restraint was atraumatic because the rats could turn around with some difficulty. Nociceptive thresholds were measured via the perpendicular application of von Frey monofilaments (North Coast Medical Co., Ltd., USA) to the periorbital region until it buckled slightly, and it was held for 3 to 6 s or until a positive response was observed. The thresholds were determined using the “up-down” method.16–18 A positive response was recorded when the rat withdrew its face from the von Frey monofilament. Rats that did not respond to the maximum filament strength (26 g) were assigned 26 g as their periorbital nociceptive threshold for analysis.
Tissue preparation
The rats were sacrificed after the final dural stimulation, and sampling was performed 24 h after the final dural stimulation to avoid the measurement of acute changes. Rats were perfused with 200 ml ice-cold PBS, and blood samples (4 ml per rat) were taken from the right external jugular vein into ice-cold tubes containing EDTA (1 mg/ml) and aprotinin (500 IU/ml) to inhibit proteinase activity. All blood samples were separated after centrifugation at 4℃ for 10 min at 4000 r/min. The TG and TNC tissues were excised. All plasma and tissue samples were stored at −80℃ until further processed.
Radioimmunoassay analysis
In the pretest experiment, we examined the levels of both PACAP38 and PACAP27 using radioimmunoassay (RIA) analysis in the blood plasma. However, the preliminary experiment results revealed that PACAP27 was not measurable in rat plasma. Thus, only PACAP38 plasma levels were measured in subsequent experiments. All nervous tissues from the TG and TNC were analyzed for the two biologically active forms of PACAP (PACAP38 and PACAP27) using RIA. The tissue samples were weighed and homogenized in 1 ml of ice-cold bidistilled water using a manual Potter-Elvehjem homogenizer. Tissue homogenates were centrifuged at 4℃ for 15 min at 12,000 r/min, and 100 µl samples of supernatants were used for RIA measurements. PACAP38 and PACAP27 levels were measured using a commercially available RIA kit (Phoenix Biotech Co., Ltd., USA; PACAP38: RK-052-05, 100–12,800 pg/ml; PACAP27: RK-052-02, 10–1280 pg/ml). Protein extraction from plasma and RIA procedures were performed according to the manufacturer’s instruction.
Western blot analysis
Western blot analyses were used to measure the expression of PACAP and related receptors (PAC1, VPAC1, and VPAC2) proteins in the TG and the TNC. Proteins were extracted after homogenization using a protease inhibitor cocktail and centrifugation. Supernatants were stored at −20℃ until the total protein concentration of the tissue was measured using a BCA protein assay kit (B9643, Sigma-Aldrich Co., USA) with bovine serum albumin as a standard. All selective antibodies against PACAP (sc-25439), PAC1 receptor (sc-30018), VPAC1 receptor (sc-30019), and VPAC2 receptor (sc-30020) were purchased from Santa Cruz Biotechnology, Inc. (CA, USA). All primary antibodies were diluted 1:500. The horseradish peroxidase-conjugated anti-rabbit IgG (sc-2030, 1:1000 dilution) was used as the secondary antibody at 1:10000. β-actin was used as a non-regulated reference protein. Blots were developed using an enhanced chemiluminescent technique, and relative band densities were quantified using Quantity-One software.
Real-time polymerase chain reaction analysis
The mRNA expression of PAC1, VPAC1, and VPAC2 receptors were determined in the TG and TNC using real-time polymerase chain reaction (PCR) analysis. Total RNA was isolated from frozen tissues using Trizol reagent according to the manufacturer’s instructions. Reverse transcription was performed using the SuperScript TM III First-strand synthesis system with 2 µg total RNA and 1 µl reverse transcriptase. Real-time PCR to quantify PAC1, VPAC1, and VPAC2 gene expression was performed using SYBR Green I dye (Power SYBR Green PCR Kit, ABI Co., Ltd, USA). GAPDH was used as a non-regulated reference gene. The following primer pairs specific for rat GAPDH, PAC1, VPAC1, and VPAC2 genes were used: PAC1(F) 5′-GGTGAGATGGTCCTTGTAAGC-3′, PAC1(R) 5′-CCCACAAGCATCGAAGTAGT-3′; VPAC1(F) 5′-CAAGGATATGGCCCTCTTCA-3′, VPAC1(R) 5′-TGATGAACACACTGGGCACT-3′; VPAC2(F) 5′- CAGATGTTGGTGGCAATGAC-3′, VPAC2(R) 5′- CCTGGAAGGAACCAACACAT-3′; GAPDH(F) 5′- ACTCACCTTTGGTCAATCCC -3′, GAPDH(R) 5′- TCTTTAGGGATCCAGGCATTG -3′. The reactions were incubated at 95℃ for 10 min, followed by 40 cycles at 95℃ for 15 s, 58℃ for 15 s, and 72℃ for 35 s. Melting curve analysis was performed automatically after each run. We used the well-established ΔCt comparative method to assess different expression levels. We used samples from PBS-Con rats as our positive samples, and samples from IS-7D, IS-14D, and IS-21D groups were considered unknown samples.
Statistical analysis
Data are expressed as the means ± SD. The SPSS statistical package version 19.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses, and graphical representations were created using GraphPad Prism 5 (GraphPad Software, San Diego, CA). Differences between experimental group means were assessed using one-way analysis of variance. A repeated measure analysis of variance was used to compare differences in periorbital nociceptive thresholds between groups. Changes in mRNA expression levels were calculated as fold changes from the respective control group. The level of acceptance for a significant difference in all statistical tests was p < 0.05.
Results
Periorbital nociceptive threshold analysis
Periorbital nociceptive thresholds were measured every other day just before IS or PBS infusion. The baseline thresholds in IS-7D, IS-14D, IS-21D, and PBS-Con groups, measured in the habituation period before surgery, were not significant different (p = 0.628). No significant differences were detected among the four groups on the first day (p = 0.914) and the third day (p = 0.454). Figure 1 showed that the periorbital nociceptive thresholds in the IS-7D, IS-14D, and IS-21D groups gradually dropped after the repeated IS infusion (IS-7D group: 18.67 ± 5.68 g–11.33 ± 2.92 g; IS-14D group: 20.50 ± 6.03 g–9.00 ± 1.09 g; IS-21D group: 20.5 ± 6.03 g–5.33 ± 2.42 g), while the changes in PBS-Con group (22.33 ± 5.68 g–16.83 ± 4.49 g) were not obvious. After five days of administration, the thresholds in three IS groups were significantly lower than the PBS-Con group (p = 0.047 on Day 5), and these differences increased over time (p = 0.006 on Day 21, IS-21D vs. PBS-Con group).
PACAP alterations in plasma, TG, and TNC using RIA
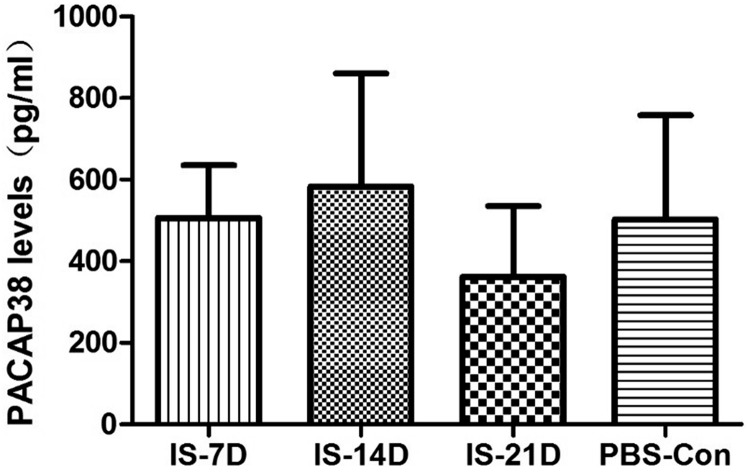
RIA analysis investigated PACAP levels in the plasma, TG, and TNC after repetitive IS stimulation for different numbers of days. PACAP27 level was not measurable in rat plasma in preliminary experiments, so only plasma PACAP38 level was measured in formal experiments. There was no significant difference in PACAP38 levels in plasma between groups (p = 0.381; Figure 2). Plasma PACAP38 levels in the IS-21D group (362.33 ± 173.39 pg/ml) were slightly lower than the PBS-Con group (503.05 ± 254.83 pg/ml), but not significantly (p = 0.289).
Figure 2.
Comparison of plasma PACAP38 levels between groups using RIA. Plasma PACAP38 levels were measured using RIA. No significant differences were observed between the IS-7D, IS-14D, IS-21D, and PBS-Con groups. PACAP27 level was not measurable in rat plasma in preliminary experiments, so only plasma PACAP38 level was measured in formal experiments.
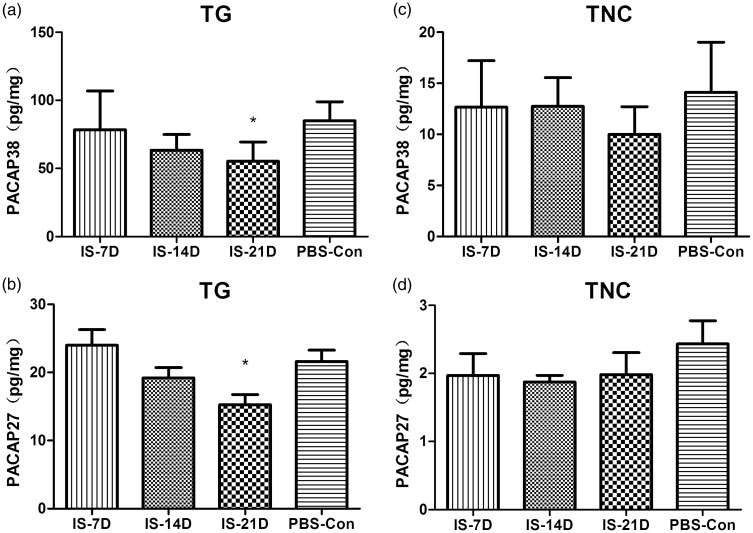
PACAP38 and PACAP27 levels were measured in the TG and TNC using RIA. PACAP38 (55.26 ± 14.23 pg/mg) and PACAP27 (15.25 ± 3.64 pg/mg) levels in the TG of the IS-21D group were significantly lower than the PBS-Con group (85.03 ± 13.93 pg/mg, p = 0.041 for PACAP38; 21.62 ± 4.02 pg/mg, p = 0.019 for PACAP27), but there was no significant difference between the IS-7D or IS-14D group and the PBS-Con group (Figure 3(a) and (b)). No significant differences in PACAP38 or PACAP27 levels were observed between groups in the TNC (p = 0.336 for PACAP38, p = 0.534 for PACAP27; Figure 3(c) and (d)). The concentrations of PACAP38 were approximately five times higher than PACAP27 in the TG and TNC regions, and PACAP38 and PACAP27 levels in the TG were 10 times higher than the TNC.
Figure 3.
Comparison of PACAP38 and PACAP27 levels in the TG and TNC. PACAP38 (a) and (c) and PACAP27 (b) and (d) levels were measured in the TG and TNC tissues using RIA. In the TG, PACAP38 (a) and PACAP27 (b) levels in the IS-21D group were significantly lower than the PBS-Con group (*p < 0.05, IS-21D group vs. the PBS-Con group), while there was no significant difference between the IS-7D or IS-14D group and the PBS-Con group. In the TNC, neither PACAP38 (c) nor PACAP27 (d) levels exhibited significant differences between the IS-7D, IS-14D, IS-21D, and PBS-Con groups.
TG: trigeminal ganglia; TNC: trigeminal nucleus caudalis; PACAP: pituitary adenylate cyclase-activating polypeptide.
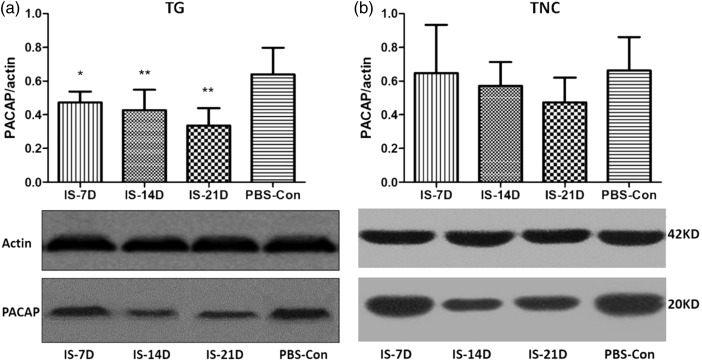
Confirmation of PACAP expression in TG and TNC using Western blot
Western blot analysis was performed to confirm the changes in peptide expression. A significant decrease of PACAP expression level was observed in the TG tissue of the IS-7D, IS-14D, and IS-21D groups compared to the PBS-Con group (p = 0.002), and the lowest PACAP levels were measured in the IS-21D group (Figure 4(a)). No significant difference in protein expression levels was observed between groups in the TNC (p = 0.370, Figure 4(b)). PACAP levels in the IS-21D group in the TNC were slightly lower than the PBS-Con group, but not significantly (p = 0.09, Figure 4(b)).
Figure 4.
Comparison of PACAP expression levels in the TG and TNC. Western blot analysis was used to confirm the changes in peptide expression in the TG (a) and TNC (b). Graph (a) shows the lowest levels of PACAP in the IS-21D group in TG tissue. *p < 0.05, IS-7D group vs. the PBS-Con group; **p < 0.01, IS-14D and IS-21D group vs. the PBS-Con group. No significant difference in protein expression levels was observed between groups in the TNC (b).
TG: trigeminal ganglia; TNC: trigeminal nucleus caudalis; PACAP: pituitary adenylate cyclase-activating polypeptide.
Alterations in PACAP-related receptor protein expression in the TG and TNC
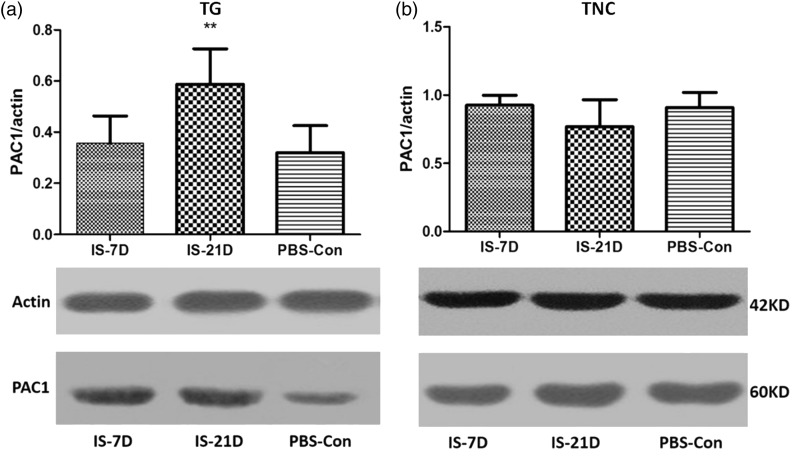
According to the PACAP results of RIA that the decrease in the PACAP38 level in the TG was observed only in the IS-21D group than PBS-Con group, PACAP-related receptor protein and mRNA expression in the TG and TNC were performed only in three groups (IS-7D, IS-21D, and PBS-Con groups).
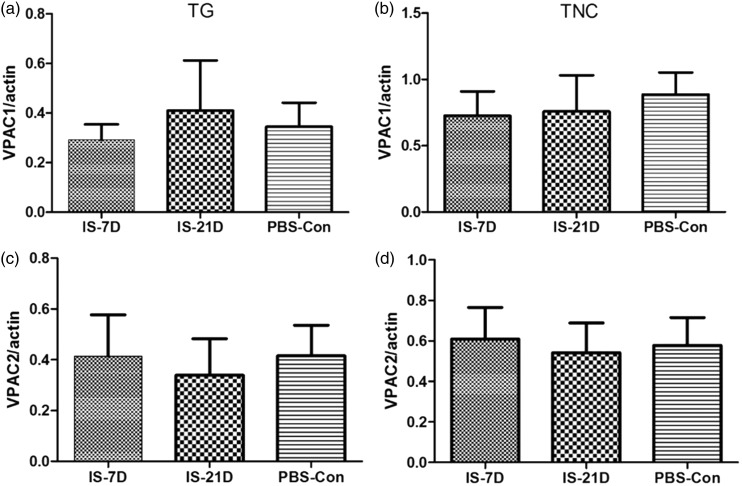
Western blot analyses of PACAP-related receptor proteins demonstrated significant increases in PAC1 receptor expression levels in the IS-21D group compared to the PBS-Con group in TG tissue (p = 0.03, Figure 5(a)). However, no significant difference in PAC1 receptor expression levels was observed between groups in the TNC tissue (p = 0.128, Figure 5(b)). VPAC1 and VPAC2 receptor levels were normal in the IS groups compared to the PBS-Con group in the TG and TNC (p = 0.345 and p = 0.411 for VPAC1 in the TG and TNC, respectively, Figure 6(a) and (b); p = 0.587 and p = 0.297 for VPAC2 in the TG and TNC, respectively, Figure 6(c) and (d)).
Figure 5.
Comparison of PAC1 receptor expression in the TG and TNC. According to the PACAP results of RIA that the decrease in the PACAP38 level in the TG were observed only in the IS-21D group, PACAP-related receptor expression in the TG and TNC were performed only in three groups (IS-7D, IS-21D, and PBS-Con groups). Western blot analyses demonstrated significant increases in PAC1 receptor expression levels in the IS-21D group; **p < 0.01, vs. the PBS-Con group in the TG tissue (a). No significant difference in PAC1 receptor expression levels was observed between groups in the TNC tissue (b).
TG: trigeminal ganglia; TNC: trigeminal nucleus caudalis; PACAP: pituitary adenylate cyclase-activating polypeptide.
Figure 6.
Comparison of VPAC1 and VPAC2 receptor expression in the TG and TNC. VPAC1 and VPAC2 receptor expression levels were measured in the TG (A-VPAC1; C-VPAC2) and TNC (BVPAC1; D-VPAC2) using Western blot analysis. No significant differences were observed between the IS-7D, IS-21D, and PBS-Con groups.
TG: trigeminal ganglia; TNC: trigeminal nucleus caudalis.
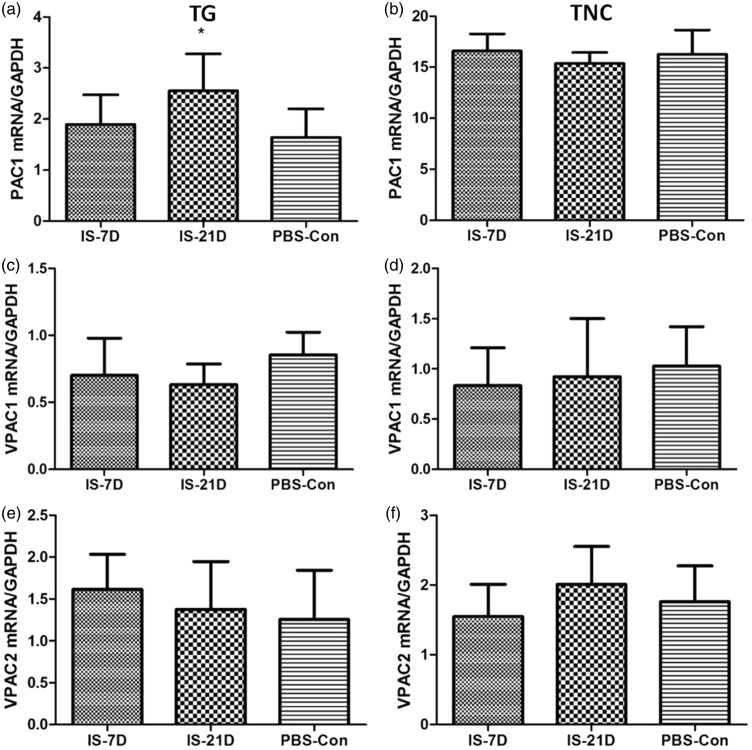
Alterations in PACAP-related receptor mRNA expression in the TG and TNC
Alterations in PAC1, VPAC1, and VPAC2 receptor gene were evaluated using real-time PCR analyses in the IS-7D, IS-21D, and PBS-Con groups. Our findings demonstrated a higher expression level of PAC1 receptor mRNA in the IS-21D group than the PBS-Con group in TG tissue (p = 0.035, Figure 7(a)). PAC1 receptor mRNA expression levels did not differ between groups in the TNC tissue (p = 0.481, Figure 7(b)). Similarly, VPAC1 and VPAC2 receptor mRNA expression levels were normal in the IS groups compared with the PBS-Con group in TG and TNC (p = 0.196 and p = 0.767 for VPAC1 in the TG and TNC, respectively, Figure 7(c) and (d); p = 0.511 and p = 0.131 for VPAC2 in the TG and TNC, respectively, Figure 7(e) and (F)). These results were confirmed at the protein level using Western blot analyses.
Figure 7.
Comparison of mRNA expression of PAC1, VPAC1, and VPAC2 receptors in the TG and TNC.
Real-time PCR analysis was used to confirm the alteration in expression of PAC1, VPAC1, and VPAC2 receptors. Graph (a) shows a higher expression level of PAC1 receptor mRNA in TG tissue; *p < 0.05, IS-21D group vs. the PBS-Con group. PAC1 receptor mRNA expression levels did not differ between groups in the TNC tissue (b). VPAC1 and VPAC2 receptor mRNA expression levels were normal in the IS groups compared with the PBS-Con group in the TG (C-VPAC1; E-VPAC2) and TNC (D-VPAC1; F-VPAC2).
TG: trigeminal ganglia; TNC: trigeminal nucleus caudalis.
Discussion
The present study established a rat model of CM using repeated IS stimulation to the dura mater surrounding the SSS, and changes of periorbital nociceptive threshold have been first detected to confirm central sensitization formation. After the repeated IS infusion, all rats in the three IS groups exhibited significantly lower periorbital nociceptive thresholds than the PBS-Con group, and these differences increased over time, which suggests the development of central sensitization.
Although the periorbital nociceptive thresholds of all rats in the three IS groups were significantly lower than the PBS-Con group after five days of IS administration, the decrease in the PACAP38 level by RIA analyses and the increase in the PAC1 expression by real-time PCR analyses in the TG were observed only in the IS-21D group. We speculated that the alteration of periorbital nociceptive thresholds was more susceptive than the changes of protein expression. In addition, as a confirmatory experiment, Western blot analysis was also performed to confirm the changes in PACAP expression in the TG and TNC, and significant decrease of PACAP expression was observed in the IS-7D, IS-14D, and IS-21D group in the TG, and the lowest PACAP levels were measured in the IS-21D group. Combined with the different RIA and Western-blot results, it is confirmed that the PACAP38 levels decreased with the IS infusion days increasing and consistent with the decreased periorbital nociceptive thresholds.
The trigeminovascular system provides an important link between vascular and neuronal elements. This system is the major afferent pain pathway between the cranial vessels and brainstem. Previous studies demonstrated that IS application to the dura activated and sensitized the trigeminovascular system.11,12,19 The establishment of peripheral and central sensitization was verified 2 h after IS stimulation of the dura,20 which is important in the pathogenesis of migraine. The present study demonstrated peripheral and central changes in PACAP metabolism in the plasma, TG, and TNC, which are most two important regions related to the trigeminovascular system in the nociceptive afferent pathway. IS recurrently activated trigeminal nociceptors. Migraine is driven by the activation and sensitization of peripheral trigeminal neurons that innervate the meninges, and peripheral activation may produce activation and subsequent sensitization.21
The present findings demonstrated that PACAP level was significantly decreased in plasma and TG after repetitive chronic IS stimulation. Our previous human study observed decreased plasma PACAP levels in patients with migraine during the interictal period,9 which is similar to our present findings of chronic changes in the recurrent migraine-like headache rat model. Animal and human studies demonstrate that recurrent migraine attacks may cause a chronic depletion of PACAP in the trigeminovascular system. Tuka et al.10 reported increased PACAP concentrations in the plasma and TNC 90 and 180 min after a single stimulation of the rat trigeminovascular system. Another human study reported increased PACAP levels in the plasma in spontaneous migraine attacks compared to the interictal period.9 However, the increased results are contradictory to our present results, and the divergent results observed may be explained by the differences in the activation mechanisms of the animal model and the different period of migraine. Tuka et al.10 observed PACAP38 changes different minutes after a single nitroglycerol injection or electrical stimulation of the TG, and the present study performed repeated IS stimulation for 7 to 21 days and sampled tissues 24 h after the final infusion to avoid measurement of the acute changes. Therefore, endogenous PACAP levels may increase in the acute period of trigeminovascular system activation in rats and during the ictal period of migraine attack but decrease during the interictal and chronic period of migraine. Similar to our findings, concentrations of PACAP27 were also not detectable in the rat plasma by mass spectrometry according to Tuka et al.10 However, the relationships of the acute high PACAP38 level and the chronic depletion of PACAP in the pathogenesis of migraine are not known.
Experimental animal studies demonstrated that systemic PACAP administration produced photophobia and meningeal vasodilatation in wild-type mice but not PACAP-knockout mice in a model of nitroglycerol-induced trigeminovascular activation,22 which suggests that PACAP is a key mediator of light aversion and meningeal blood flow regulation in chemical TS activation in mice. Systemic infusion of exogenous PACAP in humans delayed migraine-like headache approximately 6 h after injection only in patients with migraine, and healthy controls only described an initial mild headache that did not meet migraine criteria.23 Notably, intravenous PACAP and VIP-dilated cranial arteries, but only PACAP, and not VIP, induced migraine-like attacks in patients with migraine.24 The two homologous peptides (PACAP and VIP) share the VPAC1 and VPAC2 receptors, but PAC1 receptor is selective for PACAP.4 Therefore, the PAC1 receptor may play a key role in the process of PACAP triggering migraine, which may lead to new novel treatment approaches for the management of migraine. Pharmacological studies have developed new PAC1 receptor antagonist and agonist: PACAP6-38 could block the receptor function from binding to PAC1 receptor, while Maxadilan, a 61-amino acid vasodilatory peptide, could specifically activate the PAC1 receptor.25 The present study further demonstrated significant increases of the expression of PAC1 receptor protein and mRNA in the TG, but no significant changes in VPAC1 and VPAC2 receptor expression. Taken together, these results further support the important role of PACAP-PAC1 signaling in the IS-induced migraine, and this hypothesis may also explain the apparently different clinical functions on the triggering migraine attacks between PACAP and VIP. In the future, PAC1 receptor antagonist PACAP6-38 and agonist Maxadilan will be applied to the further study which is important to help to confirm the roles of PACAP-PAC1 signaling in the pathogenesis of migraine.
Conclusion
In conclusion, our data provide evidences supporting alterations in PACAP and its related receptors after repeated chemical dural stimulation in rats. The present findings suggest the potential involvement of PACAP and PACAP-related receptors in the development of migraine, suggesting PAC1 receptor as a candidate anti-migraine target. However, the exact role of PACAP-related receptors in the pathogenesis of migraine remains unclear. Tuning of PACAP-PAC1 signaling pathway may represent a novel avenue for the treatment of migraine, and further pharmacological research is necessary.
Author Contributions
All authors read and approved the final manuscript. Xun Han performed experiments, analyzed data, prepared figures, and drafted the manuscript. Ye Ran and Min Su analyzed data, prepared figures, and drafted the manuscript. Yinglu Liu and Wenjing Tang analyzed data and prepared figures. Shengyuan Yu and Zhao Dong designed and monitored this work and edited the manuscript. Shengyuan Yu and Zhao Dong contributed equally to this paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grants 81471147, 81671077, 81471146, 81500966, 81500943, and 81600952), Beijing Science and Technology Project (grant Z161100002616013), the Capital Development Scientific Research (grant 2014-4-5013), and Beijing Natural Science Foundation (grant 7162178).
References
- 1.Miyata A, Arimura A, Dahl RR, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 1989; 164: 567–574. [DOI] [PubMed] [Google Scholar]
- 2.Vaudry D, Gonzalez BJ, Basille M, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 2000; 52: 269–324. [PubMed] [Google Scholar]
- 3.Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 2009; 61: 283–357. [DOI] [PubMed] [Google Scholar]
- 4.Harmar AJ, Fahrenkrug J, Gozes I, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 2012; 166: 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdling A, Sheykhzade M, Maddahi A, et al. VIP/PACAP receptors in cerebral arteries of rat: characterization, localization and relation to intracellular calcium. Neuropeptides 2013; 47: 85–92. [DOI] [PubMed] [Google Scholar]
- 6.Knutsson M, Edvinsson L. Distribution of mRNA for VIP and PACAP receptors in human cerebral arteries and cranial ganglia. Neuroreport 2002; 13: 507–509. [DOI] [PubMed] [Google Scholar]
- 7.Tajti J, Uddman R, Edvinsson L. Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia 2001; 21: 96–101. [DOI] [PubMed] [Google Scholar]
- 8.Tuka B, Helyes Z, Markovics A, et al. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013; 33: 1085–1095. [DOI] [PubMed] [Google Scholar]
- 9.Han X, Dong Z, Hou L, et al. Interictal plasma pituitary adenylate cyclase-activating polypeptide levels are decreased in migraineurs but remain unchanged in patients with tension-type headache. Clinica Chimica Acta 2015; 450: 151–154. [DOI] [PubMed] [Google Scholar]
- 10.Tuka B, Helyes Z, Markovics A, et al. Peripheral and central alterations of pituitary adenylate cyclase activating polypeptide-like immunoreactivity in the rat in response to activation of the trigeminovascular system. Peptides 2012; 33: 307–316. [DOI] [PubMed] [Google Scholar]
- 11.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol 2004; 55: 27–36. [DOI] [PubMed] [Google Scholar]
- 12.Burstein R, Yamamura H, Malick A, et al. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 1998; 79: 964–982. [DOI] [PubMed] [Google Scholar]
- 13.Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia 2013; 33: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 14.Stucky NL, Gregory E, Winter MK, et al. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache 2011; 51: 674–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 16.Dixon WJ. Efficient analysis of experimental observations. Ann Rev Pharmacol Toxicol 1980; 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 17.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosc Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 18.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci 1994; 14: 2708–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ter Horst GJ, Meijler WJ, Korf J, et al. Trigeminal nociception-induced cerebral Fos expression in the conscious rat. Cephalalgia 2001; 21: 963–975. [DOI] [PubMed] [Google Scholar]
- 20.Jakubowski M, Levy D, Goor-Aryeh I, et al. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache 2005; 45: 850–861. [DOI] [PubMed] [Google Scholar]
- 21.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain 2001; 89: 107–110. [DOI] [PubMed] [Google Scholar]
- 22.Markovics A, Kormos V, Gaszner B, et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis 2012; 45: 633–644. [DOI] [PubMed] [Google Scholar]
- 23.Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009; 132: 16–25. [DOI] [PubMed] [Google Scholar]
- 24.Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia 2008; 28: 226–236. [DOI] [PubMed] [Google Scholar]
- 25.Schytz HW, Olesen J, Ashina M. The PACAP receptor: a novel target for migraine treatment. Neurotherapeutics 2010; 7: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]