Abstract
Ethiopia is one of the few African countries where Plasmodium vivax is co-endemic with P. falciparum. Malaria transmission is seasonal and transmission intensity varies mainly by landscape and climate. Although the recent emergence of drug resistant parasites presents a major issue to malaria control in Ethiopia, little is known about the transmission pathways of parasite species and prevalence of resistant markers. This study used microsatellites to determine population diversity and gene flow patterns of P. falciparum (N = 226) and P. vivax (N = 205), as well as prevalence of drug resistant markers to infer the impact of gene flow and existing malaria treatment regimes. Plasmodium falciparum indicated a higher rate of polyclonal infections than P. vivax. Both species revealed moderate genetic diversity and similar population structure. Populations in the northern highlands were closely related to the eastern Rift Valley, but slightly distinct from the southern basin area. Gene flow via human migrations between the northern and eastern populations were frequent and mostly bidirectional. Landscape genetic analyses indicated that environmental heterogeneity and geographical distance did not constrain parasite gene flow. This may partly explain similar patterns of resistant marker prevalence. In P. falciparum, a high prevalence of mutant alleles was detected in codons related to chloroquine (pfcrt and pfmdr1) and sulfadoxine-pyrimethamine (pfdhps and pfdhfr) resistance. Over 60% of the samples showed pfmdr1 duplications. Nevertheless, no mutation was detected in pfK13 that relates to artemisinin resistance. In P. vivax, while sequences of pvcrt-o were highly conserved and less than 5% of the samples showed pvmdr duplications, over 50% of the samples had pvmdr1 976F mutation. It remains to be tested if this mutation relates to chloroquine resistance. Monitoring the extent of malaria spread and markers of drug resistance is imperative to inform policy for evidence-based antimalarial choice and interventions. To effectively reduce malaria burden in Ethiopia, control efforts should focus on seasonal migrant populations.
Author summary
Sub-Saharan Africa is home to nearly 90% of malaria cases. In Ethiopia, two-thirds of the population lives in areas at risk of malaria infection. Malaria spread via human migrations and emergence of drug resistant parasites are major issues to malaria control in this country. Our study used microsatellite markers to determine gene flow patterns of P. falciparum and P. vivax in different parts of Ethiopia. We found that gene flow occurred across broad geographical distance and that environmental heterogeneity did not appear to influence gene flow. Unconstrained parasite gene flow may partly explain similar patterns of resistance marker prevalence across the country. While no mutation was detected in pfK13 that relates to artemisinin resistance in P. falciparum, over 50% of our P. vivax samples had pvmdr1 976F mutation that may relate to chloroquine resistance. This merits further clinical observations and/or in vitro testing. Our findings heighten the concern of chloroquine resistance for P. vivax malaria after more than a decade-application and suggests alternative treatment regime to alleviate the problem. Broadly, malaria control efforts should focus on seasonal migrant populations to effectively reduce malaria burden in Ethiopia.
Introduction
Despite considerable progress towards malaria control, two-thirds of the population in Ethiopia, i.e., approximately 66 million people, reside in areas of low or high malaria transmission [1]. Apart from human factors such as population mobility, urbanization, and agricultural development, emergence of drug resistant parasites and insecticide resistance present a major hurdle to malaria control programs in Ethiopia and worldwide [2]. Reports of emerging Plasmodium vivax resistance to chloroquine (CQ) in Ethiopia threaten the efficacy of P. vivax treatment [3–6]. Also, the well-documented emergence of P. falciparum resistance to artemisinin in Southeast Asia may endanger current malaria treatment programs in Ethiopia, given that both CQ and sulfadoxine-pyrimethamine (SP) resistance originated in Southeast Asia and spread quickly to East Africa [7]. Thus, knowing how malaria parasites spread as well as monitoring prevalence of drug resistant markers in high-risk areas are important to informing antimalarial interventions.
While P. vivax is the most widespread human malaria parasite, it is rare in Africa where P. falciparum predominates. Due to its low prevalence in the continent, little is known about the transmission patterns of P. vivax in Africa. Ethiopia is unique in that P. vivax is co-endemic with P. falciparum at approximately equal case incidence rates. Other African countries with significant P. vivax infections are Eritrea, Sudan, and Madagascar [1]. Although Ethiopia carries a substantial malaria burden, information on the transmission dynamics and spread of drug resistance across the country is scarce.
P. vivax and P. falciparum exhibit different biological and epidemiological features. Compared to P. falciparum, P. vivax has a broader temperature tolerance, an early onset of gametocyte development, and a dormant life cycle stage, the hypnozoite, in the host liver that can cause relapse. Relapse infections may present opportunities for P. vivax to exchange and disseminate alleles at any time of the year rather than only the transmission season [8]. Population genetic diversity and structure are thus expected to be different between P. vivax and P. falciparum even when the two species coexist. For instance, in Cambodia [9], the Indo-West Pacific [10–12], and the Brazilian Amazonia [13], P. vivax revealed a higher microsatellite diversity than its sympatric P. falciparum. A similar contrast was observed in Papua New Guinea where P. vivax showed a higher AMA1 gene diversity than P. falciparum [14]. Globally, both P. falciparum and P. vivax in Africa were markedly differentiated from those in Southeast Asia and Oceania [15,16], reflecting a clear continental disjunction. While P. vivax is genetically most diverse in Southeast Asia [17,18], P. falciparum diversity is the highest in East and West Africa compared to Southeast Asia and Oceania [19,20]. Such differences could be tightly associated with the historical levels of transmission intensity. Comparing genetic diversity and structure between the two species at the same endemic setting would shed light on the biological relevance on malaria epidemiology.
Apart from transmission dynamics, the biological differences between P. falciparum and P. vivax have added a layer of complexity to antimalarial treatment programs in Ethiopia. First-line treatment for P. falciparum is arthemether-lumefantrine (AL), which replaced SP in 2005 due to increasing and widespread SP resistance. While CQ was withdrawn in 1998 due to a high prevalence of CQ resistance in P. falciparum, it remains the first-line treatment for P. vivax in Ethiopia [2]. Genetic markers for CQ (pfcrtT76), SP (pfdhfrI51-R59-N108 + pfdhpsG437-E540), and artemisinin (Kelch13-propeller region) resistance in P. falciparum have been well documented [21–24]. For P. vivax, although there is no clear evidence that variants in pvcrt-o and pvmdr1 are associated with CQ resistance, mutations including T958M, Y976F and F1076L in pvmdr1, as well as a K-10 insertion (lysine (K) insertion on chromosome 10) in pvcrt-o have been suggested as possible genetic markers [25,26]. Mutation frequency of these genes largely depends on the level of drug usage and the extent of the spread of resistant genotypes. For instance, the pfcrtT76 mutation frequency was almost 100% among clinical and asymptomatic P. falciparum samples from 2004–2012 in south-central Ethiopia [27–29]. In the case of mixed infections with the two species where only P. vivax diagnosed, P. falciparum is still exposed to CQ despite the change of P. falciparum first-line treatment more than a decade ago. On the contrary, the frequency of pfdhfr and pfdhps quintuple mutations had decreased significantly from 2005 to 2008 since the withdrawal of SP in 2005 [30,31], indicative of relaxed selection in the P. falciparum populations. Mutations in the kelch (K13)-propeller region are markers of artemisinin resistance [24]. Resistance-associated pfK13 mutations are widely prevalent in Southeast Asia but those mutations have not yet been common in Africa [32]. Recently, a new nonsynonymous mutation at pfK13 position 579 (M579I) was detected in a P. falciparum strain that was indigenous to Equatorial Guinea and shown to be artemisinin resistant based on in vitro testing [33]. Careful surveillance of pfK13-propeller region mutations in Ethiopia will be especially useful to detecting the spread of artemisinin resistance from Southeast Asia to Africa.
This study examined and compared population diversity and gene flow patterns between P. falciparum and P. vivax in the northern, eastern, and southern parts of Ethiopia with low to moderate level of malaria transmission. Specifically, we investigated if landscape heterogeneity impacts parasite gene flow by testing the association between landscape factors and population genetic structure. We tested three competing hypotheses of factors that may influence gene flow: 1) factors related to vector ecology (land cover and precipitation); 2) factors related to human movement (distance to roads); and 3) factors related to environment (elevation, which is tightly correlated with temperature). Further, we inferred how gene flow pattern relates to the prevalence of drug resistance markers. This knowledge will help inform how malaria parasites and drug resistance spread, how P. vivax and P. falciparum epidemiology influences genetic structures, as well as antimalarial drug efficacy in Ethiopia. Monitoring for markers of antimalarial drug resistance is imperative to informing public health interventions.
Materials and methods
Ethics statement
Scientific and ethical clearance was obtained from the institutional scientific and ethical review boards of Jimma and Addis Ababa Universities in Ethiopia and University of California, Irvine, USA. Written informed consent/assent for study participation was obtained from all consenting heads of households, parents/guardians (for minors under age of 18), and each individual who was willing to participate in the study.
Study areas and sample collection
Clinical samples from six study sites representing the northern highland (MA: Mankush and BU: Bure), eastern Rift Valley (SR: Shewa Robit and ME: Metehara), and southern basin area (JM: Jimma and HA: Halaba) of Ethiopia were collected during the peak transmission season (September-November) of 2014 (Fig 1; S1 Table). This area encompasses an elevation gradient from ca. 50m in the basin to over 2,500m in the highlands west of the Great Rift Valley. Finger-prick blood samples were collected from malaria symptomatic (who has fever with axillary body temperature > 37.5°C and with confirmed asexual stages of malaria parasite based on microscopy) or febrile patients visiting the health centers or hospitals at each of the study sites. Thick and thin blood smears were prepared for microscopic examination and three to four spots of blood, equivalent to ~50 μl, from each individual were blotted on Whatman 3MM filter paper. Parasite DNA was extracted from dried blood spots by the Saponin/Chelex method [34]. Nested and quantitative PCR were performed to identify and confirm parasite species of the infected samples [35]. A total of 226 and 205P. falciparum and P. vivax samples (ranged from 18–58 samples per site) were included in microsatellite analyses.
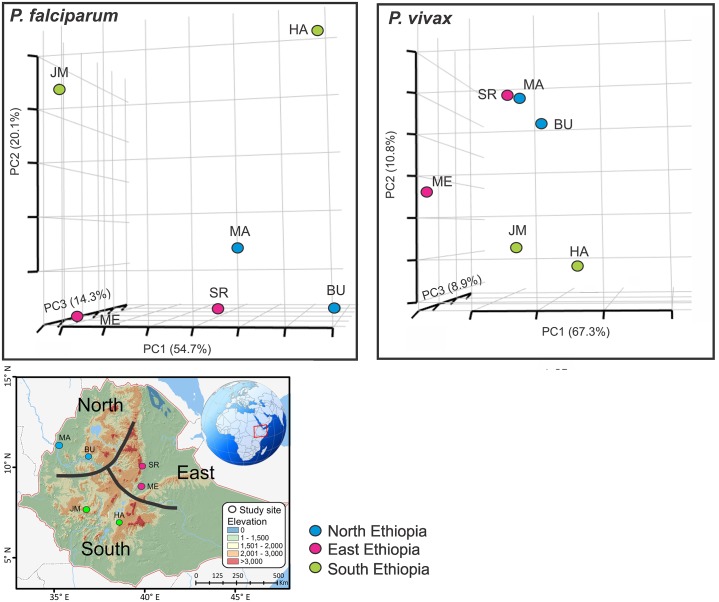
Fig 1. Three dimensional scatter plots of pairwise DS values (an analog of FST) showing the genetic relatedness of Plasmodium falciparum and P. vivax among sites.
The first three axes that contain nearly 90% of the total variation are shown. Locations of the studied sites from different parts of Ethiopia are presented in the map below as well as S1 Table.
Microsatellite genotyping
Thirteen single-copy microsatellites with tri- or tetranucleotide repeats, which mapped to 14 chromosomes, were typed for P. falciparum and P. vivax, respectively (S2 Table). Alleles were PCR-amplified with the published oligonucleotide primers [36–38]. For each PCR reaction, 2 μl of genomic DNA were used with 2 mM MgCl2, 2 μM of each primer (all forward primers were labeled with fluorescent dyes; Applied Biosystems, Foster City, CA), and 10μl of 2×DreamTaq Green PCR Master Mix (Thermo Scientific, Waltham, MA) in a final volume of 20 μl. PCR cycling conditions were as follows: 2 min, 94°C; (30 sec, 94°C; 40 sec, 58°C; 50 sec, 72°C) for 40 cycles; 5 min, 72°C. After PCR amplification, products were pooled into four groups based on size differences: TAA87+PFPK2+POLY2+9735, TAA42+TAA81+TAA109, PE87a+PFG377+POLYα, TAA60+TA80+TA116 for P. falciparum; MS1+MS3+MS4+MS5, MS8+MS9+MS16, MS10+MS12+MS15, MS20+Pv1.501+Pv3.27 for P. vivax (S2 Table). The pooled products were separated on an ABI 3730 sequencer and all allele sizes were determined and visualized in Peak Scanner. To avoid background signal and potential artifacts, a threshold of 500 relative fluorescent units was set for peak detection. For each sample, the dominant allele and any alleles with a minimum of 33% height of the dominant allele were scored [36].
Data analyses
Linkage disequilibrium and genetic diversity
All analyses were performed separately on the P. falciparum and P. vivax datasets. To examine whether the microsatellite loci represent an independent set of markers of the parasite genome, linkage disequilibrium (LD) was tested by Fisher’s exact test for each pair of loci with GenePop version 4.2, using the Markov chain method with 100 batches and 10,000 iterations per batch [39]. Significance values were adjusted by sequential Bonferroni correction for multiple comparisons. In addition, mulltilocus LD was assessed among the parasite samples for each of the populations using the web-based LIAN 3.5 [40]. The standardized index of association (IAS), which measures the strength of linkage disequilibrium and views as a function of the rate of recombination among samples, was calculated with 10,000 random permutations of the data.
The percentage of polyclonal infections (i.e., samples with more than one allele at any given locus) as well as multiplicity of infections (MOI: the number of genetically distinct clones present within a host) were estimated for each of the study sites, respectively, for P. falciparum and P. vivax. For each sample, MOI was scored as the maximum number of alleles observed when all loci were taken into account and the average MOI was calculated for each population.
Genotypic variation was calculated in GenoDive version 2.0b27 [41]. We first calculated genetic distances using the method of Smouse and Peakall, a squared Euclidean distance based on the number of times a certain allele was found in the two individuals [42]. The minimal distance class was set as threshold to identify the following: (i) the number of multilocus genotypes (G); (ii) Simpson’s diversity index (D), also known as Nei’s genetic diversity corrected for sample size that ranges from zero (where two randomly chosen individuals in a population share a single genotype) to one (where individuals have different genotypes); and (iii) genotype evenness (E) that ranges from zero (where one or a few genotypes dominate in a population) to one (where all genotypes are of equal frequency in a population). In addition, the number of effective alleles and expected heterozygosity were estimated for each study site.
Population structure and isolation-by-distance
A model-based Bayesian method implemented in STRUCTURE v2.3.4 was performed to examine partitioning of individuals to genetic clusters [43]. The number of clusters (K) was determined by simulating a range of K values from 1 (no genetic differentiation among all sites) to 6 (all sites were genetically differentiated from one another). The posterior probability of each value was then used to detect the modal value of ΔK, a quantity related to the second order rate of change with respect to K of the likelihood function [44]. Posterior probability values were estimated using a Markov Chain Monte Carlo (MCMC) method. A burn-in period of 500,000 iterations followed by 106 iterations of each chain was performed to ensure convergence of the MCMC. Each MCMC chain for each value of K was run ten times with the ‘independent allele frequency’ option that allows individuals with ancestries in more than one group to be assigned into one cluster. Individuals were assigned into K clusters according to membership coefficient values (Q) ranged from 0 (lowest affinity to a cluster) to 1 (highest affinity to a cluster). The partitioning of clusters was visualized with DISTRUCT [45]. Neighboring-joining trees were constructed using T-REX [46,47] to show the genetic relatedness among P. falciparum and P. vivax samples. The squared Euclidean distance, which is based on the number of times a certain allele found in two individuals [48], was used for tree constructions. The resulted trees were visualized in FigTree v1.4.2.
An FST analysis was conducted using θ, an FST-estimator in SPAGeDi v1.2e [49]. FST values were tested for significance using 10,000 permutations. Genetic differentiation among sites was displayed by multidimensional scaling plot based on the estimated DS values (an analog of FST) in R v3.3.0. Furthermore, an analysis of molecular variance (AMOVA) was used to determine the hierarchical distribution of genetic variance within and among populations, as well as among regions (north, east, and south of Ethiopia) using GENALEX [50]. The relationships between genetic distances (DS values) and Euclidean geographical distance (estimated from spatial coordinates using R for multivariate and spatial analysis; [51]) were examined by Mantel tests (10,000 randomizations) and reduced major axis (RMA) regression in the Isolation By Distance v3.23 [52].
Bottlenecks and migration rates
Signature of genetic bottleneck was detected with BOTTLENECK v1.2.02 [53]. Only sites with a sample size of 20 or above were included for statistical significance. Two tests were performed using three different mutation models: the infinite alleles model (IAM), the stepwise mutation model (SMM), and a combination of those two extreme hypotheses, the two-phase model (TPM). First was the overall distribution of allele frequency classes. Second was the Wilcoxon-signed rank test to compare the number of loci that present a heterozygosity excess to the number of such loci expected by chance only.
Frequency of gene flow among populations was estimated for each parasite species by a maximum-likelihood analysis implemented in Migrate-N v2.4.4 [54]. Parameters including Θ (defined as 4Neμ, where Ne is the effective population size and μ is the mutation rate per generation and site) and M (m/μ, where m is the immigration rate scaled by mutation rate) were estimated. Four independent runs were conducted with the Brownian motion model using 10 short chains with 5,000 sampled genealogies and three long chains with 50,000 sampled genealogies to obtain the mean and range of Θ and M values. In addition, we inferred migration rate using a Bayesian approach implemented in BayesAss v3 [55], which is not dependant on the assumption of equilibrium and can be used with populations that are not in migration-drift or Hardy-Weinberg equilibrium. A MCMC algorithm was used to estimate the posterior probability distribution of the proportion of migrants from one population to another. We performed the analyses with 9×106 iterations, with a burn-in of 106 iterations, and a sampling frequency of 2,000 to ensure the parameters of the model were converged. The correlation between migration rate and geographical distance was tested for all pairs of populations.
Landscape genetics
To test for the effects of landscape factors on gene flow between populations, we performed a landscape genetic analysis as follows. First, we created landscape resistance surfaces based on our predictions of the factors influencing gene flow of Plasmodium species, specifically factors influencing vector ecology (land cover and precipitation), human movement (distance to roads), and Plasmodium biology (elevation, which is tightly correlated with temperature). A resistance surface is a spatial layer in which each cell in a grid is assigned a value that represents the degree to which that cell constrains gene flow or movement [56]. These values were often based on numerous assumptions about relationships between a landscape or environmental feature and the ability of a given organism to move through that feature. Landscape resistance surfaces were derived from publicly available data: NASA MODIS MCD12Q2 for land cover (forest, shrubland, woody savanna, savanna, grassland, cropland, and sparsely vegetated) [57,58]; NASA SRTM v4.1 for elevation [59]; WorldClim v1.4 for precipitation [60]; and Roads Africa shapefile in ArcGIS for distance to roads. All raster files were resampled to a resolution of 1km in ArcGIS 10. Next, we used ResistanceGA to optimize landscape resistance surfaces based on our genetic data [61]. ResistanceGA uses a genetic algorithm to unbiasedly assign landscape resistance values to continuous or categorical data. Circuitscape v.4.05 was used to measure resistance distance between populations [62]. Circuitscape relies on electrical circuit theory to predict landscape connectivity and incorporates all possible pathways between populations into the resistance distance measure. To test the fit of resistance surfaces in relation to the genetic data, linear mixed effects models with the maximum likelihood population effects (MLPE) were fit in lme4 [63]. Finally, Akaike information criterion with a penalty for extra parameters (AICc) was calculated from the linear mixed effect model and used as the means for model selection.
Resistance gene sequencing
Five gene regions that are putatively associated with CQ (pfcrt and pfmdr1), SP (pfdhps and pfdhfr), and artemisinin (pfK13) resistance were sequenced with P. falciparum samples. Polymorphisms were examined for the following codons: pfcrt–codon76; pfmdr1–codons 86, 184, 1042, and 1246; pfdhps–codons 396, 436, 437; pfdhfr–codons 51, 59, 108; pfK13–codons 476, 493, 519, 532, 539, 543, 578, 579, 580, 582, and 590. In addition, two gene regions that are putatively associated with CQ resistance (pvcrt-o and pvmdr1) were sequenced with P. vivax samples. Polymorphisms were examined for the following: pvcrt-o–a (AAG) insertion at codon 10 (K10 insert), codon 117; pvmdr1–codons 958, 976, and 1076. Amplification was conducted in a 20μl reaction mixture containing 3μl of genomic DNA, 12.5μl of 2×DreamTaq Green PCR Master Mix, and 10 nmol of forward and reverse primers based on the published protocols [21–23,32,64]. PCR products were then purified the by the SAP-ExoI method (Affymetrix, Santa Clara, CA) and sequenced in both directions by Sanger sequencing (GENEWIZ).
Pfmdr1 and Pvmdr1 gene copy estimation
The pfmdr1 gene copy number of P. falciparum was assessed by real-time PCR. Genomic DNA of P. falciparum clones 3D7 (which has a single copy of pfmdr1) was used as a calibrator and pfβ-tubulin, a house-keeping gene, was used as an internal control. The primers for pfmdr1 and β-tubulin were described previously [65]. For P. vivax, the Salvador I strain was used as a calibrator and the pvaldolase gene, which is known to be a single copy gene in P. vivax, was used as an internal control using the published primers [66].
Amplification was performed in triplicate in a total volume of 20 μl containing 10μl of SYBR Green PCR Master Mix, 0.75 μl of each of the sense and anti-sense primers (10 μM), 20 ng of genomic DNA and 3.5 μl of water. Reaction was performed in CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad), with an initial denaturation at 95°C for 3 min, followed by 45 cycles at 94°C for 30 sec, 55°C for 30 sec, and 68°C for 1 min with a final 95°C for 10 sec. This was then followed by a melting curve step of temperature ranged from 65°C to 95°C with 0.5°C increment to determine the melting temperature of each amplified product. A negative control with no template was used in each run. Each sample was run in triplicates and the Ct values and melting temperature were recorded at the end of the reactions. The average and standard deviation of the three Ct values were calculated, and the average value was accepted if the standard deviation was lower than 0.32. The 2ΔΔCt±SD method for relative quantification was used to estimate the gene copy number [66] and the results were expressed as the N-fold copy number of the targeted gene in relative to the reference. Fisher’s exact test (given small sample size) was used to test for significant differences in mutation prevalence and gene copy number among the study populations. All statistical analyses were performed in R (R Core Team 2013).
Results
Linkage disequilibrium and complexity of infections
No significant LD was detected for all pairwise combinations of microsatellite loci among the P. falciparum and P. vivax samples (Bonferroni corrected P>0.05). However, when all locus were pooled together in the analyses, P. falciparum in general showed a higher level of linkage and/or rate of recombination (IAS values ranged from 0.005 in Bure (BU) to IAS = 0.13 in Halaba (HA); all sites IAS = 0.03, P<0.05 except BU) than P. vivax (IAS values ranged from 0.003 in Mankush (MA) and Jimma (JM) to 0.02 in Shewa Robit (SR); all sites IAS = 0.001, P>0.05; Table 1).
Table 1. Linkage disequilibrium and complexity of infection among P. falciparum and P. vivax samples by study sites.
‘ns’ denotes non-significant; ‘*’ denotes P<0.05.
| Site | IAS | # of polyclonal/total infections (%) | Mean MOI, median (range) | |
|---|---|---|---|---|
| P. falciparum | ||||
| BU | 0.005ns | 3/42 (7.1%) | 1.09, 1 (1–3) | |
| MA | 0.018* | 4/36 (11.1%) | 1.14, 1 (1–3) | |
| ME | 0.055* | 3/46 (6.5%) | 1.07, 1 (1–2) | |
| SR | 0.051* | 1/33 (3.0%) | 1.03, 1 (1–2) | |
| HA | 0.126* | 3/18 (16.7%) | 1.17, 1 (1–2) | |
| JM | 0.033* | 6/51 (11.8%) | 1.14, 1 (1–3) | |
| All sites | 0.030* | 20/226 (8.8%) | 1.10, 1 (1–3) | |
| P. vivax | ||||
| BU | 0.005ns | 2/39 (5.1%) | 1.03, 1 (1–2) | |
| MA | 0.004ns | 1/19 (5.3%) | 1.05, 1 (1–2) | |
| ME | 0.017ns | 1/21 (4.8%) | 1.05, 1 (1–2) | |
| SR | 0.025ns | 0/21 (0%) | 1, 1 (1) | |
| HA | 0.008ns | 2/47 (4.3%) | 1.04, 1 (1–2) | |
| JM | 0.004ns | 3/58 (5.2%) | 1.07, 1 (1–3) | |
| All sites | 0.001ns | 9/205 (4.4%) | 1.04, 1 (1–3) |
Compared to P. falciparum (8.8%; 20/226; Table 1), P. vivax indicated a lower rate of polyclonal infections (4.3%; 9/205). Polyclonal samples were observed in all sites for P. falciparum, with the highest rate of polyclonal infections in the southern lowlands (HA: 16.7% and JM: 11.8%). For P. vivax, polyclonal infections ranged from 5.3% in Bure (BU) to 0% in Shewa Robit (SR), despite a slightly smaller sample size. Likewise, MOI for P. falciparum from all sites (mean MOI = 1.10; Table 1) was significantly higher than that of P. vivax, (mean MOI = 1.04, P<0.01), indicative of a higher complexity within P. falciparum infections.
Among all the polyclonal infections, 18 were bi-clonal of which two equally dominant alleles were detected in a single locus. We separated the genotypes of the two strains and included them in the analyses. For the 11 samples that showed >1 alleles in two or more loci, we were unable to confidently differentiate the genotypes of the different strains and thus these samples were discarded in the analyses (S3 Table).
Genetic diversity comparison
Both P. falciparum and P. vivax revealed similar levels of allelic and genotypic diversity (Table 2). Nevertheless, genotypic evenness in P. vivax from the highlands (E = 0.75; BU and MA) was significantly lower than the other samples (P<0.01; two-tailed t-test; Table 2). This suggested a less even distribution of genotypes in these populations and that some genotypes were more common than the others. AMOVA indicated that most of the genetic variation was within populations in both P. falciparum and P. vivax (>84%; S4 Table). In P. falciparum, a greater proportion of variation was found among regions (13% among the north, east and south Ethiopia) than among populations in a region (3%); whereas in P. vivax, the proportion of variation among regions (8%) and populations (7%) were comparable and significant.
Table 2. Comparison of Plasmodium falciparum and P. vivax genetic diversity measures based on microsatellite markers.
| Species; sites | Sample size | Genotypic diversity | Gene diversity | |||
|---|---|---|---|---|---|---|
| G | D | E | Ne | He | ||
| P. falciparum | ||||||
| North Ethiopia | ||||||
| Bure | 42 | 36.75 | 0.97 | 0.94 | 2.99 | 0.59 |
| Mankush | 36 | 32.55 | 0.97 | 1 | 4.17 | 0.71 |
| East Ethiopia | ||||||
| Metehara | 46 | 44.08 | 0.98 | 0.98 | 3.04 | 0.56 |
| Shewa Robit | 33 | 29.43 | 0.97 | 0.95 | 3.05 | 0.57 |
| South Ethiopia | ||||||
| Halaba | 18 | 10.11 | 0.83 | 1 | 2.13 | 0.52 |
| Jimma | 51 | 47.29 | 0.98 | 0.96 | 3.60 | 0.66 |
| Total | 226 | 198 | 0.95 | 0.97 | 2.81 (±0.34) | 0.66 (±0.05) |
| P. vivax | ||||||
| North Ethiopia | ||||||
| Bure | 39 | 24.14 | 0.96 | 0.75 | 4.93 | 0.73 |
| Mankush | 19 | 12.08 | 0.67 | 0.75 | 2.46 | 0.66 |
| East Ethiopia | ||||||
| Metehara | 21 | 14.02 | 0.88 | 0.72 | 3.64 | 0.69 |
| Shewa Robit | 21 | 19.31 | 0.89 | 0.93 | 3.07 | 0.72 |
| South Ethiopia | ||||||
| Halaba | 47 | 40 | 0.98 | 0.91 | 3.78 | 0.59 |
| Jimma | 58 | 35.53 | 0.96 | 0.92 | 5.56 | 0.77 |
| Total | 205 | 144 | 0.91 | 0.81 | 3.16 (±0.40) | 0.69 (±0.06) |
G: Number of multilocus genotypes corrected for sample size
D: Simpson's diversity index corrected for sample size
E: Genotypic evenness;
Ne: Number of effective alleles (Nielsen et al. 2003);
He: Expected heterozygosity corrected for sample size (Nei 1978)
Genetic clustering of samples
Populations in north and east Ethiopia were slightly differentiated from those in the south (Fig 1). This pattern was shown in both P. falciparum and P. vivax, though populations were more scattered in P. falciparum. The two northern populations (BU and MA) were genetically close to samples in the eastern Rift Valley (SR and ME; Fig 1).
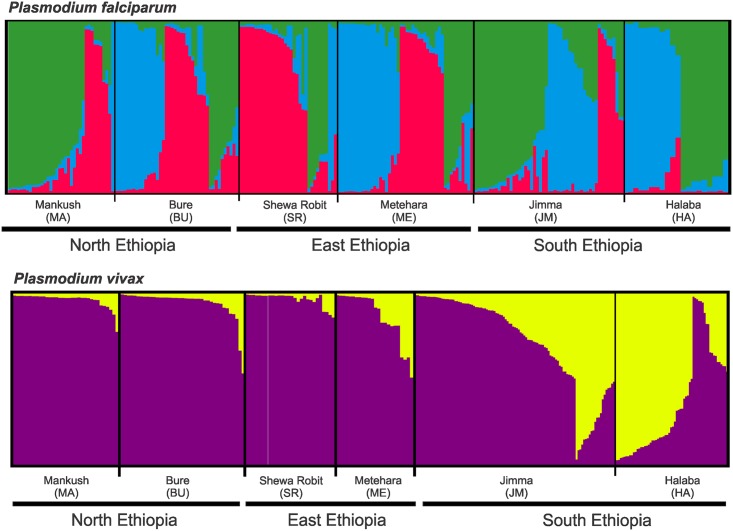
In P. falciparum, three most probable genetic clusters were detected by STRUCTURE analyses (Fig 2), but these clusters did not clearly represent geographical regions. The red cluster was most apparent in the northern (MA and BU) and eastern (SR and ME) populations, but less significant in the southern populations (JM and HA). All three genetic clusters were found in sites BU and ME, but the blue cluster was almost absent in MA and SR (Fig 2). In P. vivax, samples from north and east Ethiopia constituted predominantly the purple cluster, contrast with those from the south that constituted an admixture of the purple and yellow clusters. Unlike P. falciparum, the genetic composition between the northern and eastern populations was largely similar.
Fig 2. Bayesian inferences of the K clusters estimated by STRUCTURE among Plasmodium falciparum and P. vivax samples.
The most probable clusters are labeled by different colors, and individuals are represented as columns. Within each column (individual), the extent of the component colors indicates the magnitude of the membership coefficient (Q) corresponding to each cluster. Q values of respective clusters are presented in S5 Table.
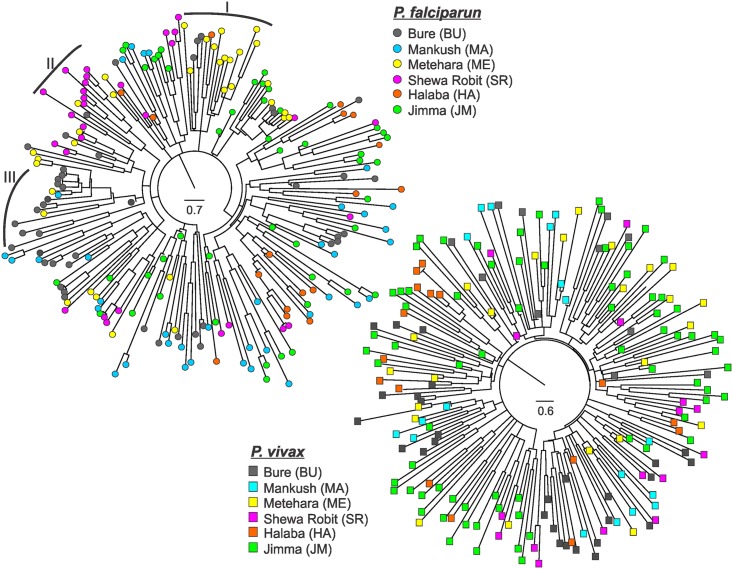
Neighbor-joining trees did not indicate clear distinction among the P. falciparum and P. vivax population samples (Fig 3). Both trees had relatively short internodes but long terminal branches, which suggested that the parasite lineages were rapidly diverged from one another and that frequent gene exchange occurred among the populations. In P. falciparum, there were a number of subclades where parasites from the same population were genetically closely related (e.g., subclades I, II, and III in Fig 3A). However, in P. vivax, samples from different sites were clustered together in the same clade without clear differentiation (Fig 3B).
Fig 3. Neighbor-joining trees showing the genetic relatedness among Plasmodium falciparum and P. vivax samples.
(A) Genetic relatedness among P. falciparum samples from the six study sites, shown by different color circle. Subclade-I represents a genetic cluster of samples predominantly from Metehara that were closely related; subclade-II represents a cluster of samples predominantly from Shewa Robit; and subclade-III represents a cluster of samples predominantly from Bure. (B) Genetic relatedness among P. vivax samples from the six study sites, shown by different color square.
Distance and landscape factors
Mantel tests indicated no significant association between geographical and genetic distances among populations of P. falciparum (R2 = 0.14, P>0.05) and P. vivax (R2 = 0.09, P>0.05), respectively. These results suggest that parasite gene flow was not limited by geographical distance. Further, for both P. falciparum and P. vivax, we found that none of the tested landscape factors explained pairwise genetic distance (FST) among populations more than the Euclidean distance alone based on AICc (Table 3). These results indicated that the differences in land cover, elevation, precipitation, and distance to roads (a proxy for accessibility) did not significantly influence parasite gene flow and that our study populations were clearly connected (S1 Fig).
Table 3. Model fitness of resistance surfaces to pairwise FST values calculated using ResistanceGA.
AICc: corrected Akaike Information Criterion; ω: Akaike weight.
| Species | Landscape surface | AICc | ΔAICc | ω |
|---|---|---|---|---|
| P. falcipaum | ||||
| Euclidean distance | -50.21 | 0 | 0.36 | |
| Distance to roads | -49.90 | 0.31 | 0.32 | |
| Elevation | -48.99 | 1.22 | 0.19 | |
| Precipitation | -48.22 | 1.99 | 0.13 | |
| Land cover | -15.78 | 34.43 | 0 | |
| P. vivax | ||||
| Euclidean distance | -43.26 | 0 | 0.41 | |
| Precipitation | -42.05 | 1.21 | 0.22 | |
| Elevation | -41.95 | 1.32 | 0.21 | |
| Distance to roads | -41.35 | 1.91 | 0.16 | |
| Land cover | -7.13 | 36.14 | 0 | |
Demographic change and migrations
All populations of P. falciparum showed a normal L-shape distribution in allele frequency (Table 4), suggesting that these populations did not experience a recent severe bottleneck. In P. vivax, allele frequency was shown with a shifted mode in site SR (east Ethiopia), indicative of a significant genetic bottleneck. In addition, a significant excess of heterozygosity was observed in this population as well as other northern and eastern populations (ME and BU) under the IAM and SMM mutation models, suggestive of a deviation in the mutation-drift equilibrium.
Table 4. Summary of the parameters and results based on BOTTLENECK analyses.
| Species | Site; sample size | Mode shift | Mutation model | Heterozygote excess |
|---|---|---|---|---|
| P. falciparum | ||||
| North Ethiopia | ||||
| Bure (n = 42) | ||||
| Normal L-shaped | IAM | P = 0.11 | ||
| SMM | 0.02* | |||
| TPM | 0.50 | |||
| Mankush (n = 36) | ||||
| Normal L-shaped | IAM | 0.05 | ||
| SMM | 0.12 | |||
| TPM | 0.05 | |||
| East Ethiopia | ||||
| Metehara (n = 46) | ||||
| Normal L-shaped | IAM | 0.52 | ||
| SMM | 0.002** | |||
| TPM | 0.13 | |||
| Shewa Robit (n = 33) | ||||
| Normal L-shaped | IAM | 0.25 | ||
| SMM | 0.26 | |||
| TPM | 0.50 | |||
| South Ethiopia | ||||
| Jimma (n = 51) | ||||
| Normal L-shaped | IAM | 0.54 | ||
| SMM | 0.25 | |||
| TPM | 0.21 | |||
| P. vivax | ||||
| North Ethiopia | ||||
| Bure (n = 39) | ||||
| Normal L-shaped | IAM | 0.56 | ||
| SMM | 0.01* | |||
| TPM | 0.55 | |||
| East Ethiopia | ||||
| Metehara (n = 21) | ||||
| Normal L-shaped | IAM | 0.02* | ||
| SMM | 0.41 | |||
| TPM | 0.21 | |||
| Shewa Robit (n = 21) | ||||
| Shifted | IAM | 0.03* | ||
| SMM | 0.35 | |||
| TPM | 0.12 | |||
| South Ethiopia | ||||
| Halaba (n = 47) | ||||
| Normal L-shaped | IAM | 0.08 | ||
| SMM | 0.50 | |||
| TPM | 0.27 | |||
| Jimma (n = 58) | ||||
| Normal L-shaped | IAM | 0.17 | ||
| SMM | 0.05 | |||
| TPM | 0.24 | |||
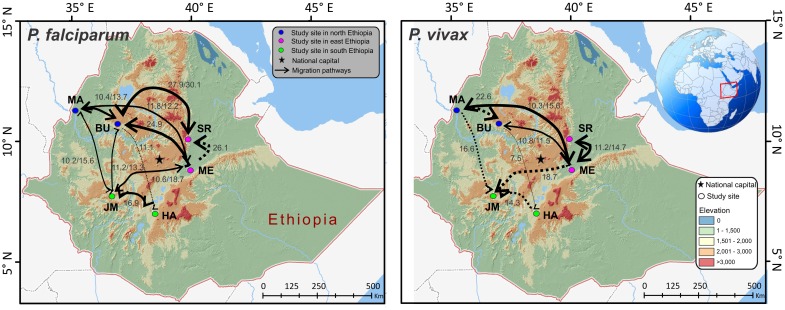
Based on Migrate-N analyses, both P. falciparum and P. vivax showed a relatively small effective population size (Θ = 0.1–0.6 and 0.17–0.88, respectively; S5 Table), which suggested that the effect of drift was unequivocally as significant as migration. Given that most values of M (m/μ) were >1, the effect of migration (m) was larger than the effect of mutation (μ). For P. falciparum, the effective number of migrants per generation Nem ranged from 0.12–8.58. The greatest migration was observed between the north and east Ethiopia (e.g., from BU to SR: M 30.12 and Nem 8.58) and these north-east migrations were frequent and mostly bidirectional. While migrations between the north-east and south Ethiopia appeared to be less significant, these migrations in most cases were bidirectional (Fig 4). For P. vivax, the effective number of migrants per generation Nem ranged from 0.12–3.58. The greatest migration was between the northern populations (e.g., from MA to BU: M 22.58 and Nem 3.58) followed by the migration from the north and east to south Ethiopia (e.g., from ME to JM: M 18.77 and Nem 2.02; from MA to JM: M 16.60 and Nem 1.96). Interestingly, the migrations to the south were primarily unidirectional (Fig 4). BayesAss analyses supported the estimates of migration rates from Migrate-N. No significant correlations were found between migration rate and geographical distance in P. falciparum (R2 = 0.13, P = 0.09) and P. vivax (R2 = 0.02, P = 0.38; S2 Fig).
Fig 4. Migratory pathways and rates of Plasmodium falciparum and P. vivax among the study sites in Ethiopia.
The intensity of gene flow is indicated by the thickness of migration paths. Solid lines denote bi-directional migration and dotted lines denote uni-directional migration. Only migration rate (M: immigration rate scaled by mutation rate) with a value of ≥10 are indicated on the maps. All values are presented in S6 Table.
Resistance gene marker polymorphisms
Among the 226 P. falciparum and 204 P. vivax samples, we successfully amplified and obtained complete resistance gene data in 199 (88%) and 185 (90%) of the samples (S7 Table). Samples with incomplete data were excluded in the analyses.
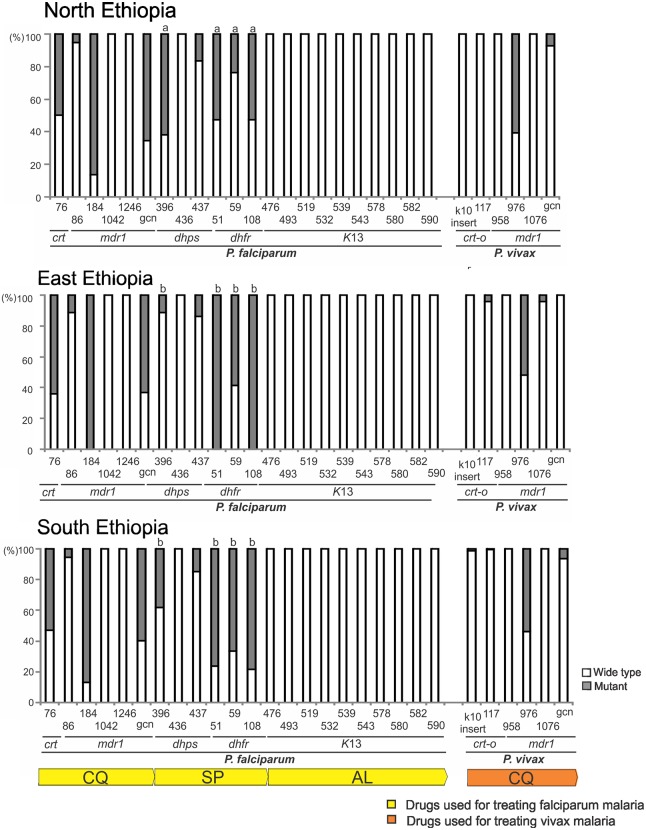
Plasmodium falciparum samples from north, east and south Ethiopia all revealed a similar pattern of mutations in pfcrt and pfmdr1, the genes that associated with chloroquine resistance. In pfcrt, about 54–62% of the samples were shown with a mutant 76T genotype (north: 37/65 = 57%; east: 45/72 = 62.5%; south: 34/62 = 54.8%; Fig 5). While the majority of P. falciparum samples showed the wild type N86, N1042, and D1246 of pfmdr1, over 85% (north: 56/65 = 86%; east: 72/72 = 100%; south: 53/62 = 85.5%; Fig 5) of the samples had the mutant 184F genotype. Also, qPCR data indicated that over 60% of the samples had two or more copies of the pfmdr1 gene (north: 42/65 = 64.6%; east: 46/72 = 63.9%; south: 38/62 = 61.3%; Fig 5). The rate of mutations observed in pfcrt and pfmdr1 was not significantly different among populations.
Fig 5. Frequency of mutations in gene codons related to antimalarial drug resistance (bottom bar) among Plasmodium falciparum and P. vivax samples from the north, east, and south Ethiopia.
The proportion of wild type (white) and mutants (gray) for each codon position was indicated in each column of the respective resistant gene associated with the antimalarial drug (bottom bar). GCN denotes gene copy number of pfmdr1 and pvmdr1 (white: single copy; gray: ≥ two copies). Significant difference was detected in the mutation frequency of codons in pfdhps and pfdhfr among the study sites, as indicated by a small letter above the column.
By contrast, the pattern of mutation in genes pfdhps and pfdhfr that associated with SP resistance appeared to vary among geographical regions in Ethiopia (Fig 5). For instances, 62% (40/65) of P. falciparum in the northern populations had the mutant 396K of pfdhps, which was significantly higher than that in the eastern (8/72 = 11%) and southern populations (24/62 = 38%). While both the eastern and southern populations showed a preponderance of pfdhfr mutations in codons 51 (51I genotype; east: 72/72 = 100%; south: 48/62 = 77.4%), 59 (59R; east: 43/72 = 59.7%; south: 41/62 = 66%) and 108 (108N; east: 72/72 = 100%; south: 49/62 = 79%), the northern populations showed a significantly lower rate of mutations in these positions (51I: 34/65 = 52.3%; 59R: 15/65 = 23.2%; 108N: 34/65 = 52.3%; P<0.05). Amplification and sequencing of the entire pfK13 indicated that this gene was highly conserved among all the P. falciparum samples. No polymorphisms were detected at the codon positions putatively related to artemisinin resistance (Fig 5; S7 Table).
For P. vivax samples from the north, east and south Ethiopia, all revealed a similar pattern of mutations in pvcrt-o and pvmdr1, the genes that associated with chloroquine resistance. Sequences of these two genes were highly conserved among the samples. Almost all had the wild type genotype except pvmdr1 codon 976 where over 50% of the samples had the mutant 976F genotype (north: 35/52 = 67.3%; east: 20/38 = 52.6%; south: 52/93 = 55.9%; Fig 5). qPCR data indicated that less than 4% of the samples had two or more copies of the pvmdr1 gene.
Discussion
Ethiopia is a unique malaria endemic country in sub-Saharan Africa where P. falciparum and P. vivax coexist. The present study showed that both species revealed similar population structure. The northern and eastern populations of P. falciparum and P. vivax were genetically closely related and slightly distinct from the southern populations. While parasite gene flow was most frequent between the northern highlands and the highland-fringe areas along the Rift Valley, the southern basin populations were not excluded. We did not find a significant association between any landscape factors and population genetic structure. This result may be caused by the chosen metric for human movement (distance to roads), which does not fully capture the seasonal migration patterns that coincide with harvest season (i.e., September to November). The seasonal migration from highland to lowland areas for agricultural harvest appears to most closely reflect that of the observed gene flow patterns. Since the end of civil war in 1991, seasonal migration of Ethiopians from highland and highland-fringe areas to lowlands has increased due to the growth of large-scale agricultural development projects [67]. Sugarcane production and coffee plantations in the lowlands are important sources of employment and income to people who live in the highlands where agricultural activities are scarce. It is possible that agricultural employments in the eastern Rift Valley and southern basin areas elicit seasonal human migrations and consequently enable spread of P. falciparum and P. vivax across broad areas without landscape or distance barriers [68,69]. For example, the lowland districts of which populations can increase by 20–30% as a result of the arrival of tens of thousands of farmers during the harvest season [70,71]. Hence, in addition to maintaining control efforts in the community, seasonal migrant populations should not be ignored in order to effectively reduce malaria burden in Ethiopia. This may include close monitoring of malaria symptoms when seasonal migrant farmers return to their home village after the harvest season, as well as offering additional instructions and aids on prevention and prompt treatment of malaria.
At the gene level, P. falciparum and P. vivax revealed a comparable level of diversity within and among populations. The mean He in our P. falciparum populations (0.66) was similar to those reported in less endemic regions such as the Caribbean (He = 0.61) [72] and Indonesia (He = 0.53) [11]. Compared to endemic areas in South Pacific and Southeast Asia e.g., Papua New Guinea (He = 0.84) [12], central Vietnam (He = 0.88) [73], Cambodia (He = 0.84) [9] as well as a previous study in south-central Ethiopia (He = 0.82) [74], the mean He in our P. vivax populations (0.69) was slightly lower but similar to that in Asendabo (He = 0.70) [15], which is about 50km away from our study site Jimma in southern Ethiopia. The moderate and comparable genetic diversity observed in both species in Ethiopia contrasts with those reported in South Pacific and Southeast Asia where P. vivax showed a higher microsatellite diversity and less fragmented gene pool than the sympatric P. falciparum [9–12]. In countries or areas where malaria transmission is intense and stable, relapse could be common. This may, in turn, facilitate recombination and local spread of P. vivax leading to higher within-population diversity and lower among-population differentiation compared to P. falciparum. By contrast, our study sites in Ethiopia represent areas of low to moderate transmission settings where the relapse rate of P. vivax is largely unknown [35,75]. Malaria there displays a strong seasonal pattern with a lag time varying from a few weeks at the beginning of the rainy season to more than a month at the end of the rainy season [76]. The rainy season is relatively short in the highland and highland-fringe areas, and thus transmission season is usually short-lived [77]. These apparent seasonal and/or landscape differences not only influence the behavior and distribution of vector mosquitoes and the length of the parasite life cycle [78], but also the patterns of human movement and settlement that can in turn determine transmission dynamics of malaria. For instances, although P. vivax infections can occur periodically throughout the year, human migration is seasonal in Ethiopia and this could limit the spread of relapse P. vivax. Also, the long arid or semi-arid season particularly in the highlands may constrain gametocyte development of P. vivax or local transmission even when relapse occurs. A recent study in southern Ethiopia indicated an approximately 9.4% (ranged from 6.4–13.6% by sites) of P. vivax patients showed recurrent infection by day 28 [5,6]. However, because relapses can occur as early as 21 days following initial treatment, it is unclear how many of the recurrent cases were due to relapse. Given that our P. falciparum and P. vivax samples were collected at the same time during the peak transmission season, the lack of contrast between P. falciparum and P. vivax population structure and diversity may suggest similar demographic factors and a less significant impact of relapse. Future study should investigate the incidence of relapse among transmission settings and how such influences parasite population diversity.
The overall polyclonal infection rates observed in P. falciparum (8.8%) and P. vivax (4.3%) were low in our study area. The proportion of polyclonal P. vivax infections was similar to that reported in areas of low endemic setting such as Central China (2–19%) [79], but considerably lower than hypo-endemic areas such as Vietnam (71.4%; [80]), Sri Lanka (68%; [81]), Colombia (60–80%; [82]), and the Amazon Basin in Brazil (50%; [83]). Likewise, the polyclonal rate of P. falciparum was comparable to low transmission areas such as Haiti (12.9%) [72] and southern China (10–23%) [84] that are approaching elimination phase, but lower than endemic countries in West Africa such as Gambia and Senegal (36–50%) [85,86], East Africa such as Kenya (70–90%) [87], Papua New Guinea (39–45%) [88], and Southeast Asia such as Malaysia (65%) [89] and Cambodia (47%) [9].The higher polyclonality in P. falciparum than in P. vivax may imply a potentially large P. falciparum reservoir present in asymptomatic hosts that emerges during the transmission/rainy season [90]. By contrast, because P. vivax infections can occur periodically throughout the year in Ethiopia, our samples may reflect only a fraction of the existing P. vivax gene pool. Although relapse and early production and circulation of gametocytes in P. vivax infection can lead to increased opportunities for recombination, drug-sensitive clones (both blood-stage parasites or hypnozoites) could be eliminated during the non-transmission season by antimalarial treatment and resulted in reduced within-host diversity [8,82]. The lack of demographic data and drug use history of our patients limits our exploration of immunity and other factors on the observed MOI. It is important to note that microsatellites have the potential to underestimate polyclonality due to their lower sensitivity and specificity in detecting minority alleles compared to amplicon deep sequencing [91]. Despite the concern of relapse by P. vivax, the asymptomatic P. falciparum and P. vivax reservoirs that remain undetected during non-transmission season or in low endemic areas could pose a long-term impact on local transmission.
Ethiopia adopted AL as the first-line treatment for uncomplicated P. falciparum in 2004 in response to increased resistance to CQ and SP [2]. Unlike the Greater Mekong Subregion of SE Asia where delayed ACTs response has been reported, AL is shown to be highly effective in clearing parasite and fever within three days of drug treatment in Ethiopia [92]. The high efficacy of AL concords with our finding of predominantly wild type pfK13 genotypes in P. falciparum from the northern, eastern, and southern populations. Although CQ and SP have not been used for P. falciparum treatment in the last decade, the high prevalence of mutations in pfcrt 76, pfmdr1 184, pfdhps 396, and pfdhfr 51 and 108 across Ethiopia suggested that strong selection may still exist possibly by the use of CQ for treating P. vivax malaria, as well as SP for intermittent preventive treatment (IPT) on pregnant women as part of the antimalarial schemes in sub-Saharan Africa [93]. It is concerning that the high prevalence of SP resistance mutations observed in the present study may indeed influence the outcome or effectiveness of IPT [94]. Another explanation for the high frequency of CQ and SP resistance mutations is the spread of resistance parasites from one population to another. Microsatellites indicated a substantial admixture of P. falciparum genotypes between north and east Ethiopia. It is possible that resistant parasites can spread via frequent human movements and become locally selected.
The observations of an alarming level of CQ resistance prevalence in Papua New Guinea [95], India [96], SE Asia [97,98], and South America [99] after a decade-application deepen the concern for the appearance of CQ resistance to P. vivax in Ethiopia. Recent reports of therapeutic failure of CQ (5.76–13%) [3–6] as well as high rates (9–32%) of recurrent infections subsequent to CQ usage in different parts of Ethiopia [6,100] have threatened the efficacy of P. vivax treatment. The highly conserved sequences of pvcrt-o as well as the predominantly wild type T958M, F1076L, and single copy of pvmdr1 suggest that these attributes may not be relevant to CQ resistance. By contrast, we detected a high prevalence of pvmdr1 976F mutation, which is considerably higher than that reported in India (22%) where the observed resistance genotypes were confirmed by in vitro drug sensitivity testing [101]. Thus, one possible explanation for the high 976F prevalence in our study area is that this mutation may associate with emerging CQ resistance. Such association merits further clinical observations and/or in vitro testing to confirm its functional significance. Moreover, it is yet unclear whether our P. vivax samples were relapse or recrudescent infections. In Cambodia, 89% of the P. vivax samples that were isolated from patients with recurrent/relapse infections within a 42-day follow-up had pvmdr1 976F mutation [102]. This reiterates the importance of distinguishing recrudescent from relapse infections in order to clarify the implications of the observed mutations and accurately elucidate resistance prevalence of P. vivax. Given that chloroquine monotherapy has been the recommended regimen for P. vivax malaria in Ethiopia for the past decades [2], selection as well as parasite gene flow may explain the emergence and spread of resistance genotypes across the country. Alternative P. vivax treatment regimes such as ACT or CQ in combination with primaquine are suggested to prolong the efficacy of CQ and prevent/reduce relapse in Ethiopia.
In summary, P. falciparum and P. vivax revealed moderate levels of genetic diversity and similar population structure in Ethiopia. Human migrations may promote parasite gene flow while environmental heterogeneity and geographical distance did not appear to be a major gene flow barrier. To effectively reduce malaria burden in Ethiopia, control efforts should focus on seasonal migrant populations. Unconstrained parasite gene flow may partly explain similar patterns of resistance marker prevalence across the country. Our findings are paramount to monitoring the emergence and spread of antimalarial drug resistance and offer evidence-based guidelines to existing treatment regimes.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
The scale of 1–10 (right) indicates the level of connectivity, e.g., areas withyellow represent the highest level of connectivity. Study sites were indicated by black dots.
(EPS)
(EPS)
Acknowledgments
We are greatly indebted to technicians and the staffs from Jimma and Addis Ababa Universities for sample collection, the communities and hospitals for their support and willingness to participate in this research, and undergraduate students from University of California Irvine for assisting data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by grants from the National Institutes of Health (R01 AI050243, U19 AI129326 and D43 TW001505) and the Thematic research program, Addis Ababa University. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. World Malaria Report (2015) WHO, Geneva.
- 2.Federal ministry of health (2012) National malaria guidelines. 3rd ed Addis Ababa, Ethiopia: Federal ministry of health. [Google Scholar]
- 3.Yeshiwondim AK, Tekle AH, Dengela DO, Yohannes AM, Teklehaimanot A (2010) Therapeutic efficacy of chloroquine and chloroquine plus primaquine for the treatment of Plasmodium vivax in Ethiopia. Acta Trop 113:105–113. doi: 10.1016/j.actatropica.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 4.Yohannes AM, Teklehaimanot A, Bergqvist Y, Ringwald P (2011). Confirmed vivax resistance to chloroquine and effectiveness of artemether-lumefantrine for the treatment of vivax malaria in Ethiopia. Am J Trop Med Hyg 84:137–140. doi: 10.4269/ajtmh.2011.09-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ketema T, Getahun K, Bacha K (2011) Therapeutic efficacy of chloroquine for treatment of Plasmodium vivax malaria cases in Halaba district, South Ethiopia. Parasite Vector 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Getachew S, Thriemer K, Auburn S, Abera A, Gadisa E, et al. (2015) Chloroquine efficacy for Plasmodium vivax malaria treatment in southern Ethiopia. MalarJ 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talisuna AO, Karema C, Ogutu B, Juma E, Logedi J, et al. (2012) Mitigating the threat of artemisinin resistance in Africa: improvement of drug-resistance surveillance and response system. Lancet Infect Dis 12:888–896. doi: 10.1016/S1473-3099(12)70241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White NJ (2011) Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J 10:297 doi: 10.1186/1475-2875-10-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orjuela-Sáncheza P, Sác JM, Michelle C, Brandia C, Priscila T, et al. (2013) Higher microsatellite diversity in Plasmodium vivax than in sympatric Plasmodium falciparum populations in Pursat, western Cambodia. Exp Parasitol 134:318–326. doi: 10.1016/j.exppara.2013.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray KA, Dowd S, Bain L, Bobogare A, Wini L, et al. (2013) Population genetics of Plasmodium falciparum and Plasmodium vivax and asymptomatic malaria in Temotu Province, Solomon Islands. Malar J 12:429 doi: 10.1186/1475-2875-12-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noviyanti R, Coutrier F, Utami RAS, Trimarsanto H, Tirta YK, et al. (2015) Contrasting transmission dynamics of co-endemic Plasmodium vivax and P. falciparum: Implications for malaria control and elimination. PLoS Negl Trop Dis 9:e0003739 doi: 10.1371/journal.pntd.0003739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennison C, Arnott A, Tessier N, Tavul L,Koepfli C, et al. (2015) Plasmodium vivax populations are more genetically diverse and less structured than sympatric Plasmodium falciparum populations. PLoS Negl Trop Dis 9:e0003634 doi: 10.1371/journal.pntd.0003634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira MU, Kaunaweera ND, de Silva-Nunes M, da Silva NS, Wirth DF, et al. (2007) Population structure and transmission dynamics of Plasmodium vivax in rural Amazonia. J Infect Dis 195:1218–1226. doi: 10.1086/512685 [DOI] [PubMed] [Google Scholar]
- 14.Arnott A, Wapling J, Mueller I, Ramsland PA, Siba PM, et al. (2014) Distinct patterns of diversity, population structure and evolution in the AMA1 genes of sympatric Plasmodium falciparum and Plasmodium vivax populations of Papua New Guinea from an area of similarly high transmission. Malar J 13:233 doi: 10.1186/1475-2875-13-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunawardena S, Karunaweera ND, Ferreira MU, Phone-Kyaw M, Pollack RJ, et al. (2010) Geographic Structure of Plasmodium vivax: Microsatellite Analysis of Parasite Populations from Sri Lanka, Myanmar, and Ethiopia. Am J Trop Med Hyg.82:235–242. doi: 10.4269/ajtmh.2010.09-0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, et al. (2012) Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487:375–9. doi: 10.1038/nature11174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schousboe ML, Ranjitkar S, Rajakaruna RS, Amerasinghe PH, Konradsen F, et al. (2014) Global and local genetic diversity at two microsatellite loci in Plasmodium vivax parasites from Asia, Africa and South America. Malar J 13:392 doi: 10.1186/1475-2875-13-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koepfli C, Rodrigues PT, Antao T, Orjuela-Sánchez P, Van den Eede P, et al. (2015) Plasmodium vivax diversity and population structure across four continents. PLoS Negl Trop Dis 9:e0003872 doi: 10.1371/journal.pntd.0003872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campino S, Auburn S, Kivinen K, Zongo I, Ouedraogo JB, et al. (2011) Population genetic analysis of Plasmodium falciparum parasites using a customized Illumina Golden Gate genotyping assay. PLoS ONE 6:e20251 doi: 10.1371/journal.pone.0020251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry AE, Waltmann A, Koepfli C, Barnadas C, Mueller I (2015) Uncovering the transmission dynamics of Plasmodium vivax using population genetics. Pathog Glob Health 109: 142–152. doi: 10.1179/2047773215Y.0000000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowman AF, Morry MJ, Biggs BA, Cross GA, Foote SJ (1988) Amino acid changes linked to pyrimethamine resistance in the dihydrofolatereductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci USA 85:9109–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triglia T, Menting JG, Wilson C, Cowman AF (1997) Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci USA 94:13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidhu AB, Verdier-Pinard D, Fidock DA (2002) Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210–213. doi: 10.1126/science.1074045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, et al. (2014) A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips EJ, Keystone JS, Kain KC (1996) Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin Infect Dis 23:1171–3. [DOI] [PubMed] [Google Scholar]
- 26.Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, et al. (2007) Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One 2:e1089 doi: 10.1371/journal.pone.0001089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schunk M, Kumma WP, Miranda IB, Osman ME, Roewer S, et al. (2006) High prevalence of drug-resistance mutations in Plasmodium falciparum and Plasmodium vivax in southern Ethiopia. MalarJ 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mula P, Fernández-Martínez A, de Lucio A, Ramos JM, Reyes F, et al. (2011) Detection of high levels of mutations involved in anti-malarial drug resistance in Plasmodium falciparum and Plasmodium vivax at a rural hospital in southern Ethiopia. MalarJ 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G (2014) High prevalence of pfcrt-CVIET haplotype in isolates from asymptomatic and symptomatic patients in south-central Oromia, Ethiopia. Malar J 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hailemeskel E, Kassa M, Taddesse G, Mohammed H, Woyessa A, et al. (2013) Prevalence of sulfadoxine–pyrimethamine resistance-associated mutations in dhfr and dhps genes of Plasmodium falciparum three years after SP withdrawal in Bahir Dar, Northwest Ethiopia. Acta Trop 128:636–41. doi: 10.1016/j.actatropica.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 31.Tessema SK, Kassa M, Kebede A, Mohammed H, Leta GT, et al. (2015) Declining trend of Plasmodium falciparum dihydrofolatereductase (dhfr) and dihydropteroate synthase (dhps) mutant alleles after the withdrawal of Sulfadoxine-Pyrimethamine in North Western Ethiopia. PLoS ONE 10:e0126943 doi: 10.1371/journal.pone.0126943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, et al. (2016) A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 374:2453–64. doi: 10.1056/NEJMoa1513137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidiein L (2017) Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med 376:991–993. doi: 10.1056/NEJMc1612765 [DOI] [PubMed] [Google Scholar]
- 34.Bereczky S, Martensson A, Gil JP (2005) Short report: Rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am J Trop Med Hyg 72:249–51. [PubMed] [Google Scholar]
- 35.Lo E, Yewhalaw D, Zhong D, Zemene E, Degefa T, et al. (2015) Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among Duffy-positive and Duffy-negative populations in Ethiopia. Malar J 14:84 doi: 10.1186/s12936-015-0596-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson TJC, Su XZ, Bockarie M, Lagog M, Day KP (1999) Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitol 119:113–125. [DOI] [PubMed] [Google Scholar]
- 37.Karunaweera N, Ferreira M, Hartl D, et al. (2007) Fourteen polymorphic microsatellite DNA markers for the human malaria parasite Plasmodium vivax. Mol Ecol Notes 7:172–175. [Google Scholar]
- 38.Koepfli C, Mueller I, Marfurt J, et al. (2009) Evaluation of Plasmodium vivax genotyping markers for molecular monitoring in clinical trials. J Infect Dis 199:1074.–. doi: 10.1086/597303 [DOI] [PubMed] [Google Scholar]
- 39.Raymond M, Rousset F (1995) GENEPOP: a population genetic software for exact test and ecumenicism. J Hered 86:248–249. [Google Scholar]
- 40.Haubold B, Hudson RR (2000) LIAN 3.0: detecting linkage disequilibrium in multilocus data. Bioinformatics 16:847–849. [DOI] [PubMed] [Google Scholar]
- 41.Meirmans PG, Van Tienderen PH. (2004) GenoType and GenoDive: two programs for the analysis of genetic diversity of asexual organisms. MolEcol Note 4:792–4. [Google Scholar]
- 42.Smouse PE, Peakall R (1999) Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561–73. [DOI] [PubMed] [Google Scholar]
- 43.Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. MolEcol 14:2611–2620. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg NA (2004) Distruct: a program for the graphical display of population structure. MolEcol Note 4:137–8. [Google Scholar]
- 46.Makarenkov V (2001) T-Rex: Reconstructing and visualizing phylogenetic trees and reticulation networks, Bioinformatics 17:664–668. [DOI] [PubMed] [Google Scholar]
- 47.Boc A, Diallo Alpha B, Makarenkov V (2012) T-REX: a web server for inferring, validating and visualizing phylogenetic trees and networks, Nucleic Acids Res 40:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smouse PE, Peakall R (1999) Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561–573. [DOI] [PubMed] [Google Scholar]
- 49.Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. MolEcol Note 2:618–20. [Google Scholar]
- 50.Peakall R, Smouse PE (2006) Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. MolEcol Note 6:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casgrain P, Legendre P (2004) The R Package for Multivariate and Spatial Analysis v4.0d10. Université de Montréal, Montréal, Canada. http://www.bio.umontreal.ca/casgrain/en/labo/R/v4/
- 52.Bohonak AJ (2002) IBD (Isolation By Distance): a program for analyses of isolation by distance. J Hered 93:153–4. [DOI] [PubMed] [Google Scholar]
- 53.Piry S, Luikart G, Cornuet JM (1999) BOTTLENECK: a computer program for detecting recent reductions in effective size using allele frequency data. J Hered 90:502–3. [Google Scholar]
- 54.Beerli P (2004) Migrate: Documentation and program, part of LAMARC version 2.4.4. http://evolution.gs.washington.edu/lamarc.html
- 55.Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163:1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spear SF, Balkenhol N, Fortin MJ, et al. (2010) Use if resistance surfaces for landscape genetic studies: considerations for parameterization and analysis. Mol Ecol 19:3576–3591. doi: 10.1111/j.1365-294X.2010.04657.x [DOI] [PubMed] [Google Scholar]
- 57.Friedl MA, Sulla-Menashe D, Tan B, et al. (2010) MODIS Collection 5 global land cover: Algorithm refinements and characterization of new datasets. Remote Sens Environ 114:168–182. [Google Scholar]
- 58.Channan S, Collins K, Emanuel WR (2014) Global mosaics of the standard MODIS land cover type data, University of Maryland and the Pacific Northwest National Laboratory, College Park, Maryland, USA. [Google Scholar]
- 59.Jarvis A, Reuter HI, Nelson A, et al. (2008) Hole-filled SRTM for the globe version 4. the CGIAR-CSISRTM 90m database http://srtm.csi.cgiar.org
- 60.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int JClimatol 25:1965–1978. [Google Scholar]
- 61.Peterman WE, Connette GM, Semlitsch RD, et al. (2014) Ecological resistance surfaces predict fine-scale genetic differentiation in a terrestrial woodland salamander. Mol Ecol 23:2402–2413. doi: 10.1111/mec.12747 [DOI] [PubMed] [Google Scholar]
- 62.MaRae BH (2006) Isolation by resistance. Evolution 60:1551–1561. [PubMed] [Google Scholar]
- 63.Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–6. http://CRAN.R-project.org/package=lme4.
- 64.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, et al. (2000) The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarialsmefloquine and artemisinin. Mol Biochem Parasitol 108:13–23. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira I, do Rosário VE, Cravo P (2006) Real-time quantitative PCR with SYBR Green I detection for estimating copy numbers of nine drug resistance candidate genes in Plasmodium falciparum. Malar J 5:1 doi: 10.1186/1475-2875-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, et al. (2007) Chloroquine resistant Plasmodium vivax: In vitro characterization and association with molecular polymorphisms. PLoS ONE 2:e1089 doi: 10.1371/journal.pone.0001089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schicker RS, Hiruy N, Melak B, Gelaye W, Bezabih B, et al. (2015) Avenue-based survey of malaria, anemia and mobility patterns among migrant farm workers in Amhara region, Ethiopia. PLoS ONE 10:e0143829 doi: 10.1371/journal.pone.0143829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martens P, Hall L (2000) Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis 6:103–9. doi: 10.3201/eid0602.000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deressa W, Ali A, Berhane Y (2006) Review of the interplay between population dynamics and malaria transmission in Ethiopia. Ethiop J Health Develop 20:137–44. [Google Scholar]
- 70.Yukich JO, Taylor C, Eisele TP, Reithinger R, Nauhassenay H, et al. (2013) Travel history and malaria infection risk in a low-transmission setting in Ethiopia: a case control study. Malar J 12:33 doi: 10.1186/1475-2875-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alemu K, Worku A, Berhane Y, Kumie A (2014) Men traveling away from home are more likely to bring malaria into high altitude villages, northwest Ethiopia. PLoS ONE 9:e95341 doi: 10.1371/journal.pone.0095341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carter TE, Malloy H, Existe A, Memnon G, St. Victor Y, et al. (2015) Genetic Diversity of Plasmodium falciparum in Haiti: Insights from Microsatellite Markers. PLoS ONE 10:e0140416 doi: 10.1371/journal.pone.0140416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van den Eede P, Erhart A, Van der Auwera G, et al. (2010) High complexity of Plasmodium vivax infections in symptomatic patients from a rural community in central Vietnam detected by microsatellite genotyping. Am J Trop Med Hyg 82:223–227. doi: 10.4269/ajtmh.2010.09-0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Getachew S, To S, Trimarsanto H, Thriemer K, Clark TG, et al. (2015) Variation in complexity of infection and transmission stability between neighboring populations of Plasmodium vivax in Southern Ethiopia. PLoS ONE 10:e140780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou G, Yewhalaw D, Lo E, Zhong D, Wang X, et al. (2016) Analysis of asymptomatic and clinical malaria in urban and suburban settings of southwestern Ethiopia in the context of sustaining malaria control and approaching elimination. Malar J 15:250 doi: 10.1186/s12936-016-1298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teklehaimanot HD, Lipsitch M, Teklehaimanot A, Schwartz J (2004) Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia I. Patterns of lagged weather effects reflect biological mechanisms. Malar J 3:41 doi: 10.1186/1475-2875-3-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Midekisa A, Beyene B, Mihretie A, Bayabil E, Wimberly MC(2015) Seasonal associations of climatic drivers and malaria in the highlands of Ethiopia. Parasite Vector 8:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Craig MH, Snow RW, Sueur D (1999) A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol 15:105–11. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Auburn S, Cao J, Trimarsanto H, Zhou H, et al. , (2014) Genetic diversity and population structure of Plasmodium vivax in Central China. Malar J 13:262 doi: 10.1186/1475-2875-13-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen VH, Delgado-Ratto C, Thanh PV, et al. (2016) Population genetics of Plasmodium vivax in four rural communities in central Vietnam. PLoS Negl Trop Dis 10:e0004434 doi: 10.1371/journal.pntd.0004434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gunawardena S, Ferreira MU, Kapilananda GMG, et al. (2014) The Sri Lankan paradox: high genetic diversity in Plasmodium vivax populations despite decreasing levels of malaria transmission. Parasitol 141:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Imwong M, Snounou G, Pukrittayakamee S, et al. (2007) Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis 195:927–933. doi: 10.1086/512241 [DOI] [PubMed] [Google Scholar]
- 83.Batista CL, Barbosa S, Da Silva Bastos M, et al. (2015) Genetic diversity of Plasmodium vivax over time and space: a community–based study in rural Amazonia. Parasitol 142:374–384. [DOI] [PubMed] [Google Scholar]
- 84.Wei G, Zhang L, Yan H, Zhao Y, Hu J et al. (2015) Evaluation of the population structure and genetic diversity of Plasmodium falciparum in southern China. Malar J 14:283 doi: 10.1186/s12936-015-0786-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mobegi VA, Loua KM, Ahouidi AD, Satoguina J, Nwakanma DC, et al. (2012) Population genetic structure of Plasmodium falciparum across a region of diverse endemicity in West Africa. Malar J 11:223 doi: 10.1186/1475-2875-11-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salgueiro P, Vicente JL,Figueiredo RC, Pinto J (2016) Genetic diversity and population structure of Plasmodium falciparum over space and time in an African archipelago. Infect Genet Evol 43:252–260. doi: 10.1016/j.meegid.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 87.Gatei W, Kariuki S, Hawley W, Kuile F, Terlouw D, et al. (2010) Effects of transmission reduction by insecticidetreated bed nets (ITNs) on parasite genetics population structure: I. The genetic diversity of Plasmodium falciparum parasites by microsatellite markers in western Kenya. Malar J 9:353 doi: 10.1186/1475-2875-9-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schultz L, Wapling J, Mueller I, Ntsuke PO, Senn N, et al. Multilocus haplotypes reveal variable levels of diversity and population structure of Plasmodium falciparum in Papua New Guinea, a region of intense perennial transmission. Malar J 9:336 doi: 10.1186/1475-2875-9-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Razak MA, Sastu UR, Norahmad NA, Abdul-Karim A, Muhammad A, et al. (2016) Genetic diversity of Plasmodium falciparum populations in malaria declining areas of Sabah, East Malaysia. PLoS ONE 11:e0152415 doi: 10.1371/journal.pone.0152415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nkhoma SC, Nair S, Cheeseman IH, Rohr-Allegrini C, Singlam S, et al. (2012) Close kinship within multiple-genotype malaria parasite infections. Proc BiolSci 279:2589–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin JT, Hathaway NJ, Saunders DL, et al. (2015) Using amplicon deep sequencing to detect genetic signatures of Plasmodium vivax relapse. J Infect Dis 212:999–1008. doi: 10.1093/infdis/jiv142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wudneh F, Assefa A, Nega D, Mohammed H, Solomon H, et al. (2016) Open-label trial on efficacy of artemether/lumefantrine against the uncomplicated Plasmodium falciparum malaria in Metema district, Northwestern Ethiopia. Ther Clin Risk Manag 12:1293–1300. doi: 10.2147/TCRM.S113603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vallely A, Vallely L, Changalucha J, Greenwood B,Chandramohan D (2007) Intermittent preventive treatment for malaria in pregnancy in Africa: What's new, what's needed? Malar J 6:16 doi: 10.1186/1475-2875-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Braun V, Rempis E, Schnack A, Decker S, Rubaihayo J, et al. (2015) Lack of effect of intermittent preventive treatment for malaria in pregnancy and intense drug resistance in western Uganda. Malar J 14:372 doi: 10.1186/s12936-015-0909-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schuurkamp GJ, Spice PE, Kereu RK, Bulungol PK, Rieckmann KH (1992) Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg 86:121–12. [DOI] [PubMed] [Google Scholar]
- 96.Dua VK, Kar PK, Sharma VP (1996) Chloroquine resistant P. vivax in India. Trop Med Intl Health 1:816–19. [DOI] [PubMed] [Google Scholar]
- 97.Guthmann JP, Pittet A, Lesage A, Imwong M, Lindegardh N, et al. (2008) Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Intl Health 13:91–98. [DOI] [PubMed] [Google Scholar]
- 98.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, et al. (2008) Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: A prospective study in Papua, Indonesia. PLoS Med 5:e128 doi: 10.1371/journal.pmed.0050128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonçalves LA, Cravo P, Ferreira MU (2012) Emerging Plasmodium vivax resistance to chloroquine in South America: an overview. Mem Inst Oswaldo Cruz 109:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang J, Alemayehu BH, Reithinger R, Tekleyohannes SG, Teshi T, et al. (2013) In vivo efficacy of artemether-lumefantrine and chloroquine against Plasmodium vivax: a randomized open label trial in Central Ethiopia. PLoS ONE 8:e63433 doi: 10.1371/journal.pone.0063433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh G, Singh R, Urhehar AD (2016) Simple molecular methods for early detection of chloroquine drug resistance in Plasmodium vivax and Plasmodium falciparum. J Clin Diagnos Res 10:DC19–DC23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin JT, Patel JC, Kharabora O, Sattabongkot J, Muth S, et al. (2013) Plasmodium vivax isolates from Cambodia and Thailand show high genetic complexity and distinct patterns of P. vivax multidrug resistance gene 1 (pvmdr1) polymorphisms. Am J Trop Med Hyg 88:1116–1123. doi: 10.4269/ajtmh.12-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
The scale of 1–10 (right) indicates the level of connectivity, e.g., areas withyellow represent the highest level of connectivity. Study sites were indicated by black dots.
(EPS)
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.