ABSTRACT
Centrally positioned in nuclear RNA metabolism, the exosome deals with virtually all transcript types. This 3′–5′ exo- and endo-nucleolytic degradation machine is guided to its RNA targets by adaptor proteins that enable substrate recognition. Recently, the discovery of the ‘Poly(A) tail exosome targeting (PAXT)’ connection as an exosome adaptor to human nuclear polyadenylated transcripts has relighted the interest of poly(A) binding proteins (PABPs) in both RNA productive and destructive processes.
KEYWORDS: Nuclear RNA degradation, poly(A) tail, poly(A) binding proteins, RNA exosome, RNA exosome adaptors, the PAXT connection
Introduction
To manage the constant and pervasive production of RNA, eukaryotic nuclei rely heavily on RNA degradation systems. These aid in the processing of mature RNA from precursors and in the removal of transcriptional and RNA processing by-products, transcripts produced in excess and otherwise nuclear retained RNA. A chief player here is the 3′–5′ exo- and endo-nucleolytic RNA exosome, conserved in all studied eukaryotes.1-5 This multisubunit protein complex resides in both the nucleus and the cytoplasm, where it deals with most known RNA biotypes.6 The nuclear exosome, relevant for this article, is composed of an inert doughnut-shaped core of 9 proteins (EXO-9) and achieves its catalytic activity from associated ribonucleases; the exonuclease hRRP6/Rrp6p and the exo- and endo-nuclease hRRP44 (DIS3)/Rrp44p, positioned on opposite sides of the central core channel (Fig. 1).
Figure 1.
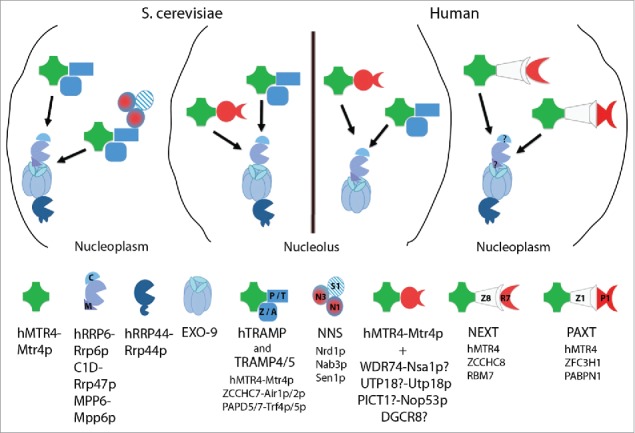
RNA exosome adaptors in S. cerevisiae and human nuclei. Principle overview of the main factors interacting with, and providing target specificity to, the exosome in S. cerevisiae (left) and human (right) nuclei (see text for details). hMTR4-Mtr4p (green) offers a main anchor linking the exosome to its different adaptors (indicated by black arrows). Question marks indicate possible interactions of Nsa1p and PICT1, UTP18 and DGCR8 with Mtr4p and hMTR4, respectively, motivated by protein homologies. Localization and function of C1D and MPP6 in the human nucleoplasm remain an open question (as question marks indicate). Note that proteins with the symbol ‘p’ derive from S. cerevisiae.
Although, the exosome might target some of its substrates directly, available data suggest that it uses various RNA-binding adaptor complexes. Indeed, a functional scheme with a ‘ready-to-use’ RNA decay machine connected to a suitable adaptor, providing substrate specificity, is emerging as a general theme. In line with this, we recently described a new human exosome adaptor, which targets polyadenylated nuclear RNAs. Consequently, we coined this adaptor the ‘poly(A) tail exosome targeting (PAXT) connection’.7 Here we discuss organizational strategies for exosomal targeting of nuclear RNA, possible biologic implications of the PAXT connection and its relation to fellow nuclear RNA decay pathways.
Organization of exosome co-factors and adaptors
In all species studied, proper function of both nuclear and cytoplasmic exosomes critically depends on RNA helicase activities.8 Human examples of such ATP-dependent exosome-associated helicases are the cytoplasmic and nuclear proteins SKI2 (SKIV2L) and hMTR4 (SKIV2L2), respectively. hMTR4/Mtr4p is a DExH superfamily 2 (SF2) helicase conserved from yeast to man and its proposed role is to unwind secondary structure and facilitate the injection of RNA substrate into the channels of the nuclear exosome.9-13 However, besides this key activity, hMTR4/Mtr4p also provides a universal anchor for nuclear exosome adaptors (Fig. 1). The N-terminal domain of hMTR4/Mtr4p is responsible for exosome interaction, in particular via hRRP6/Rrp6p and C1D/Rrp47p, an hRRP6/Rrp6p co-factor.14,15 This is for example relevant within nucleoli of human cells, where hRRP6 and C1D participate in pre-rRNA processing.16 Like C1D/Rrp47p, the M-phase phosphoprotein 6 protein, MPP6/Mpp6p, appears to serve as a general exosome co-factor,17-19 but although C1D, MPP6/Mpp6p and Mtr4p display affinities for RNAs harbouring poly(G), poly(C/U) and poly(A) tracts, respectively,9,16-18 it is unclear whether this directly guides the exosome to some of its RNA targets or whether it reflects supportive roles for the exosome in, for example, substrate organization or unwinding.
Recently, other proteins have emerged as clear cases of direct adaptors between substrate RNA and the nuclear exosome through their interactions with hMTR4/Mtr4p. In an apparent simple arrangement, the S. cerevisiae RNA-binding proteins, Nop53p and Utp18p, bind the so-called arch domain of Mtr4p, facilitating 5.8S rRNA processing and the degradation of 5′external transcribed spacer (5′ETS) RNAs, respectively (Fig. 1).20 In particular, Nop53p cross-links close to the 3′end of mature 5.8S rRNA on its 35S pre-rRNA precursor and adjacent to an Mtr4p cross-linking site, which is lost upon Nop53p depletion. Whether the human counterparts of Nop53p and Utp18p, PICT1 and UTP18, harbour similar hMTR4 interaction motifs and functions remains to be investigated. Another nucleolar example is the hMTR4-interacting protein WDR74, which is somewhat similar to the S. cerevisiae ribosome biogenesis factor Nsa1p and suggested to recruit hMTR4 and the exosome to 12S rRNA for its processing into 5.8S rRNA.21 Finally, the human RNA binding protein DGCR8 is suggested to target the nucleolar exosome to the degradation of mature snoRNA and telomerase RNA (hTR).22 However, an involvement here of hMTR4 for exosomal anchoring has not been directly analyzed.
In the nucleoplasm of human cells, a common organization of 2 exosome adaptors has been described in our laboratory. In both instances, an RNA-binding protein, believed to provide target specificity to the exosome, connects to hMTR4 via a large bridging protein (Fig. 1). In case of the trimeric nuclear exosome targeting (NEXT) complex, the zinc-finger protein ZCCHC8 bridges hMTR4 to RBM7, which binds RNA rather promiscuously, reflecting the broad substrate range of NEXT toward early unprocessed transcripts.7,23-26 In case of the PAXT connection, the zinc-finger protein ZFC3H1 bridges hMTR4 to the nuclear poly(A) binding protein PABPN1, which primarily binds polyadenylated RNA species,7,27,28 Noteworthy, however, the tight MTR4-ZFC3H1 dimer interacts with PABPN1 in a more transient and partly RNA-dependent manner, suggesting a role for yet unknown factors in yielding an active PAXT connection. In contrast, the proteins of the hMTR4-ZCCHC8-RBM7 trimer can be co-precipitated in virtually equal stoichiometries.26 As a possible way of engaging NEXT and PAXT with certain capped RNAs, both assemblies physically associate with components of the cap binding complex (CBC) and its associated factors ARS2 and ZC3H18.7,23
A particularly complex architecture of an exosome linkage to its RNA substrate is reflected by the 3-way organization of the Trf4p-Air1p/2p-Mtr4p polyadenylation (TRAMP4),29-31 Nrd1p-Nab3p-Sen1p (NNS)32 and exosome complexes in the S. cerevisiae nucleoplasm (Fig. 1). Here, the NNS complex is recruited to short sequence motifs (GUAA/G and UCUUG recognized by Nrd1p and Nab3p, respectively) in the nascent RNA.32 This elicits transcription termination, through the helicase activity of Sen1p, and mediates a direct interaction between Nrd1p and the RNA exosome, mediated by TRAMP4. An interaction which establishes a ‘hand-over’ of substrate to the exosome as exemplified by its complete degradation of cryptic unstable transcript (CUTs) or its 3′end processing of stable sn(o)RNA.33-38 A functional homolog of the NNS complex appears to be lacking in mammalian cells, but the above mentioned ARS2 protein might serve a similar role in linking transcription termination to exosome activity.23,39
A distinct Trf5p-Air1p-Mtr4p polyadenylation (TRAMP5) complex also exists in S. cerevisiae nuclei (Fig. 1).40-42 In addition to the substrates mentioned above, TRAMP4/5 complexes aid the exosome in the degradation of 23S pre-rRNA, aberrant 5S rRNA forms and hypomethylated initiator tRNAMet as well as in the processing of diverse pre-rRNA species.43-45 While Trf4p and Trf5p endow TRAMP4 and TRAMP5 with poly(A) polymerases activity, the Air1p and Air2p zinc-knuckle proteins bind RNA. Together with the helicase activity of Mtr4p, these capacities of TRAMP4/5 are suggested to aid in the exosomal degradation of structured substrates, which may require subsequent rounds of 3′adenylation by Trf4/5p to reach completion.46 In this sense, TRAMP complexes may be best described as exosome co-factors, still requiring adaptors for proper RNA targeting. Except for the NNS complex connection of TRAMP4, it is not clear how TRAMP4/5 complexes are recruited to transcript substrates.
TRAMP structural organization and activity is conserved in S. pombe and in human cells through the Mtr4-Air1-Cid14 and hMTR4-ZCCHC7-PAPD5/7 complexes, respectively.26,47-49 In these species, TRAMP complexes appear to largely reside in nucleoli, serving the exosome in the processing and degradation of pre-rRNA species. However, in S. pombe, TRAMP components also participate in the degradation of heterochromatic transcripts via the recruitment of the exosome and the RNA interference (RNAi) machinery.47 Moreover, some hMTR4-dependent exosome adaptor schemes are conserved in S. pombe, where, the hMTR4-related protein, Mtr4-like protein 1 (Mtl1), interacts with the ZFC3H1-homologous Red1 protein to form a scaffold coined the Mtl1-Red1 core (MTREC) complex.50-52 MTREC further associates with other proteins, including the S. pombe PABPN1 homolog Pab2, suggesting the existence of a PAXT-like activity. ZCCHC8 and RBM7 do not appear to be conserved in S. pombe, whereas the A. thaliana hMTR4-like helicase, HEN2, localizes to the nucleoplasm and co-purifies with 2 proteins related to human ZCCHC8 and one protein related to RBM7.53,54 Continued exploration of exosome-targeting components and strategies across eukaryotic species will reveal how nuclear exosomes achieve their substrate specificities and how changing environmental conditions may balance the use of different adaptor complexes according to substrate abundances and cellular needs.
Poly(A) binding proteins (PABPs) in RNA metabolism
Eukaryotic PABPs are both nuclear and cytoplasmic at steady-state, but often shuttle between these compartments.55 The recent description of PAXT highlights the versatility of nuclear PABPs across species. In eukaryotic nuclei, multiple PABPs cover the poly(A) tail, which reaches a length of ∼80–90 nt in S. cerevisiae and ∼250 nt in human cells. In addition, PABPN1, and possibly also the S. cerevisiae nuclear PABP Nab2p, bind the nascent tail during its synthesis.56-61 Notably, mechanistic differences may exist, as Nab2p is not a homolog of PABPN1, which seems to be absent in S. cerevisiae. Regardless, these PABP-scaffolds on nuclear poly(A) tails were proposed to serve a protective function, shielding the newly produced RNA against nucleolytic attack while directing RNAs with any business in the cytoplasm for nuclear export.62,63 It is now clear, however, that PABPs may also invoke RNA degradation by directly recruiting ribonucleolytic activities.
In S. cerevisiae, Nab2p protects newly synthesized mRNA against exosomal degradation, likely during the distributive phase of the polyadenylation reaction where the growing tail is most vulnerable.59 Significantly, Nab2p also connects to mRNA export factors,63 perhaps further aiding in moving the transcript along its productive path and escaping nuclear decay. However, for transcripts with longer nuclear residence times, like certain pools of pre-mRNA, Nab2p appears to attract the exosome machinery, causing transcript turnover.64 Such double-faced activity of a PABP, pending the maturation level of the RNA, might provide an in-built nuclear timer ensuring that transcripts failing to exit the nucleus in a timely manner will be eliminated (Fig. 2).65 PABPN1 holds similar properties as Nab2p; it binds the nascent poly(A) tail,56,57,66 it is implicated in RNA export62 and it participates in nuclear RNA decay.55 The latter activity was suggested before the discovery of the PAXT connection, when it was described that PABPN1 and the poly(A) polymerases α and γ contribute to nuclear exosome degradation of RNA via the so-coined ‘PABPN1 and PAP-mediated RNA decay (PPD)’ pathway.27,28,67 We suggest that physical interactions demonstrated by the PAXT connection explain, at least part of, the biology of the PPD pathway. However, it should be kept in mind that nuclear RNA decay pathways are still insufficiently characterized and that central factors might engage with more than one type of nucleolytic activity (see below). Regardless, it seems plausible that the concept of one individual PABP affecting nuclear levels of polyadenylated RNA both positively and negatively is conserved between S. cerevisiae and human cells.
Figure 2.
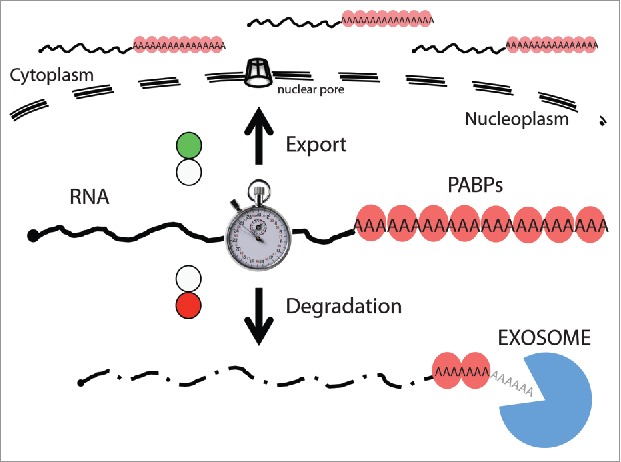
PABPs provide timing of RNA nuclear residence. Proceeding their poly(A) tail recognition by nuclear PABPs (red balloons), processed RNAs are exported to the cytoplasm (indicated by green light). In contrast, export-impaired or -delayed RNAs are turned over (red light). Thus, PABPs ‘sense’ the nuclear exposure time (represented by a the timer) of polyadenylated RNAs and provide the platform for nuclear RNA decay.
Surprisingly, the S. pombe homolog of PABPN1, Pab2, is not involved in the RNA polyadenylation reaction, perhaps being obsolete due to the short size of S. pombe poly(A) tails (∼40 nt) and/or the high processivity of the Pla1 poly(A) polymerase in fission yeast.56,68,69 Yet, Pab2 does engage in nuclear exosome-related activities like the degradation of meiosis-specific transcripts and unspliced pre-mRNAs via MTREC51,70,71 as well as the 3′end trimming of extended snoRNA precursors.72 Instead, an RNA protective function was recently suggested for the fission yeast homolog of S. cerevisiae Nab2, spNab2, which competes with Pab2 for specific polyadenylated transcripts to prevent exosome-mediated decay.73 This highlights the fact that genomes of studied model organisms encode more than just a single PABP and that these may have redundant or even opposing functions; both Pab2 and spNab2 deleted S.pombe cells are viable and produce mRNAs with normal poly(A) tail lengths, motivating the finding that the fission yeast homolog of S. cerevisiae Pab1, the cytoplasmic spPabp, complements essential functions of Pab2- and spNab2-null cells.74 Similarly, overexpression of the normally cytoplasmic Pab1p rescues the viability of genetic or conditional Nab2p depletion in S. cerevisiae and Pab1p likely also binds to nascent poly(A) tails.59,63,75 Finally, the zinc-finger protein ZC3H14, a human homolog of Nab2p, was recently ascribed an RNA protective function,76 and like Nab2p, ZC3H14 was suggested to control poly(A) tail length and modulate processing of pre-mRNAs.77
An additional degradative role in RNA maturation has also recently been ascribed to PABPN1. Interestingly this occurs in a PAXT-independent manner and instead of the nuclear exosome involves the poly(A)-specific ribonuclease PARN.78-80 A particularly well-characterized substrate for this pathway is the hTR, which uses the canonical poly(A) polymerases α and γ, PABPN1 and PARN for its maturation - in their absence mature hTR forms are significantly decreased.79 Consistent with PABPN1 activity outside of the PAXT connection, depletion of the PAXT subunit ZFC3H1 has no effect on mature hTR levels (Fig. 3A). The RNA exosome does nevertheless take part in hTR biology in that it aids in the degradation of 3′extended hTRs derived from imperfect 3′end processing events.80 However, in this case the NEXT complex is engaged (Fig. 3B), consistent with targeting of these RNA species not being dependent on prior polyadenylation.
Figure 3.
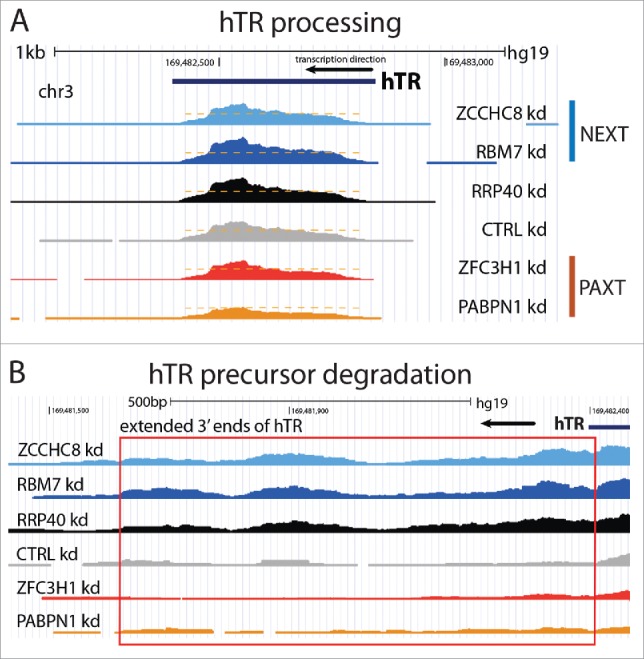
Processing and degradation of hTR. Screen shots of the hTR gene and RNAseq data from.7 (A) hTR maturation depends on the PAXT-independent poly(A) tail recognition by PABPN1 and the ensuing 3′end trimming by PARN (see text for details). Accordingly, depletion of PABPN1 (‘PABPN1 Kd’), but not ZFC3H1 (‘ZFC3H1 kd’), decreases levels of mature hTR. Orange dashed lines indicate the maximum RNA signal levels in PABPN1 depletion conditions. (B) Extended hTR 3′ends (highlighted by red box) of impaired hTR precursors are targets for NEXT-dependent exosome degradation in the nucleoplasm.
The ability of PABPN1 to engage in RNA productive and destructive processes raises several questions. While nuclear residence time may well determine whether poly(A) tail-bound PABPN1 gets a chance to associate with decay activity or not, it is less clear what controls whether PABPN1 associates with the exosome or with PARN. Does this decision govern whether the transcript is targeted for processing or complete decay? Also, how do functional RNAs requiring nuclear stability counteract nucleolytic targeting? Perhaps they avoid polyadenylation altogether, as exemplified by sn(o)RNAs or by creating structures incompatible with PABPN1 binding or exosomal activity. Clearly more research is required to detail aspects of PABP function in controlling cellular poly(A) RNA levels. Still, our realization that these proteins function dually in production and decay provides a biologic framework in which this can be done.
Disclosure of potential conflicts of interest
No potential conflicts of interests were disclosed.
Acknowledgments
We thank Agnieszka Tudek, Manfred Schmid, Michal Lubas and Domenico Libri for fruitful comments on this article.
Funding
This work was supported by the ERC (grant 339953), the Danish National Research Council, the Lundbeck- and the Novo Nordisk-Foundations.
References
- 1.Chlebowski A, Lubas M, Jensen TH, Dziembowski A. RNA decay machines: The exosome. Biochim Biophys Acta 2013; 1829(6–7):552-60; PMID:23352926; https://doi.org/ 10.1016/j.bbagrm.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 2.Januszyk K, Lima CD. The eukaryotic RNA exosome. Curr Opin Struct Biol 2014; 24:132-40; PMID:24525139; https://doi.org/ 10.1016/j.sbi.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilchert C, Wittmann S, Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat Rev Mol Cell Biol 2016; 17(4):227-39; PMID:26726035; https://doi.org/ 10.1038/nrm.2015.15 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P. Exosome substrate targeting: The long and short of it. Biochem Soc Trans 2014; 42(4):1129-34; PMID:25110014; https://doi.org/ 10.1042/BST20140088 [DOI] [PubMed] [Google Scholar]
- 5.Schneider C, Tollervey D. Threading the barrel of the RNA exosome. Trends Biochem Sci 2013; 38(10):485-93; PMID:23910895; https://doi.org/ 10.1016/j.tibs.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudipati RK, Xu Z, Lebreton A, Séraphin B, Steinmetz LM, Jacquier A, Libri D. Extensive degradation of RNA precursors by the exosome in wild-type cells. Mol Cell 2012; 48(3):409-21; PMID:23000176; https://doi.org/ 10.1016/j.molcel.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meola N, Domanski M, Karadoulama E, Chen Y, Gentil C, Pultz D, Vitting-Seerup K, Lykke-Andersen S, Andersen JS, Sandelin A, et al.. Identification of a nuclear exosome decay pathway for processed transcripts. Mol Cell 2016; 64(3):520-33; PMID:27871484; https://doi.org/ 10.1016/j.molcel.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 8.Hardwick SW, Luisi BF. Rarely at rest: RNA helicases and their busy contributions to RNA degradation, regulation and quality control. RNA Biol 2013; 10(1):56-70; PMID:23064154; https://doi.org/ 10.4161/rna.22270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein J, Ballin JD, Patterson DN, Wilson GM, Toth EA. Unique properties of the Mtr4p-poly(A) complex suggest a role in substrate targeting. Biochemistry 2010; 49(49):10357-70; PMID:21058657; https://doi.org/ 10.1021/bi101518x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein J, Patterson DN, Wilson GM, Toth EA. Characterization of the essential activities of Saccharomyces cerevisiae Mtr4p, a 3′->5′ helicase partner of the nuclear exosome. J Biol Chem 2008; 283(8):4930-42; PMID:18096702; https://doi.org/ 10.1074/jbc.M706677200 [DOI] [PubMed] [Google Scholar]
- 11.de la Cruz J, Kressler D, Tollervey D, Linder P. Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J 1998; 17(4):1128-40; PMID:9463390; https://doi.org/ 10.1093/emboj/17.4.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairman-Williams ME, Guenther UP, Jankowsky E. SF1 and SF2 helicases: Family matters. Curr Opin Struct Biol 2010; 20(3):313-24; PMID:20456941; https://doi.org/ 10.1016/j.sbi.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weir JR, Bonneau F, Hentschel J, Conti E. Structural analysis reveals the characteristic features of Mtr4, a DExH helicase involved in nuclear RNA processing and surveillance. Proc Natl Acad Sci U S A 2010; 107(27):12139-44; PMID:20566885; https://doi.org/ 10.1073/pnas.1004953107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell P, Petfalski E, Houalla R, Podtelejnikov A, Mann M, Tollervey D. Rrp47p is an exosome-associated protein required for the 3′ processing of stable RNAs. Mol Cell Biol 2003; 23(19):6982-92; PMID:12972615; https://doi.org/ 10.1128/MCB.23.19.6982-6992.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuch B, Feigenbutz M, Makino DL, Falk S, Basquin C, Mitchell P, Conti E. The exosome-binding factors Rrp6 and Rrp47 form a composite surface for recruiting the Mtr4 helicase. EMBO J 2014; 33(23):2829-46; PMID:25319414; https://doi.org/ 10.15252/embj.201488757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schilders G, van Dijk E, Pruijn GJ. C1D and hMtr4p associate with the human exosome subunit PM/Scl-100 and are involved in pre-rRNA processing. Nucleic Acids Res 2007; 35(8):2564-72; PMID:17412707; https://doi.org/ 10.1093/nar/gkm082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milligan L, Decourty L, Saveanu C, Rappsilber J, Ceulemans H, Jacquier A, Tollervey D. A yeast exosome cofactor, Mpp6, functions in RNA surveillance and in the degradation of noncoding RNA transcripts. Mol Cell Biol 2008; 28(17):5446-57; PMID:18591258; https://doi.org/ 10.1128/MCB.00463-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schilders G, Raijmakers R, Raats JM, Pruijn GJ. MPP6 is an exosome-associated RNA-binding protein involved in 5.8S rRNA maturation. Nucleic Acids Res 2005; 33(21):6795-804; PMID:16396833; https://doi.org/ 10.1093/nar/gki982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Pellarin R Fridy PC, Fernandez-Martinez J, Thompson MK, Li Y, Wang QJ, Sali A Rout MP, Chait BT. A strategy for dissecting the architectures of native macromolecular assemblies. Nat Methods 2015; 12(12):1135-8; PMID:26436480; https://doi.org/ 10.1038/nmeth.3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoms M, Thomson E, Baßler J, Gnädig M, Griesel S, Hurt E. The exosome is recruited to RNA substrates through specific adaptor proteins. Cell 2015; 162(5):1029-38; PMID:26317469; https://doi.org/ 10.1016/j.cell.2015.07.060 [DOI] [PubMed] [Google Scholar]
- 21.Hiraishi N, Ishida Y, Nagahama M. AAA-ATPase NVL2 acts on MTR4-exosome complex to dissociate the nucleolar protein WDR74. Biochem Biophys Res Commun 2015; 467(3):534-40; PMID:26456651; https://doi.org/ 10.1016/j.bbrc.2015.09.160 [DOI] [PubMed] [Google Scholar]
- 22.Macias S, Cordiner RA, Gautier P, Plass M, Cáceres JF. DGCR8 acts as an adaptor for the exosome complex to degrade double-stranded structured RNAs. Mol Cell 2015; 60(6):873-85; PMID:26687677; https://doi.org/ 10.1016/j.molcel.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen PR, Domanski M, Kristiansen MS, Storvall H, Ntini E, Verheggen C, Schein A, Bunkenborg J, Poser I, Hallais M, et al.. The human cap-binding complex is functionally connected to the nuclear RNA exosome. Nat Struct Mol Biol 2013; 20(12):1367-76; PMID:24270879; https://doi.org/ 10.1038/nsmb.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hrossova D, Sikorsky T, Potesil D, Bartosovic M, Pasulka J, Zdrahal Z, Stefl R, Vanacova S. RBM7 subunit of the NEXT complex binds U-rich sequences and targets 3′-end extended forms of snRNAs. Nucleic Acids Res 2015; 43(8):4236-48; PMID:25852104; https://doi.org/ 10.1093/nar/gkv240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubas M, Andersen PR, Schein A, Dziembowski A, Kudla G, Jensen TH. The human nuclear exosome targeting complex is loaded onto newly synthesized RNA to direct early ribonucleolysis. Cell Rep 2015; 10(2):178-92; PMID:25578728; https://doi.org/ 10.1016/j.celrep.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 26.Lubas M, Christensen MS, Kristiansen MS, Domanski M, Falkenby LG, Lykke-Andersen S, Andersen JS, Dziembowski A, Jensen TH. Interaction profiling identifies the human nuclear exosome targeting complex. Mol Cell 2011; 43(4):624-37; PMID:21855801; https://doi.org/ 10.1016/j.molcel.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu YB, Kleinman CL, Landry-Voyer AM, Majewski J, Bachand F. Polyadenylation-dependent control of long noncoding RNA expression by the poly(A)-binding protein nuclear 1. PLoS Genet 2012; 8(11):e1003078; PMID:23166521; https://doi.org/ 10.1371/journal.pgen.1003078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bresson SM, Conrad NK. The human nuclear poly(a)-binding protein promotes RNA hyperadenylation and decay. PLoS Genetics 2013; 9(10):e1003893; PMID:24146636; https://doi.org/ 10.1371/journal.pgen.1003893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falk S, Weir JR, Hentschel J, Reichelt P, Bonneau F, Conti E. The molecular architecture of the TRAMP complex reveals the organization and interplay of its two catalytic activities. Mol Cell 2014; 55(6):856-67; PMID:25175027; https://doi.org/ 10.1016/j.molcel.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K, Butler JS. Nuclear RNA surveillance: Role of TRAMP in controlling exosome specificity. Wiley Interdiscip Rev RNA 2013; 4(2):217-31; PMID:23417976; https://doi.org/ 10.1002/wrna.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, Langen H, Keith G, Keller W. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 2005; 3(6):e189; PMID:15828860; https://doi.org/ 10.1371/journal.pbio.0030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porrua O, Boudvillain M, Libri D. Transcription termination: Variations on common themes. Trends Genet 2016; 32(8):508-22; PMID:27371117; https://doi.org/ 10.1016/j.tig.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 33.Arigo JT, Carroll KL, Ames JM, Corden JL. Regulation of yeast NRD1 expression by premature transcription termination. Mol Cell 2006; 21(5):641-51; PMID:16507362; https://doi.org/ 10.1016/j.molcel.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 34.Arigo JT, Eyler DE, Carroll KL, Corden JL. Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol Cell 2006; 23(6):841-51; PMID:16973436; https://doi.org/ 10.1016/j.molcel.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 35.Thiebaut M, Kisseleva-Romanova E, Rougemaille M, Boulay J, Libri D. Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the nrd1-nab3 pathway in genome surveillance. Mol Cell 2006; 23(6):853-64; PMID:16973437; https://doi.org/ 10.1016/j.molcel.2006.07.029 [DOI] [PubMed] [Google Scholar]
- 36.Tudek A, Porrua O, Kabzinski T, Lidschreiber M, Kubicek K, Fortova A, Lacroute F, Vanacova S, Cramer P, Stefl R, et al.. Molecular basis for coordinating transcription termination with noncoding RNA degradation. Mol Cell 2014; 55(3):467-81; PMID:25066235; https://doi.org/ 10.1016/j.molcel.2014.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasiljeva L, Buratowski S. Nrd1 interacts with the nuclear exosome for 3′ processing of RNA polymerase II transcripts. Mol Cell 2006; 21(2):239-48; PMID:16427013; https://doi.org/ 10.1016/j.molcel.2005.11.028 [DOI] [PubMed] [Google Scholar]
- 38.Wlotzka W, Kudla G, Granneman S, Tollervey D. The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J 2011; 30(9):1790-803; PMID:21460797; https://doi.org/ 10.1038/emboj.2011.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallais M, Pontvianne F, Andersen PR, Clerici M, Lener D, Benbahouche Nel H, Gostan T, Vandermoere F, Robert MC, Cusack S, et al.. CBC-ARS2 stimulates 3′-end maturation of multiple RNA families and favors cap-proximal processing. Nat Struct Mol Biol 2013; 20(12):1358-66; PMID:24270878; https://doi.org/ 10.1038/nsmb.2720 [DOI] [PubMed] [Google Scholar]
- 40.Egecioglu DE, Henras AK, Chanfreau GF. Contributions of Trf4p- and Trf5p-dependent polyadenylation to the processing and degradative functions of the yeast nuclear exosome. RNA 2006; 12(1):26-32; PMID:16373491; https://doi.org/ 10.1261/rna.2207206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fasken MB, Leung SW, Banerjee A, Kodani MO, Chavez R, Bowman EA, Purohit MK, Rubinson ME, Rubinson EH, Corbett AH. Air1 zinc knuckles 4 and 5 and a conserved IWRXY motif are critical for the function and integrity of the Trf4/5-Air1/2-Mtr4 polyadenylation (TRAMP) RNA quality control complex. J Biol Chem 2011; 286(43):37429-45; PMID:21878619; https://doi.org/ 10.1074/jbc.M111.271494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt K, Xu Z, Mathews DH, Butler JS. Air proteins control differential TRAMP substrate specificity for nuclear RNA surveillance. RNA 2012; 18(10):1934-45; PMID:22923767; https://doi.org/ 10.1261/rna.033431.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houseley J, Tollervey D. Yeast Trf5p is a nuclear poly(A) polymerase. EMBO Rep 2006; 7(2):205-11; PMID:16374505; https://doi.org/ 10.1038/sj.embor.7400612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA 2006; 12(3):508-21; PMID:16431988; https://doi.org/ 10.1261/rna.2305406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.San Paolo S, Vanacova S, Schenk L, Scherrer T, Blank D, Keller W, Gerber AP. Distinct roles of non-canonical poly(A) polymerases in RNA metabolism. PLoS Genet 2009; 5(7):e1000555; PMID:19593367; https://doi.org/ 10.1371/journal.pgen.1000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 2005; 121(5):713-24; PMID:15935758; https://doi.org/ 10.1016/j.cell.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 47.Buhler M, Haas W, Gygi SP, Moazed D. RNAi-dependent and -independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell 2007; 129(4):707-21; PMID:17512405; https://doi.org/ 10.1016/j.cell.2007.03.038 [DOI] [PubMed] [Google Scholar]
- 48.Wang SW, Stevenson AL, Kearsey SE, Watt S, Bähler J. Global role for polyadenylation-assisted nuclear RNA degradation in posttranscriptional gene silencing. Mol Cell Biol 2008; 28(2):656-65; PMID:18025105; https://doi.org/ 10.1128/MCB.01531-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Win TZ, Draper S, Read RL, Pearce J, Norbury CJ, Wang SW. Requirement of fission yeast Cid14 in polyadenylation of rRNAs. Mol Cell Biol 2006; 26(5):1710-21; PMID:16478992; https://doi.org/ 10.1128/MCB.26.5.1710-1721.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egan ED, Braun CR, Gygi SP, Moazed D. Post-transcriptional regulation of meiotic genes by a nuclear RNA silencing complex. RNA 2014; 20(6):867-81; PMID:24713849; https://doi.org/ 10.1261/rna.044479.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee NN, Chalamcharla VR, Reyes-Turcu F, Mehta S, Zofall M, Balachandran V, Dhakshnamoorthy J, Taneja N, Yamanaka S, Zhou M, et al.. Mtr4-like protein coordinates nuclear RNA processing for heterochromatin assembly and for telomere maintenance. Cell 2013; 155(5):1061-74; PMID:24210919; https://doi.org/ 10.1016/j.cell.2013.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Zhu J, Schermann G, Ohle C, Bendrin K, Sugioka-Sugiyama R, Sugiyama T, Fischer T. The fission yeast MTREC complex targets CUTs and unspliced pre-mRNAs to the nuclear exosome. Nat Commun 2015; 6:7050; PMID:25989903; https://doi.org/ 10.1038/ncomms8050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lange H, Sement FM, Gagliardi D. MTR4, a putative RNA helicase and exosome co-factor, is required for proper rRNA biogenesis and development in Arabidopsis thaliana. Plant J 2011; 68(1):51-63; PMID:21682783; https://doi.org/ 10.1111/j.1365-313X.2011.04675.x [DOI] [PubMed] [Google Scholar]
- 54.Lange H, Zuber H, Sement FM, Chicher J, Kuhn L, Hammann P, Brunaud V, Bérard C, Bouteiller N, Balzergue S, et al.. The RNA helicases AtMTR4 and HEN2 target specific subsets of nuclear transcripts for degradation by the nuclear exosome in Arabidopsis thaliana. PLoS Genet 2014; 10(8):e1004564; PMID:25144737; https://doi.org/ 10.1371/journal.pgen.1004564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wigington CP, Williams KR, Meers MP, Bassell GJ, Corbett AH. Poly(A) RNA-binding proteins and polyadenosine RNA: New members and novel functions. Wiley Interdiscip Rev RNA 2014; 5(5):601-22; PMID:24789627; https://doi.org/ 10.1002/wrna.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn U, Buschmann J, Wahle E. The nuclear poly(A) binding protein of mammals, but not of fission yeast, participates in mRNA polyadenylation. RNA 2017; 23(4):473-82; PMID:28096519; https://doi.org/ 10.1261/rna.057026.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn U, Gündel M, Knoth A, Kerwitz Y, Rüdel S, Wahle E. Poly(A) tail length is controlled by the nuclear poly(A)-binding protein regulating the interaction between poly(A) polymerase and the cleavage and polyadenylation specificity factor. J Biol Chem 2009; 284(34):22803-14; PMID:19509282; https://doi.org/ 10.1074/jbc.M109.018226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minvielle-Sebastia L, Keller W. mRNA polyadenylation and its coupling to other RNA processing reactions and to transcription. Curr Opin Cell Biol 1999; 11(3):352-7; PMID:10395555; https://doi.org/ 10.1016/S0955-0674(99)80049-0 [DOI] [PubMed] [Google Scholar]
- 59.Schmid M, Olszewski P, Pelechano V, Gupta I, Steinmetz LM, Jensen TH. The nuclear polyA-binding protein nab2p is essential for mRNA production. Cell Rep 2015; 12(1):128-39; PMID:26119729; https://doi.org/ 10.1016/j.celrep.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 60.Viphakone N, Voisinet-Hakil F, Minvielle-Sebastia L. Molecular dissection of mRNA poly(A) tail length control in yeast. Nucleic Acids Res 2008; 36(7):2418-33; PMID:18304944; https://doi.org/ 10.1093/nar/gkn080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahle E. Poly(A) tail length control is caused by termination of processive synthesis. J Biol Chem 1995; 270(6):2800-8; PMID:7852352. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J 1999; 18(8):2273-83; PMID:10205180; https://doi.org/ 10.1093/emboj/18.8.2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hector RE, Nykamp KR, Dheur S, Anderson JT, Non PJ, Urbinati CR, Wilson SM, Minvielle-Sebastia L, Swanson MS. Dual requirement for yeast hnRNP Nab2p in mRNA poly(A) tail length control and nuclear export. EMBO J 2002; 21(7):1800-10; PMID:11927564; https://doi.org/ 10.1093/emboj/21.7.1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmid M, Poulsen MB, Olszewski P, Pelechano V, Saguez C, Gupta I, Steinmetz LM, Moore C, Jensen TH. Rrp6p controls mRNA poly(A) tail length and its decoration with poly(A) binding proteins. Mol Cell 2012; 47(2):267-80; PMID:22683267; https://doi.org/ 10.1016/j.molcel.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Libri D. Nuclear poly(a)-binding proteins and nuclear degradation: Take the mRNA and run? Mol Cell 2010; 37(1):3-5; PMID:20129049; https://doi.org/ 10.1016/j.molcel.2009.12.029 [DOI] [PubMed] [Google Scholar]
- 66.Kerwitz Y, Kühn U, Lilie H, Knoth A, Scheuermann T, Friedrich H, Schwarz E, Wahle E. Stimulation of poly(A) polymerase through a direct interaction with the nuclear poly(A) binding protein allosterically regulated by RNA. EMBO J 2003; 22(14):3705-14; PMID:12853485; https://doi.org/ 10.1093/emboj/cdg347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bresson SM, Hunter OV, Hunter AC, Conrad NK. Canonical poly(a) polymerase activity promotes the decay of a wide variety of mammalian nuclear RNAs. PLoS Genet 2015; 11(10):e1005610; PMID:26484760; https://doi.org/ 10.1371/journal.pgen.1005610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perreault A, Lemieux C, Bachand F. Regulation of the nuclear poly(A)-binding protein by arginine methylation in fission yeast. J Biol Chem 2007; 282(10):7552-62; PMID:17213188; https://doi.org/ 10.1074/jbc.M610512200 [DOI] [PubMed] [Google Scholar]
- 69.Lemay JF, Lemieux C, St-André O, Bachand F. Crossing the borders: poly(A)-binding proteins working on both sides of the fence. RNA Biol 2010; 7(3):291-5; PMID:20400847; https://doi.org/ 10.4161/rna.7.3.11649 [DOI] [PubMed] [Google Scholar]
- 70.Lemieux C, Marguerat S, Lafontaine J, Barbezier N, Bähler J, Bachand F. A Pre-mRNA degradation pathway that selectively targets intron-containing genes requires the nuclear poly(A)-binding protein. Mol Cell 2011; 44(1):108-19; PMID:21981922; https://doi.org/ 10.1016/j.molcel.2011.06.035 [DOI] [PubMed] [Google Scholar]
- 71.St-Andre O, Lemieux C, Perreault A, Lackner DH, Bähler J, Bachand F. Negative regulation of meiotic gene expression by the nuclear poly(a)-binding protein in fission yeast. J Biol Chem 2010; 285(36):27859-68; PMID:20622014; https://doi.org/ 10.1074/jbc.M110.150748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lemay JF, D'Amours A, Lemieux C, Lackner DH, St-Sauveur VG, Bähler J, Bachand F. The nuclear poly(A)-binding protein interacts with the exosome to promote synthesis of noncoding small nucleolar RNAs. Mol Cell 2010; 37(1):34-45; PMID:20129053; https://doi.org/ 10.1016/j.molcel.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 73.Grenier St-Sauveur V, Soucek S, Corbett AH, Bachand F. Poly(A) tail-mediated gene regulation by opposing roles of Nab2 and Pab2 nuclear poly(A)-binding proteins in pre-mRNA decay. Mol Cell Biol 2013; 33(23):4718-31; PMID:24081329; https://doi.org/ 10.1128/MCB.00887-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thakurta AG, Ho Yoon J, Dhar R. Schizosaccharomyces pombe spPABP, a homologue of Saccharomyces cerevisiae Pab1p, is a non-essential, shuttling protein that facilitates mRNA export. Yeast 2002; 19(9):803-10; PMID:12112233; https://doi.org/ 10.1002/yea.876 [DOI] [PubMed] [Google Scholar]
- 75.Dunn EF, Hammell CM, Hodge CA, Cole CN. Yeast poly(A)-binding protein, Pab1, and PAN, a poly(A) nuclease complex recruited by Pab1, connect mRNA biogenesis to export. Genes Dev 2005; 19(1):90-103; PMID:15630021; https://doi.org/ 10.1101/gad.1267005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelly SM, Leung SW, Pak C, Banerjee A, Moberg KH, Corbett AH. A conserved role for the zinc finger polyadenosine RNA binding protein, ZC3H14, in control of poly(A) tail length. RNA 2014; 20(5):681-8; PMID:24671764; https://doi.org/ 10.1261/rna.043984.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wigington CP, Morris KJ, Newman LE, Corbett AH. The polyadenosine RNA-binding protein, zinc finger cys3his protein 14 (ZC3H14), regulates the Pre-mRNA processing of a key ATP synthase subunit mRNA. J Biol Chem 2016; 291(43):22442-59; PMID:27563065; https://doi.org/ 10.1074/jbc.M116.754069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moon DH, Segal M, Boyraz B, Guinan E, Hofmann I, Cahan P, Tai AK, Agarwal S. Poly(A)-specific ribonuclease (PARN) mediates 3′-end maturation of the telomerase RNA component. Nat Genet 2015; 47(12):1482-8; PMID:26482878; https://doi.org/ 10.1038/ng.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen D, Grenier St-Sauveur V, Bergeron D, Dupuis-Sandoval F, Scott MS, Bachand F. A polyadenylation-dependent 3′ end maturation pathway is required for the synthesis of the human telomerase RNA. Cell Rep 2015; 13(10):2244-57; PMID:26628368; https://doi.org/ 10.1016/j.celrep.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 80.Tseng CK, Wang HF, Burns AM, Schroeder MR, Gaspari M, Baumann P. Human telomerase RNA processing and quality control. Cell Rep 2015; 13(10):2232-43; PMID:26628367; https://doi.org/ 10.1016/j.celrep.2015.10.075 [DOI] [PubMed] [Google Scholar]


