Abstract
Background and Objective
Optimal placement of the Argus II Retinal Prosthesis System is critical. Intraoperative OCT allows for intrasurgical visualization and confirmation of array placement. In this study, two different OCT systems were evaluated to assess the feasibility and utility of this technology during Argus II surgery.
Methods
Intraoperative OCT was performed on five patients undergoing Argus II implantation at Cole Eye Institute from June 2015 to July 2016. The EnVisu portable OCT (Bioptigen, Research Triangle Park) and microscope-integrated RESCAN 700 (Zeiss, Oberkochen, Germany) intraoperative OCT systems were utilized. The EnVisu was used in three patients and the RESCAN 700 in three of the five patients. Following array tacking, intraoperative OCT was performed over the entire array including the edges and tack.
Results
Intraoperative OCT allowed for visualization of the array/retina interface. Microscope integration of the OCT system facilitated ease of focusing, real-time feedback, surgeon-directed OCT scanning to the areas of interest, and enhanced image quality at points of interest.
Conclusion
Intraoperative imaging of the Argus II electrode array is feasible and provides information about electrode array-retina interface and distance to help guide a surgeon. Microscope-integration of OCT appears to provide an optimal and efficient approach to intraoperative OCT during Argus II array placement.
Introduction
The Argus II Retinal Prosthesis System (Argus II®) (Second Sight Medical Products, Sylmar, CA) is an FDA approved humanitarian use device for patients blind from retinitis pigmentosa. Argus I, a 16-electrode device, was implanted in 6 subjects between 2002 and 2004.1 The Argus II Retinal Prosthesis Study involved 30 subjects. During the study, Argus II was implanted between June 2007 and August 2009. It consists of 60 platinum electrodes that are 200 microns in diameter each.1 As of August 2016, there have been over 160 implants of the Argus II (personal communication with Second Sight) in the U.S., Canada, Europe, and the Middle East with the numbers continuing to grow. A trial of Argus II for severe age-related macular degeneration is currently underway in the UK.
The surgery for the Argus II System involves securing an electronics case and implant coil to the sclera via a scleral band. The electrode array is connected to the electronics case via a cable. After pars plana vitrectomy, the cable and the array are introduced into the vitreous cavity through a scleral incision and the electrode array is secured over the macula with a custom retinal tack (Second Sight Medical Products, Inc, Sylmar, CA). The extraocular components communicate with glasses and video processing unit and transmit visual data to the array.
The optimal positioning of the epiretinal device is key to successful surgery and remains a challenge. Poor positioning has been shown to lead to higher stimulus thresholds.1,2 Proper centering of the array over the macula and creating close contact between the electrodes and retinal surface were identified as common challenges in implantation by a consortium of Argus surgeons.3 Intraoperative OCT could serve as an adjunct tool in ensuring proper array positioning.
The goal of this study was to examine the feasibility of intrasurgical imaging of Argus II retinal prosthesis array after tacking using two different intraoperative OCT platforms: portable and microscope-integrated OCT. Microscope integrated imaging of Argus II array has not been previously reported.
Methods
A total of six patients underwent surgery for Argus II Retinal Prosthesis System implantation at Cole Eye Institute from June 2015 to July 2016. Intraoperative OCT was performed on five of these patients undergoing Argus II implantation. All study-related procedures were approved by the institutional review board of the Cleveland Clinic. Microscope-integrated OCT was performed as part of the DISCOVER intraoperative OCT study.4 The study adhered to the tenets of the Declaration of Helsinki. Imaging was performed using two platforms, the EnVisu portable OCT system (Bioptigen, Research Triangle Park) and RESCAN 700 (Zeiss, Oberkochen, Germany).4 All the surgeries and the intraoperative imaging were performed by the two lead surgeons (AVR and AY). The Argus implantation surgery is performed under general anesthesia and can take up to 4–5 hours. The imaging is performed immediately before and after tacking of the array to the retina, which is one of the lasts steps in the surgery.
The EnVisu portable OCT system is mounted to the surgical microscope using a custom OCT mount. Aiming of this system occurs through adjustment of the X,Y,Z planes using the microscope pedal. OCT focus is controlled manually. The OCT scan head is moved closer or further from the eye to adjust the reference delay position.
The RESCAN 700 is a microscope integrated OCT system. Aiming with the RESCAN is performed with foot pedal control of the OCT scanning beam. The surgeon may utilize a heads-up display through the oculars or an external monitor display to visualize the surgical field with an OCT overlay to target the scan to the region of interest. Focusing is performed through a digital slide control on the supplemental display screen.
Our intraoperative imaging goal was to image the entire array including all edges, the body of the array, the distance between the array and the retina and the retina immediately surrounding the array. The array measures 9 mm by 5.5 mm and consists of 60 platinum electrodes that are 200 microns in diameter each. The grasping handle is secured to the distal end of the array (closest to the optic nerve) and is used for array handing. The cable is found at the proximal end of the array and connects the array to the external components via a pars plana incision. The tack is inserted into the opening close to the cable.
In this study, images and workflow for performing intraoperative OCT were analyzed. The ease of scanning, the ability to localize and capture the structures of interest, and the ability to scan the array’s body and perimeter were evaluated.
Results
Intraoperative OCT was performed on five patients who received the Argus II implant at Cole Eye Institute. The EnVisu portable system was used in three cases and Rescan 700 was used in three cases.
Imaging was performed immediately before and after the tacking of the array to the retina, one of the last surgical steps. The mean operative time for six cases was 3 hr 59 min (range 3hr 2 min – 5 hr 1 min). The view for tacking of the array and subsequent intraoperative OCT may be compromised by corneal edema and corneal scraping was performed in three of the five patients. In all patients, corneal scraping improved both microscope and intraoperative OCT visualization.
Imaging before the tacking allowed complete examination of the macular contour for optimal positioning. Images acquired with the portable external system provided visualization of the device and the retina, but did not provide clear differentiation of points of interest. Although the retina and the device were visualized, it did not allow us to discern in detail the relationship between the array and the underlying neurosensory retina. Moreover, the lack of real-time heads-up display feedback precluded surgeon-directed OCT imaging and complete scanning along the perimeter of the array since it was hard to track which areas had been previously scanned. (Figure 1A and 2A–B)
Figure 1.

Intraoperative OCT of the body of of the Argus II electrode array.
Intraoperative OCT of the body of of the Argus II electrode array utilizing a portable OCT system (A) and microscope-integrated OCT system (C). (B) Reference scan for the microscope-integrated OCT system.
Figure 2.
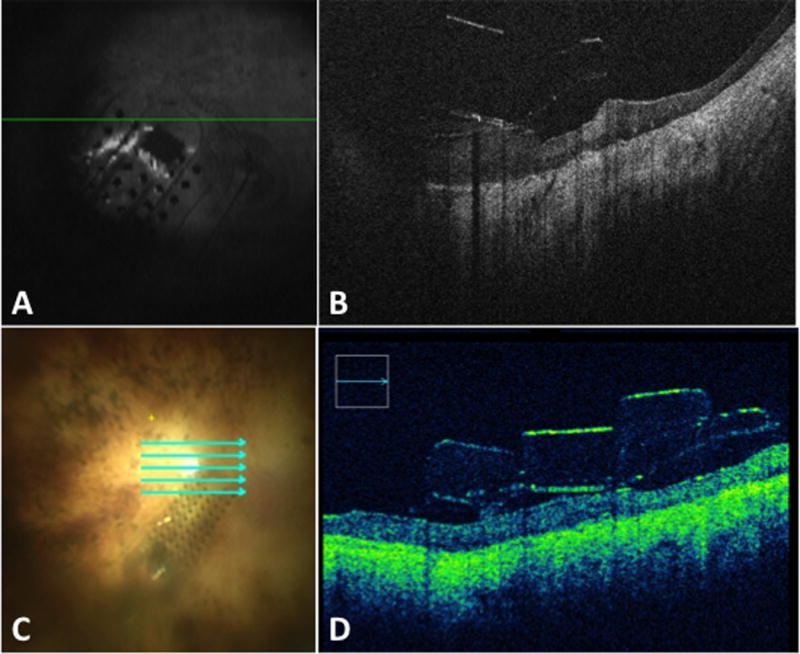
Intraoperative OCT of the edge of of the Argus II electrode array.
Intraoperative OCT of the edge of of the Argus II electrode array utilizing a portable (A–B) and microscope-integrated OCT system (C-D). (A, C) Reference scans for portable OCT system and microscope-integrated OCT system, respectively.
The microscope-integrated system allowed for real-time feedback, enhanced image quality and apparent better signal strength at all points of interest. (Supplemental Video 1) The microscope-integrated system allowed for surgeon-directed OCT scanning to areas of interest such as the optic nerve in relation to the implant edge, the distance between the electrode array and the retina at various locations, the shape of the array (the area free of electrodes and the area with electrodes), the grasping handle on the array, the tack and the cable coming off the array. (Figure 1B–C, 2 C–D, 3 A–D) Image capture allowed for easy co-localization of the OCT-image and the en face reference scan. The areas that have been scanned and those that have not could be easily tracked.
Figure 3.
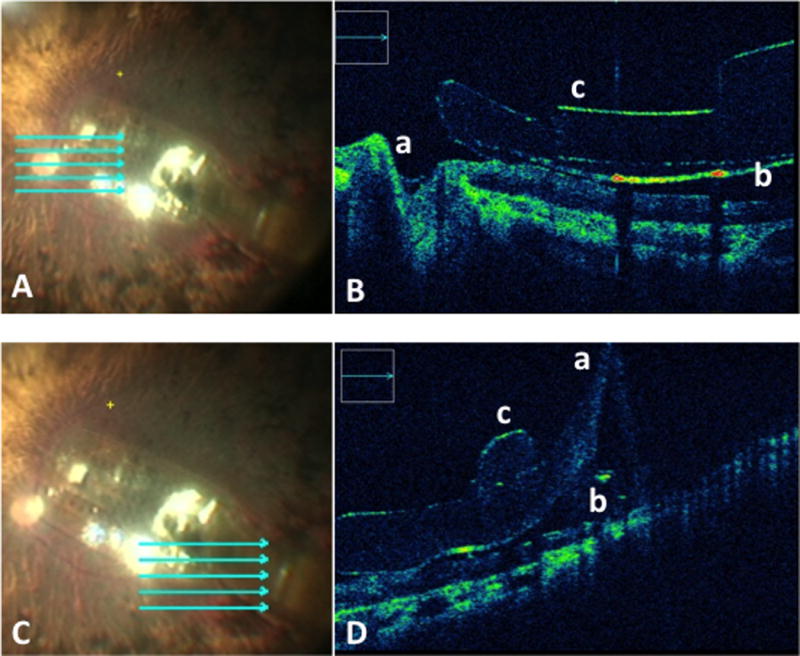
Microscope-integrated intraoperative OCT system.
Intraoperative OCT of the edges of of the Argus II electrode array utilizing a microscope-integrated OCT system. (A, C) Reference images for surgeon directed localization of the OCT scan beam. (B) Visualization of the following points of interest: optic nerve in relationship to the implant edge (a), the distance between the electrode array and the retina (b), the shape of the array (c). (D) Visualization of the following points of interest: the cable coming of the array (a), the distance between the electrode array and the retina (b), area of array next to the retinal tack (c).
We attempted to image the same areas with both OCT systems, but lack of real-time co-localization of the B-scan image with the en face image limited the ability of the portable external system to scan all areas of interest.
Discussion
The current study shows that intraoperative imaging of the Argus II electrode array is feasible and the information that it gives could advance our understanding of the way the device provides vision to patients. Both portable and microscope integrated systems allowed for intraoperative imaging of the Argus II array. However, in the case of Argus II retinal electrode array the imaging with a microscope-integrated system was more efficient and provided more useful data.
The use of intraoperative OCT could aid in both securing of the implant during surgery and in long-term study of the function of the array. Optimal positioning of the array remains a challenge.3 Pre-operative OCT is often suboptimal as these patients cannot maintain fixation due to their poor vision. Intraoperative surgeon guided OCT in a patient under general anesthesia allows for complete examination of the macular contour. After tacking is performed, the array could be manipulated to adjust the positioning slightly. Intraoperative OCT provides information about array-retina distance and array tilt and allows for immediate adjustment if necessary. A case report describing malrotation of the array utilized hand-held intraoperative OCT to confirm that the electrode array was repositioned to be in closer contact with the retina and was not elevated by the edge of a macular staphyloma.5 At our site, post-tacking adjustment of the array was necessary in one patient, in order to optimize the position of the array.
Additionally, intraoperative OCT provides data that could address the long-term functioning of the array. Array-retina distance is important for the performance of the device. Significant correlation between electrode–retina distance and electrical thresholds (the minimum electrical stimulus parameters, such as amplitude and duration, required for initiating an action potential) has been established.2 At the same time, it has been shown that there might be retinal damage when the retina is directly contacted by the electrodes.6 Moreover, it is not currently known if the electrode array changes position after the initial implantation and if there are mechanical changes in the neurosensory retina in response to implantation. The OCT analysis of a single patient implanted with the original Argus I implant showed fibrotic tissue around the tack at 3 years and anatomic distortions in specific areas under the implant that may have been caused by pressure induced by a slight tilt of the prosthesis. The latter was analyzed at 5 and 10 years after implantation, revealing no additional change in anatomic findings.7 Intraoperative OCT provides information about the initial interaction between the device and the retina. Due to the healing process, the postoperative imaging of the array is not always possible in the immediate postoperative period and, once again, limited by the fixation abilities of the patients with poor vision. A limitation of the current report is the small number of patients. However, we have been one of the busiest sites in the U.S. with a total of 6 surgeries to date.
In summary, the complexities of imaging the Argus II array appear to be more amenable to a microscope-integrated system that allowed the surgeon to direct the OCT image and manipulate the eye to maximize signal penetration and image quality. This flexibility of imaging also provided rapid feedback on multiple parameters related to the Argus II array/retina interface. Further research is needed to investigate the role for intraoperative OCT in optimizing implant fixation and overall patient outcomes.
Acknowledgments
Funding and support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE); Research to Prevent Blindness (Institutional Cole Grant); All funding sources had no input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial Disclosures: AVR: Allergan (C). AY: None. MJM: None. JR: None JPE: Bioptigen (consultant, intellectual property), Thrombogenics (consultant, research funding), Synergetics (intellectual property), Genentech (research funding), Leica (consultant), Zeiss (consultant), Alcon (consultant)
Author Contributions: Design of the study (All authors); Conduct of the study (All authors); Data collection (All authors); Data management (All authors); Data analysis (All authors); Data Interpretation (All authors); Preparation of the manuscript (AVR); Review and approval of the manuscript (All authors); Provision of patients (AVR, AY). The principal investigators (AVR, AY, JPE) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Grant Support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE); Research to Prevent Blindness RPB1580DM (Cole Eye Institutional Grant);
Footnotes
The authors had total control of all aspects of this observational case report including: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: AVR: Allergan (C) AY: None. MM: None. JR: None. JPE: Genentech (R), Regeneron (R), Thrombogenics (R,C), Leica (C), Zeiss (C), Bioptigen (P, C), Synergetics (P), Alcon (C).
References
- 1.Weiland JD, Cho AK, Humayun MS. Retinal prostheses: current clinical results and future needs. Ophthalmology. 2011 Nov;118(11):2227–2237. doi: 10.1016/j.ophtha.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja AK, Yeoh J, Dorn JD, et al. Factors Affecting Perceptual Threshold in Argus II Retinal Prosthesis Subjects. Translational vision science & technology. 2013 Apr;2(4):1. doi: 10.1167/tvst.2.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghodasra DH, Chen A, Arevalo JF, Birch DG, Branham K, Coley B, Dagnelie G, de Juan E, Devenyi RG, Dorn JD, Fisher A. Worldwide Argus II implantation: recommendations to optimize patient outcomes. BMC ophthalmology. 2016 May 6;16(1):1. doi: 10.1186/s12886-016-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers JP, Goshe J, Dupps WJ, et al. Determination of Feasibility and Utility of Microscope-Integrated Optical Coherence Tomography During Ophthalmic Surgery: The DISCOVER Study RESCAN Results. JAMA ophthalmology. 2015 Oct 1;133(10):1124–32. doi: 10.1001/jamaophthalmol.2015.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seider MI, Hahn P. Argus II retinal prosthesis malrotation and repositioning with intraoperative optical coherence tomography in a posterior staphyloma. Clinical ophthalmology (Auckland, NZ) 2015;9:2213. doi: 10.2147/OPTH.S96570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colodetti L, Weiland JD, Colodetti S, et al. Pathology of damaging electrical stimulation in the retina. Experimental eye research. 2007 Jul;85(1):23–33. doi: 10.1016/j.exer.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Yue L, Falabella P, Christopher P, Wuyyuru V, Dorn J, Schor P, Greenberg RJ, Weiland JD, Humayun MS. Ten-Year Follow-up of a Blind Patient Chronically Implanted with Epiretinal Prosthesis Argus I. Ophthalmology. 2015 Dec 31;122(12):2545–52. doi: 10.1016/j.ophtha.2015.08.008. [DOI] [PubMed] [Google Scholar]


