Abstract
This chapter summarizes recent findings regarding the central transmission of acute and chronic itch. Itch is transduced by cutaneous pruriceptors that transmit signals to neurons in the superficial spinal cord. Spinal itch-signaling circuits utilize several neuropeptides whose receptors represent novel targets to block itch transmission. Itch is relieved by scratching, which activates spinal interneurons to inhibit itch-transmitting neurons. Spinal itch transmission is also thought to be modulated by descending pathways. Itch is transmitted rostrally via ascending pathways to activate a variety of brain regions involved in sensory discrimination of affective and motor responses to itch. The pathophysiological mechanisms of chronic itch are poorly understood but likely involve sensitization of itch-signaling pathways and/or dysfunction of itch-inhibitory circuits. Improved understanding of central itch mechanisms has identified a number of novel targets for the development of antipruritic treatment strategies.
Itch is defined as an unpleasant sensation associated with the desire to scratch. Chronic itch decreases the quality of life [1] and imposes a high economic burden [2, 3]. This chapter reviews recent breakthroughs in our understanding of the central processing of itch and novel therapeutic approaches to block itch transmission or enhance the inhibition of itch at peripheral, spinal, and supraspinal sites. We also address the poorly understood pathophysiology of chronic itch and potential interventions.
Peripheral Encoding of Itch
Pruritic stimuli activate at least two distinct classes of itch-signaling nerve fibers (fig. 1). Histamine activates mechanically insensitive C-fibers [4, 5] via H1/H 4 receptors and transient receptor potential cation channel V1 (TRPV1) [6], while non-histaminergic pruritogens activate polymodal nociceptors [7, 8] via TRPA1 [9]. Figure 1 provides a list of many pruritogens and their receptors. The participation of TRP channels in itch provides an avenue to allow the small local anesthetic QX-314 to enter into pruriceptive afferent fibers and block conduction, thereby silencing itch transmission [10]. Additional details concerning peripheral mechanisms of itch may be found in the chapter by Azimi et al. [this vol., pp. 18–23].
Fig. 1.
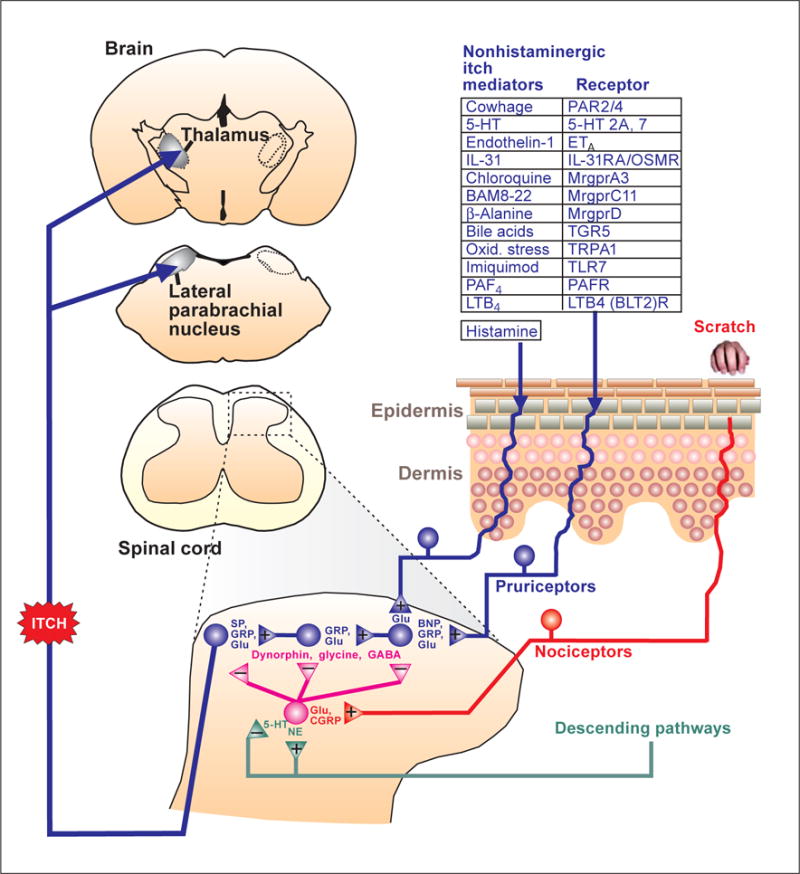
Schematic drawing of the neural pathway for itch. 5-HT = 5-Hydroxytryptamine (serotonin); CGRP = calcitonin gene-related peptide; ETA = endothelin-A receptor; Glu = glutamate; IL = interleukin; LTB4 = leukotriene B4 ; Mrgpr = Mas-related G protein-coupled receptor; NE = norepinephrine; OSMR = oncostatin M receptor; Oxid. stress = oxidative stress; PAF = platelet-activating factor; PAR = protease-activated receptor; PAFR = platelet-activating factor receptor; SP = substance P; TGR5 = G protein-coupled bile acid receptor; TLR = Toll-like receptor.
Spinal/Trigeminal Encoding of Itch
The central branches of pruriceptors terminate in superficial layers of the spinal or medullary dorsal horn to activate second-order neurons. Candidate neurotransmitters released from the central terminals of pruriceptors include glutamate [11] and the neuropeptides gastrin-releasing peptide (GRP), substance P, and brain natriuretic peptide (BNP) [12–15] (fig. 1). BNP is released from pruriceptors and is necessary for both histaminergic and nonhistaminergic itch [15]. MrgprA3-expressing pruriceptor terminals directly contact spinal neurons expressing the GRP receptor, implicating GRP as a neuropeptide released from pruriceptors [16]. GRP is also released from excitatory spinal interneurons. Neurotoxic ablation of neurons expressing neurokinin 1 (NK1), the receptor for substance P, also reduced itch behavior [14]. Nearly all spinal neurons with ascending axonal projections to the thalamus and parabrachial nucleus (see below) express NK1 [17]. These data suggest a spinal itch-signaling pathway in which BNP is released from pruriceptors to serially activate interneurons that release GRP, and then substance P, to activate NK1 receptor-expressing neurons that transmit itch signals to the brain (fig. 1). A cocktail of antagonists for NK1, GRP, and glutamate (AMPA; α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors completely inhibited scratching behavior and activation of dorsal horn neurons elicited by chloroquine (MrgprA3 agonist), whereas individual or pairs of antagonists were less effective [11]. In contrast, the AMPA antagonist alone was sufficient to block histamine-evoked itch and excitation of dorsal horn neurons. These data implicate multiple spinal neuropeptides in mediating nonhistaminergic itch, and glutamate as the primary neurotransmitter for histamine-mediated itch. The use of combinations of antagonists for NK1, GRP, BNP, and glutamate (AMPA) receptors may prove useful to relieve itch.
Scratch Inhibition of Itch
It is well known that scratching relieves itch. Scratching within or adjacent to the receptive field area of spinal neurons inhibits their pruritogen-evoked activity [18, 19]. This effect is state dependent in that only pruritogen- but not algogen-evoked firing is suppressed by scratching. The inhibitory neurotransmitters GABA (γ-aminobutyric acid) and glycine mediate scratch inhibition [20], and mice lacking spinal glycine exhibited excessive scratching [21]. A specific class of inhibitory interneurons expressing the transcription factor Bhlhb5 (and co-expressing the somatostatin 2A receptor and galanin or neuronal nitric oxide synthase) is crucial for inhibition of itch. Genetic ablation of these inhibitory interneurons resulted in abnormally increased itch behavior [22]. The inhibitory interneurons are thought to release dynorphin, which acts at κ-opioid receptors presumably expressed by itch-signaling neurons [23]. Indeed, κ-opioid agonists such as nalfurafine suppressed itch behavior in mice [23, 24] and relieved itch from chronic kidney disease in human patients [25]. In mice lacking Bhlhb5, excessive scratching was significantly attenuated and skin lesions improved following spinal transplantation of GABAergic neurons [26]. These data thus indicate that GABA, glycine, and dynorphin modulate the spinal transmission of itch signals and that agonists of these inhibitory neurotransmitters may prove useful in treating itch.
Ascending Transmission of Itch
Itch-signaling neurons send ascending axons to the contralateral ventrobasal thalamus (spinothalamic tract) and to the lateral parabrachial nucleus bilaterally (spinoparabrachial tract) (fig. 1). In primates, separate subpopulations of spinothalamic tract neurons responded to histamine versus cowhage [27], a bean plant whose seed pods have spicules containing proteases that elicit nonhistaminergic itch [28]. This distinction is less evident in rodents, whereby most spinal neurons respond to histamine as well as nonhistaminergic pruritogens (fig. 1). In rodents, many spinothalamic and spinoparabrachial neurons respond to multiple pruritogens [29–31]. Interestingly, most or all pruritogen-responsive neurons are also excited by the algogens capsaicin and mustard oil, as well as other pain-producing stimuli. This presents a problem in terms of understanding how the nervous system discriminates between itch and pain. One possibility is that pruritogen-responsive neurons signal itch (even though they can be activated by noxious stimuli), while pain is signaled by a larger population of nociceptive neurons that is insensitive to pruritogens. This is consistent with a report that capsaicin, which normally elicits pain behavior, instead elicits itch behavior in mice lacking the capsaicin-sensitive receptor TRPV1, in whom TRPV1 was genetically inserted selectively back into sensory neurons expressing MrgprA3 [16].
Descending Modulation of Itch
Spinal itch transmission is thought to be under descending modulation from the brain, although to date there is limited data. Depletion of spinal cord levels of norepinephrine increased itch behavior, indicating a role for noradrenergic pathways descending from locus coeruleus and adjacent regions to inhibit itch transmission, possibly by activating inhibitory interneurons [32] (fig. 1). Depletion of supraspinal serotonin reduced itch behavior, indicating that serotonergic pathways descending from the rostral ventromedial medulla may tonically facilitate itch [33] (fig. 1).
Supraspinal Processing of Itch
To date, little is known regarding the functional properties of neurons in the ventrobasal thalamus or parabrachial nuclei that receive direct ascending pruriceptive input. However, numerous functional imaging studies in humans have revealed a variety of brain regions that are activated during itch [34]. These include (1) the thalamus, primary and secondary somatosensory cortex, areas involved in recognition of and attention to itch, and localization and intensity rating of itch, (2) the cingulate and insular cortex, areas associated with cognition, motivation to act (scratch), and awareness of emotional state and body feeling, (3) the medial parietal cortex, posterior cingulate cortex, and precuneus, areas possibly associated with the subjective sensation of itch, and (4) motor-related areas, including the supplementary, premotor, and primary motor cortices, striatum and cerebellum, areas potentially involved in planning motor responses (e.g. scratching) to itch and affective aspects such as the desire to scratch.
Itch relief by scratching and the act of scratching itself have been suggested to be pleasurable. It is interesting that scratching during itch activates brain areas associated with reward including the midbrain striatum, medial prefrontal cortex, anterior cingulate cortex, and orbitofrontal cortex [34].
Humans often perceive itch and scratch themselves when observing other people scratching, a phenomenon called contagious itch [35]. Contagious scratching has also been observed in monkeys [36]. Interestingly, contagious itch is associated with activation of the same brain areas that are active during histamine-evoked itch [37].
Chronic Itch
Chronic itch arises from a variety of skin conditions, such as atopic dermatitis or psoriasis, from systemic kidney or liver disease, nerve damage, and many other sources, and is usually resistant to antihistamine treatment, implying dysfunction of the nonhistaminergic itch pathways. Chronic itch could be due to altered skin physiology or damage causing sensitization of pruriceptors, central sensitization of spinal/trigeminal transmission, disruption of spinal itch-inhibition, disruption of descending itch modulation, altered supraspinal processing of itch signals, or combinations thereof. Symptoms of chronic itch include ongoing (spontaneous) itch, increased itch to a normally pruritic stimulus (hyperknesis), and itch elicited by low-threshold tactile stimulation (alloknesis). In rodent models of atopic dermatitis, dry skin itch, and contact hypersensitivity, animals exhibited spontaneous scratching behavior, alloknesis, and enhanced scratching elicited by nonhistaminergic pruritogens (chloroquine, serotonin, proteases), but not histamine [38–40]. Primary and second-order sensory neurons with input from dry skin exhibited significantly enhanced responses to nonhistaminergic itch mediators, but not to histamine [38, 41], suggesting peripheral and possibly central sensitization of nonhistaminergic itch-signaling neurons in this dry skin model.
Mice lacking a subset of spinal inhibitory interneurons (see above) exhibited enhanced spontaneous scratching and hyperknesis [22], suggesting that dysfunction of spinal inhibition contributes to this genetic model of neuropathic itch.
Human patients suffering from itch of end-stage renal disease exhibited greater baseline activation in the anterior cingulate cortex, insula, claustrum, hippocampus, and nucleus accumbens, as well as reduced cowhage-evoked activation of primary somatosensory cortex and other areas, compared to healthy control subjects [42]. This suggests that chronic itch results in altered supraspinal processing of itch signaling.
Conclusions
Our understanding of the central transmission of itch has increased dramatically in recent years, revealing a number of attractive targets for future development of novel therapeutics to block itch transmission or enhance itch inhibition at peripheral, spinal, and supraspinal sites. Less is known regarding pathophysiological mechanisms underlying chronic itch. However, with the availability of animal models for many types of chronic itch, we can expect dramatic advances to be made in our knowledge of the pathophysiology of chronic itch with the advent of new evidence-based treatment strategies.
Acknowledgments
The authors’ cited work was supported by grants from the National Institutes of Health (AR063228, DE021183, AR057194).
References
- 1.Halvorsen JA, Dalgard F, Thoresen M, Bjertness E, Lien L. Itch and pain in adolescents are associated with suicidal ideation: a population-based cross-sectional study. Acta Derm Venereol. 2012;92:543–546. doi: 10.2340/00015555-1251. [DOI] [PubMed] [Google Scholar]
- 2.Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health spending? Health Aff (Mill-wood) 2004:W4-437–445. doi: 10.1377/hlthaff.w4.437. Suppl Web Exclusives. [DOI] [PubMed] [Google Scholar]
- 3.Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, Gould C, Gemmen E, Dall T. The burden of skin diseases: 2004. A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Schmelz M, Schmidt R, Bickel A, Hand-werker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, Shim B, LaMotte RH, Meyer RA. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci. 2013;16:910–918. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain. 2014;155:80–92. doi: 10.1016/j.pain.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 13.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akiyama T, Tominaga M, Carstens MI, Carstens EE. Site-dependent and state-dependent inhibition of pruritogen-responsive spinal neurons by scratching. Eur J Neurosci. 2012;36:2311–2316. doi: 10.1111/j.1460-9568.2012.08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counter-stimuli. PLoS One. 2011;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hosli L, Haenraets K, Ghanem A, Conzelmann KK, Bosl M, Zeilhofer HU. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama T, Carstens MI, Piecha D, Steppan S, Carstens E. Nalfurafine suppresses pruritogen- and touch-evoked scratching behavior in models of acute and chronic itch in mice. Acta Derm Venereol. 2015;95:147–150. doi: 10.2340/00015555-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16:3742–3747. doi: 10.1681/ASN.2005020152. [DOI] [PubMed] [Google Scholar]
- 26.Braz JM, Juarez-Salinas D, Ross SE, Basbaum AI. Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J Clin Invest. 2014;124:3612–3616. doi: 10.1172/JCI75214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser HR, Giesler GJ., Jr Characterization of pruriceptive trigeminothalamic tract neurons in rats. J Neurophysiol. 2014;111:1574–1589. doi: 10.1152/jn.00668.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen NA, Giesler GJ., Jr Response characteristics of pruriceptive and nociceptive trigeminoparabrachial tract neurons in the rat. J Neurophysiol. 2014;113:58–70. doi: 10.1152/jn.00596.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama T, Curtis E, Nguyen T, Carstens MI, Carstens E. Anatomical evidence of pruriceptive trigeminothalamic and trigeminoparabrachial projection neurons in mice. J Comp Neurol. 2015;524:244–256. doi: 10.1002/cne.23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotoh Y, Andoh T, Kuraishi Y. Noradrenergic regulation of itch transmission in the spinal cord mediated by alpha-adrenoceptors. Neuropharmacology. 2011;61:825–831. doi: 10.1016/j.neuropharm.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Zhao ZQ, Liu XY, Jeffry J, Karunarathne WK, Li JL, Munanairi A, Zhou XY, Li H, Sun YG, Wan L, Wu ZY, Kim S, Huo FQ, Mo P, Barry DM, Zhang CK, Kim JY, Gautam N, Renner KJ, Li YQ, Chen ZF. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron. 2014;84:821–834. doi: 10.1016/j.neuron.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mochizuki H, Kakigi R. Central mechanisms of itch. Clin Neurophysiol. 2015;126:1650–1660. doi: 10.1016/j.clinph.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Papoiu AD, Wang H, Coghill RC, Chan YH, Yosipovitch G. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299–1303. doi: 10.1111/j.1365-2133.2011.10318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feneran AN, O’Donnell R, Press A, Yosipovitch G, Cline M, Dugan G, Papoiu AD, Nattkemper LA, Chan YH, Shively CA. Monkey see, monkey do: contagious itch in nonhuman primates. Acta Derm Venereol. 2013;93:27–29. doi: 10.2340/00015555-1406. [DOI] [PubMed] [Google Scholar]
- 37.Holle H, Warne K, Seth AK, Critchley HD, Ward J. Neural basis of contagious itch and why some people are more prone to it. Proc Natl Acad Sci USA. 2012;109:19816–19821. doi: 10.1073/pnas.1216160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akiyama T, Nguyen T, Curtis E, Nishida K, Devireddy J, Carstens MI, Carstens E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain. 2015;156:1240–1246. doi: 10.1097/j.pain.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu K, Qu L, Shimada SG, Nie H, LaMotte RH. Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neurosci Lett. 2014;579:190–194. doi: 10.1016/j.neulet.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akiyama T, Carstens MI, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. J Neurophysiol. 2011;105:2811–2817. doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papoiu AD, Emerson NM, Patel TS, Kraft RA, Valdes-Rodriguez R, Nattkemper LA, Coghill RC, Yosipovitch G. Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol. 2014;112:1729–1738. doi: 10.1152/jn.00827.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


