Abstract
Background
Cardiac magnetic resonance imaging (CMRI) is considered to be useful for the diagnosis of myocarditis, and the Lake Louise Criteria (LLC) has been proved to be of significance as the standard of diagnosis. However, the diagnostic performance of LLC-based CMRI for myocarditis compared with endomyocardial biopsy (EMB) has not been quantitatively evaluated in a meta-analysis.
Material/Methods
The databases PubMed, Cochrane’s Library, and EMBASE were searched to identify studies on LLC and its individual components for the diagnosis of myocarditis. EMB was the control reference. The sensitivity, specificity, and positive and negative diagnostic likelihood ratios were calculated with a random-effects model. The area under the receiver operating characteristic curve (AUC) was estimated to show overall effectiveness.
Results
We included 9 cohorts (614 patients) of patients with suspected MC. The combined sensitivities, specificities, and AUCs for T1-weighed global relative enhancement were 0.66, 0.73, and 0.71; for T2-weighed edema ratio they were 0.52, 0.73, and 0.72; for the late gadolinium enhancement, they were 0.70, 0.57, and 0.67; and for LLC-based CMRI they were 0.70, 0.56, and 0.70, respectively. Subgroup analysis indicated that the sensitivities, specificities, and diagnostic accuracies of LLC and its individual component-based CMRI seemed to be similar in patients with acute or chronic myocarditis. Results of the Deeks’ funnel plot asymmetry test showed no significant publication bias among the studies.
Conclusions
CMRI based on LLC or its individual components seems to have moderate accuracy in diagnosis of acute or chronic myocarditis.
MeSH Keywords: Diffusion Magnetic Resonance Imaging, Meta-Analysis, Myocarditis
Background
Myocarditis (MC) is now considered as an inflammatory cardiovascular disease that is complicated by heterogeneous clinical presentations [1]. The incidence and mortality associated with MC is difficult to estimate since endomyocardial biopsy (EMB), the criterion standard for the diagnosis of MC, is not commonly used in clinical practice [2]. Results of autopsy studies show that MC accounts for the causes of sudden cardiac death in 2~42% of young cases [3,4], while results of studies based on biopsy showed that MC contributes to 9%~16% of causes for adult patients with dilated cardiomyopathy (DCM) [5], and more than 40% for children with DCM [6]. MC is characterized by histopathological features associated with chronic myocardial inflammation, including myocardial fibrosis, ventricular remodeling, and subsequent cardiac dysfunction, which are not specific to MC [7]. Accordingly, diagnosis of MC is an enormous challenge in cardiovascular practice [8]. Because MC can be fatal, early diagnosis of MC is important for the prevention and treatment of the disease [1,2]. The clinical manifestations of MC vary according to the severity of the disease. Moreover, the symptoms and signs of patients with MC are usually nonspecific, which underlies the importance of additional medical examinations for the diagnosis of MC [2,9].
The criterion standard for the diagnosis of MC is EMB [10]. However, the application of EMB in clinical practice is limited since it is an invasive procedure that causes discomfort and there is an increased risk of severe complications [10]. Recent studies suggested that cardiac magnetic resonance imaging (CMRI) may be a useful noninvasive diagnostic tool for patients with suspected MC [7]. Based on the results of accumulated studies, 3 CMRI-related parameters have been suggested to be of diagnostic efficacy for MC, including T1-weighed global relative enhancement (gRE), T2-weighed edema ratio of the myocardium (ER), and late gadolinium enhancement (LGE) in contrast CMRI [8,9,11]. These parameters are considered to be reflective of myocardial hyperemia, edema, necrosis, and fibrosis, which are the major histopathological characteristics of myocardium in patients with MC [12]. Moreover, a subsequent expert consensus proposed that the diagnostic efficacy of CMRI for MC could be further improved if 2 of the 3 above parameters were found to be positive, which are known as the Lake Louise Criteria (LLC) [11].
However, the LLC were based on studies with limited sample sizes [13–17]. More importantly, these studies compared the diagnostic efficacy of LLC and its individual components with clinically-diagnosed MC rather than EMB-diagnosed MC [13–17]. Therefore, the diagnostic performance of LLC-based CMRI for MC as compared with EMB deserves further evaluation. Since the proposal of LLC, some studies [18–24] have been published that investigated the potential diagnostic efficacy of LLC and its individual components-based CMRI for patients with MC. However, the results were not always consistent. The aim of the present study was to quantitatively evaluate the diagnostic performance of LLC-based CMRI for MC in a meta-analysis, as compared with the criterion standard of EMB.
Material and Methods
Database searching
We performed this systematic review and meta-analysis following the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [25] and the Cochrane Handbook [26]. The studies were identified through computerized searching of the databases PubMed (MEDLINE), Cochrane Library, and EMBASE using the terms “magnetic resonance”, “magnetic resonance imaging”, MR, MRI, or CMRI, paired with “myocarditis” and “endomyocardial biopsy” or EMB. The search was limited to studies in humans and the last search was completed on 1 August 2016. We also manually searched the reference lists of original and review articles.
Inclusion and exclusion criteria
We evaluated the full text of each article with the following inclusion criteria: (1) original study published as full-length article in English; (2) designed as prospective or retrospective cohorts; (3) including patients suspected of acute MC (AMC) or chronic MC (CMC); (4) aimed to evaluate the diagnostic performance of CMR for MC based on LLC or its individual component (gRE, ER and LGE); (5) using EMB as the referenced standard for the diagnosis of MC according to the Dallas Criteria [2]; and (6) reported the essential data so that true- and false-positive values, and true- and false-negative values, could be extracted or calculated and a 2×2 table created. The definitions of AMC and CMC were consistently applied in the original studies according to duration of symptoms from onset to hospital admission (AMC within 14 days; CMC >14 days) [24]. Specifically, Dallas Criteria defined the histological evidence of MC as inflammatory infiltrates within the myocardium associated with myocyte degeneration and necrosis of non-ischemic origin, and quantitatively defined as ≥14 leucocytes/mm2, which include up to 4 monocytes/mm2 with the presence of CD3-positive T lymphocytes ≥7 cells/mm2 [2]. Review articles and duplicate publications were excluded.
Data extraction and quality assessment
Two authors independently performed the literature search, data extraction, and quality assessment based on the inclusion criteria. Discrepancies were resolved by consensus. Data extracted from each study included: first author, published year, location, number of participants, mean ages, proportion of male patients, AMC or CMC, characteristics of CMRI scanners, and CMRI variables analyzed. True- and false-positive data, and true- and false-negative data, obtained from LLC or its individual components for the diagnosis of MC, were extracted for further evaluation. For studies that reported the results of 2 or more cohorts without overlapping participants, multiple sets of data were extracted. We evaluated the quality of the included studies using QUADAS-2 (Quality Assessment Tool for Diagnostic Accuracy Studies) [27]. Each study’s risk of bias and applicability were rated as low, high, or unclear for each domain.
Statistics
The summary sensitivity, specificity, and positive and negative diagnostic likelihood ratios (DLRs) were calculated from the 2×2 tables with corresponding 95% confidence intervals (CIs). The efficacy of LLC-based CMRI for the diagnosis of MC was measured by the diagnostic odds ratio [OR], which is the ratio of the odds of a correct diagnosis to the odds of a misdiagnosis [28]. The area under the receiver operating characteristic (AUC) curve derived from the data was taken to reflect the overall effectiveness of each quantitative method. Inter-study heterogeneity was formally tested using Cochrane’s Q test, and significant heterogeneity was defined as P<0.10. We also calculated the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance; I2 >50% was considered significant heterogeneity [29]. We used a random-effects model, rather than a fixed-effects model, to estimate the overall effect. This is because the random-effects model is a more conservative method that takes into account that study heterogeneity can vary beyond chance, and the results are thus more generalizable. Subgroup analyses were performed to summarize the sensitivity, specificity, and diagnostic accuracy of LLC and its individual component-based CMRI for the diagnosis of AMC and CMC. Deeks’ funnel plot asymmetry test was used to evaluate publication bias. Statistical analyses were performed using STATA 12.0. All statistical tests were 2-sided, with P<0.05 indicating statistical significance.
Results
Results of literature searching
Initially, 302 records were retrieved from the primary database search, and the process of study identification was summarized in Figure 1. Briefly, 21 studies were detected based on title and abstract screening, and 7 studies were finally included after reviewing the full texts of the 21 articles. Fourteen studies were further excluded because 5 were not relevant studies, 4 did not use EMB as a referenced standard, 3 did not include the outcome data, 1 was not in patients with suspected MC, and 1 was a duplicate of an included study.
Figure 1.
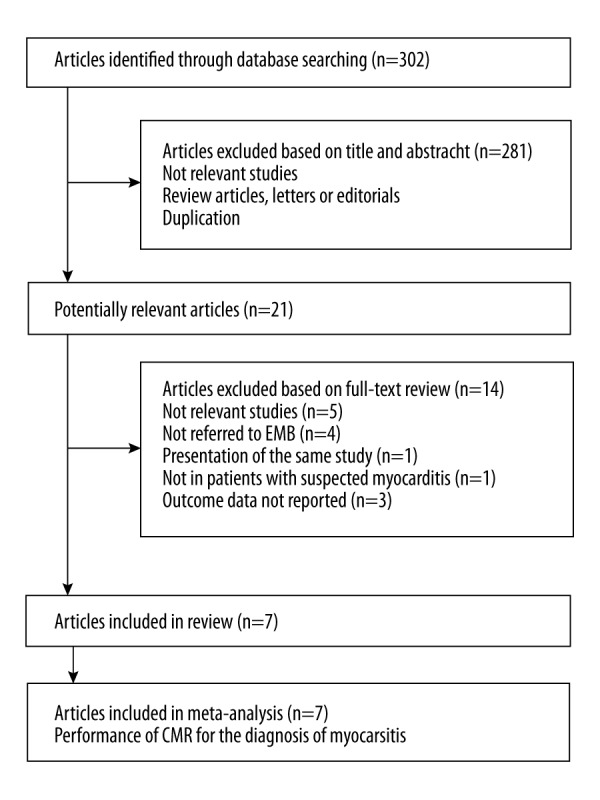
Flow diagram of search results and study selection.
Study characteristics and quality evaluation
Overall, 7 studies including 614 patients with suspected MC were included in our meta-analysis [18–24]. Since 2 of the studies [22,24] included both the cohorts of AMC and CMC, 9 cohorts were included for subsequent quantitative analyses. The characteristics of the included studies are presented in Table 1. The mean age of the patients included in each cohort varied from 40.0 to 58.4 years, with the proportions of males ranging from 32.9% to 87.0%. All of the included patients underwent CMRI with a 1.5T scanner, and 1 study also provided CMRI data with a 3.0T scanner [22]. To keep the consistency of the CMRI technique applied and avoiding repeated inclusion of the same study cohort, only the CMRI data with the 1.5T scanner were included for analysis. Biventricular sampling was applied in EMB in 3 of the studies [18,20,24]. The quality of the eligible studies was assessed according to the QUADAS-2 criteria (Table 2). All of the studies were assessed as having a low risk of bias for patient selection, index test, and reference standard, although 3 studies did not report the time intervals between CMRI examination and EMB [18,19,22].
Table 1.
Characteristics of included studies.
| Author year | Country | Patients cohort | Number of participants | Mean age (years) | Male (%) | EMB sampling | Scanner brand | CMR variables analyzed |
|---|---|---|---|---|---|---|---|---|
| Mahrholdt 2006 | Germany | Suspected MC | 128 | 41.2 | 74.5 | LGE region or BV | Siemens 1.5T | LGE |
| Yilmaz 2008 | Germany | Suspected MC | 69 | 58.4 | 32.9 | RV, LV or BV | Siemens 1.5T | LGE |
| Gutberlet 2008 | Germany | Suspected CMC | 83 | 44.8 | 33.7 | IVS | GE 1.5T | gRE, ER, LGE, LLC |
| Sramko 2013 | Czech | Suspected CMC | 42 | 43.9 | 71.4 | RV | Siemens 1.5T | gRE, ER, LGE, LLC |
| Lurz 2014-AMC | Germany | Suspected AMC | 70 | 44.0 | 87.0 | LV | Philips 1.5T | gRE, ER, LGE, LLC |
| Lurz 2014-CMC | Germany | Suspected CMC | 62 | 52.0 | 69.4 | LV | Philips 1.5T | gRE, ER, LGE, LLC |
| Bohnen 2015 | Germany | Suspected MC | 31 | 51.0 | 77.0 | RV or LV | Philips 1.5T | LGE, LLC |
| Lurz 2016-AMC | Germany | Suspected AMC | 61 | 40.0 | 83.0 | BV | Philips 1.5T | LGE, LLC |
| Lurz 2016-CMC | Germany | Suspected CMC | 68 | 46.0 | 81.0 | BV | Philips 1.5T | LGE, LLC |
AMC – acute myocarditis; CMC – chronic myocarditis; MC – myocarditis; EMB – endomyocardial biopsy; CMR – cardiac magnetic resonance; ER – edema ratio; gRE – global relative enhancement; LGE – late gadolinium enhancement.
Table 2.
Quality assessment of included studies with QUADAS-2 Scores.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Mahrholdt 2006 | Low | Low | Low | Unclear | Low | Low | Low |
| Yilmaz 2008 | Low | Low | Low | Unclear | Low | Low | Low |
| Gutberlet 2008 | Low | Low | Low | Low | Low | Low | Low |
| Sramko 2013 | Low | Low | Low | Low | Low | Low | Low |
| Lurz 2014-AMC | Low | Low | Low | Unclear | Low | Low | Low |
| Lurz 2014-CMC | Low | Low | Low | Unclear | Low | Low | Low |
| Bohnen 2015 | Low | Low | Low | Low | Low | Low | Low |
| Lurz 2016-AMC | Low | Low | Low | Low | Low | Low | Low |
| Lurz 2016-CMC | Low | Low | Low | Low | Low | Low | Low |
Meta-analysis of LLC-based CMRI for diagnosis of MC
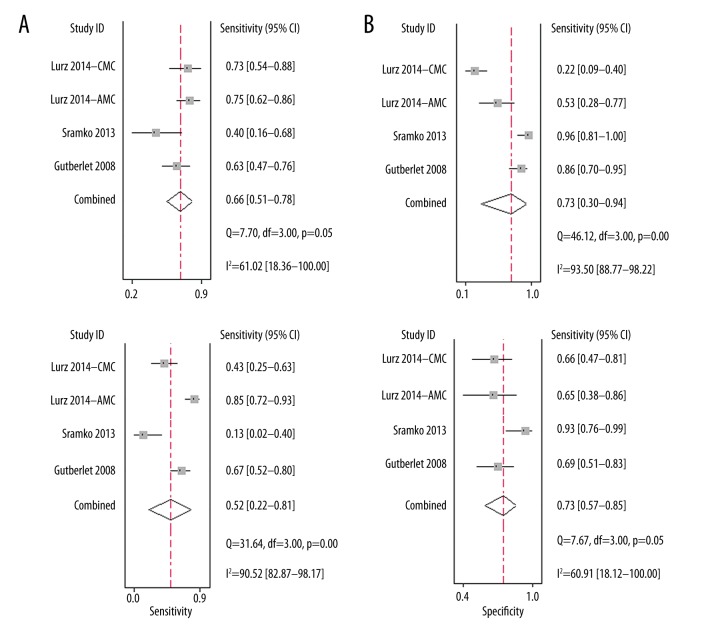
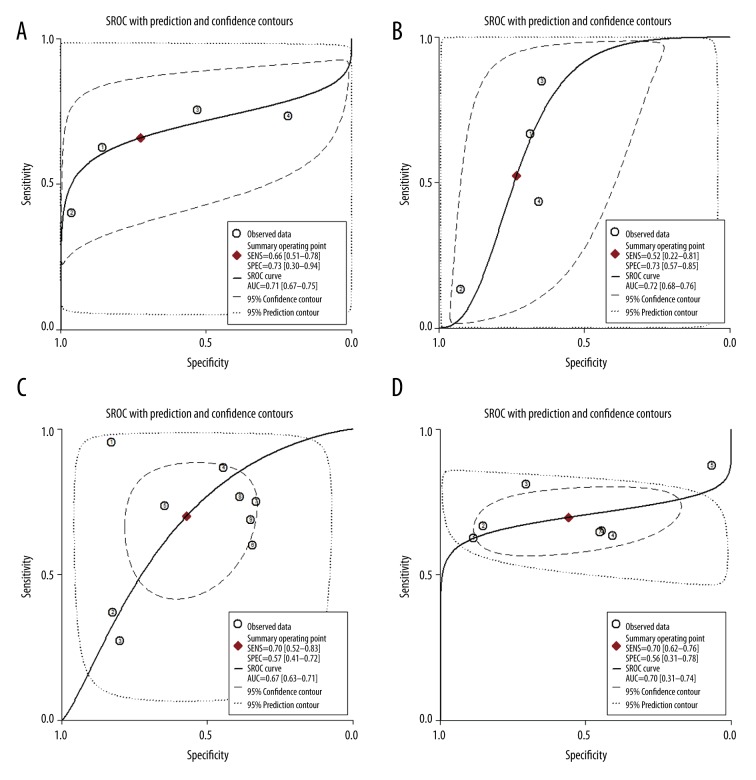
Four of the cohorts [19,21,22], comprising 257 patients with suspected MC, evaluated the performance of gRE-based CMRI for the diagnosis of MC. The summary sensitivity was 0.66 (95% CI: 0.51–0.78) and the specificity was 0.73 (95% CI: 0.30–0.94) (Figure 2A). The positive DLR was 2.40 (95% CI: 0.80–7.60) and the negative DLR was 0.47 (95% CI: 0.34–0.64). Significant heterogeneity existed among the included studies for summary sensitivity and specificity (I2=61.0% and 93.3%, respectively). The summary diagnostic odds ratio was 5.0 (95% CI: 1.0–21.0) and the summary AUC was 0.71 (95% CI: 0.67–0.75) according to the synthesized ROC curve (Figure 3A).
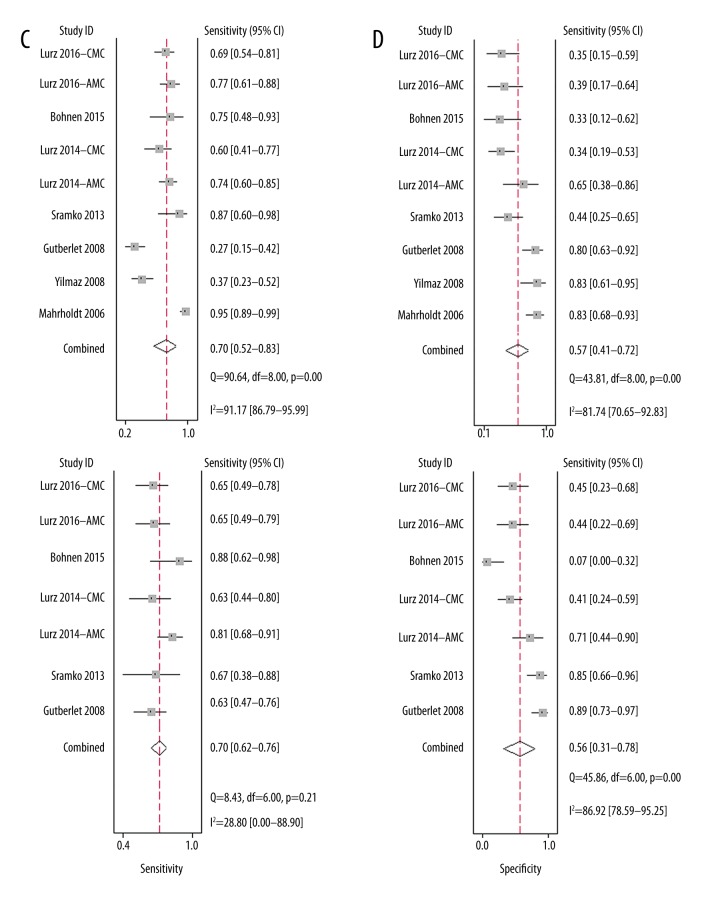
Figure 2.
Forest plots for the performance of LLC and its individual component-based CMRI for the diagnosis of MC. (A) Summary sensitivity and specificity for gRE; (B) Summary sensitivity and specificity for ER; (C) Summary sensitivity and specificity for LGE; (D) Summary sensitivity and specificity for LLC.
Figure 3.
Summary ROC curves for the performance of LLC and its individual component-based CMRI for the diagnosis of MC. (A) Summary ROC curve for gRE; (B) Summary ROC curve for ER; (C) Summary ROC curve for LGE; (D) Summary ROC curve for LLC; Summary ROC curves were based on the bivariate random-effects model.
For the diagnostic efficacy of ER-based CMRI, 4 cohorts [19,21,22] comprising 257 patients with suspected MC were included. The summary sensitivity was 0.52 (95% CI: 0.22–0.81) and the specificity was 0.73 (95% CI: 0.57–0.85) (Figure 2B). The positive DLR was 2.0 (95% CI: 1.3–3.0) and the negative DLR was 0.65 (95% CI: 0.36–1.16). Significant heterogeneity existed among the included studies for summary sensitivity and specificity (I2=90.5% and 60.9%, respectively). The summary diagnostic odds ratio was 3.0 (95% CI: 1.0–8.0), and the summary AUC was 0.72 (95% CI: 0.68–0.76) according to the synthesized ROC curve (Figure 3B).
For the diagnostic efficacy of LGE-based CMRI, all of the 9 cohorts (614 patients) of suspected MC patients were included. The summary sensitivity was 0.70 (95% CI: 0.52–0.83) and the specificity was 0.57 (95% CI: 0.41–0.72) (Figure 2C). The positive DLR was 1.6 (95% CI: 1.1–2.4) and the negative DLR was 0.52 (95% CI: 0.30–0.92). Significant heterogeneity existed among the included studies for summary sensitivity and specificity (I2=91.2% and 81.7%, respectively). The summary diagnostic odds ratio was 3.0 (95% CI: 1.0–8.0) and the summary AUC was 0.67 (95% CI: 0.63–0.71) according to the synthesized ROC curve (Figure 3C).
For the diagnostic efficacy of LLC-based CMRI, 7 cohorts [19,21–24] (417 patients with suspected MC) were included. The summary sensitivity was 0.70 (95% CI: 0.62–0.76) and the specificity was 0.56 (95% CI: 0.31–0.78) (Figure 2D). The positive DLR was 1.6 (95% CI: 0.9–2.8) and the negative DLR was 0.54 (95% CI: 0.35–0.84). Significant heterogeneity existed among the included studies for specificity (I2=86.9%). The summary diagnostic odds ratio was 3.0 (95% CI: 1.0–8.0) and the summary AUC was 0.70 (95% CI: 0.66–0.74) according to the synthesized ROC curve (Figure 3D).
Subgroup analyses for LLC-based CMRI for diagnosis of AMC or CMC
Since only 1–4 cohorts were available for the evaluation of LLC and its individual component-based CMRI for the diagnosis AMC and CMC separately, we pooled the data for summary sensitivity, specificity, and diagnostic accuracy based on the included cohorts. The results are presented in Table 3. Generally, the sensitivities, specificities, and diagnostic accuracies of gRE-, ER-, LGE-, and LLC-based CMRI seemed to be similar in patients with AMC and CMC.
Table 3.
Subgroup analyses in patients with AMC or CMC.
| AMC | CMC | |||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | |
| gRE | 0.75 | 0.53 | 0.70 | 0.62 | 0.67 | 0.65 |
| ER | 0.85 | 0.64 | 0.80 | 0.51 | 0.74 | 0.63 |
| LGE | 0.75 | 0.51 | 0.69 | 0.55 | 0.51 | 0.53 |
| LLC | 0.74 | 0.57 | 0.69 | 0.64 | 0.67 | 0.65 |
Publication bias
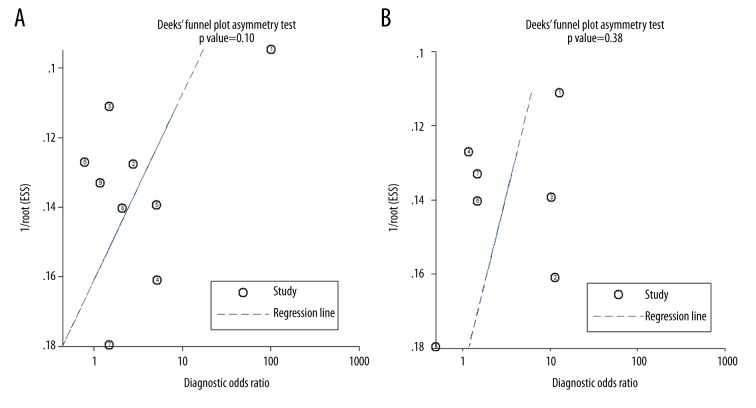
According to the Deeks’ funnel plot asymmetry test, there was no significant publication bias among the studies for estimation of diagnostic efficacies of LGE- or LLC-based CMRI for MC (P=0.10 and 0.38 respectively; Figure 4). The publication biases the diagnostic efficacies of gRE- and ER-based CMRI were difficult to estimate because a limited number of studies were included.
Figure 4.
(A, B) Deeks’ funnel plots for the assessment of publication biases for the estimations of the diagnostic efficacies of LGE- or LLC-based CMRI for MC.
Discussion
By pooling the results of 7 diagnostic studies using EMB as the referenced standard, our meta-analysis showed only moderate diagnostic efficacies of LLC and its individual component-based CMRI for MC. Specifically, the AUCs for gRE, ER, LGE, and LLC were 0.71, 0.72, 0.67, and 0.70 respectively. Moreover, subgroup analysis suggested that the sensitivities, specificities, and diagnostic accuracies of LLC and its individual component-based CMRI seemed to be similar in patients with AMC and CMC. These results suggest that compared with EMB, the diagnostic performance of LLC and its individual component-based CMRI in patients with suspected MC is moderate. Our results highlight the need for development of novel CMRI-related parameters and novel imaging techniques for the diagnosis of MC.
Our study has clinical implications. To the best of our knowledge, this is the first meta-analysis to systematically evaluate the diagnostic performance of LLC-based CMRI in patients with suspected MC using EMB as the standard. Indeed, the previously proposed LLC for CMRI diagnosis of MC were based on small-scale studies, which mostly compared the CMRI results with clinical data [13–17]. The CMRI parameters used in the LLC were generally 3 histopathological features of myocardial inflammation, which may lack sensitivity and specificity for MC as compared with patients with other cardiovascular diseases [7,8,11]. Firstly, gRE, which indicates T1-weighed global relative enhancement and reflects myocardial hyperemia, has been shown to be nonspecific for MC [30]. Because the signal intensity of skeletal muscle is used for the normalization of the gRE ratio, gRE in patients complicated with skeletal muscle diseases may become falsely negative [31]. Secondly, ER, which indicates the T2-weighed edema ratio and reflects the extent of myocardial edema, has also been suggested to be nonexclusive in MC and in other diseases with myocardial interstitial injury [32]. In addition, technique shortcomings, such as low signal-to-noise ratio, also limited the diagnostic accuracy of ER [33]. Lastly, LGE, which indicates late gadolinium enhancement in contrast CMRI and reflects myocardial necrosis and fibrosis, also lacks specificity and sensitivity. Since myocardial necrosis and fibrosis could be observed in many other cardiovascular diseases with myocardial injury and remodeling, LGE was not specific for MC [34,35]. Moreover, for patients with moderate MC, the extent of myocardial necrosis and fibrosis may be not significant enough to be detected by LGE. In summary, based on these limitations of the components of LLC, it was not surprising to find that CMRI based on LLC and its components were moderately accurate for diagnosis in patients with suspected MC. Although a previous consensus proposed to combine these 3 techniques and apply 2 of 3 positive as the diagnostic standard to improve overall diagnostic accuracy [11], results of our meta-analysis did not support that CMRI based on LLC had better diagnostic efficacy than CMRI based on the individual components.
Some previous studies suggest that CMRI based on LLC may confer better diagnostic accuracy in patients with AMC than in those with CMC [22,24], because patients with AMC typically have more significant histopathological features of MC, such as myocardial hyperemia, edema, necrosis, and fibrosis. Although limited data could be included, our subgroup analyses by pooling the data from AMC and CMC studies did not support that CMRI based on LLC has better diagnostic performance in AMC, indicating that the diagnostic efficacy of LLC-based CMRI may be consistently moderate in AMC and CMC. These results should be interpreted with caution, and studies with adequate statistical power are needed to further confirm our findings.
Results of our study highlights the need to develop novel imaging strategies for the diagnosis of MC. Some recent studies have suggested that T1 and T2 mapping may be superior to the conventional LLC-based CMRI in patients with suspected MC [23,24], especially in those with CMC. Additionally, the performance of some other functional imaging methods, such as 19F MRI for the detection of immune cell infiltration [36], deserves further evaluation.
Our study has limitations which should be considered when interpreting the results. Firstly, although we used EMB as the referenced standard for the diagnosis of MC, this method has possible limitations as a criterion diagnostic standard for MC, because the diagnostic efficacy of EMB has been shown to be hampered by the potential sampling error [37] and associated lower sensitivity of the diagnostic strategy [38], as well as the influence of high interobserver variability in the interpretation of biopsy samples [39]. To overcome these limitations, some of our included studies applied biventricular biopsy. Secondly, significant heterogeneities among the included studies were detected for most of the outcomes. Differences in study characteristics, such as the duration of symptom-onset of CMRI examination, comorbidities of the patients, and protocols for CMRI analysis, may have contributed to heterogeneity. Finally, the limited number of available studies and included patients prevent us from subsequently discovering the source of the heterogeneity, and the AUCs for the diagnostic efficacy of CMRI in patients with AM or CM could not be estimated separately because limited datasets were available. However, with the limitations of EMB used in clinical studies, performing a meta-analysis like ours is an important strategy to overview the diagnostic performance of LLC-based CMRI as compared with EMB.
Conclusions
Results of our meta-analysis show that CMRI based on LLC or its individual components seems to have moderate efficacy in the diagnosis of MC in clinical practice. Novel CMRI-related parameters and imaging techniques are still needed for the diagnosis of MC.
Footnotes
Source of support: Departmental sources
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Schultheiss HP, Kuhl U, Cooper LT. The management of myocarditis. Eur Heart J. 2011;32:2616–25. doi: 10.1093/eurheartj/ehr165. [DOI] [PubMed] [Google Scholar]
- 2.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48. 2648a–2648d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 3.Gore I, Saphir O. Myocarditis; A classification of 1402 cases. Am Heart J. 1947;34:827–30. doi: 10.1016/0002-8703(47)90147-6. [DOI] [PubMed] [Google Scholar]
- 4.Basso C, Calabrese F, Corrado D, et al. Postmortem diagnosis in sudden cardiac death victims: Macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001;50:290–300. doi: 10.1016/S0008-6363(01)00261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–75. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 6.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz A, Ferreira V, Klingel K, et al. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail Rev. 2013;18:747–60. doi: 10.1007/s10741-012-9356-5. [DOI] [PubMed] [Google Scholar]
- 8.Biesbroek PS, Beek AM, Germans T, et al. Diagnosis of myocarditis: Current state and future perspectives. Int J Cardiol. 2015;191:211–19. doi: 10.1016/j.ijcard.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Pollack A, Kontorovich AR, Fuster V, et al. Viral myocarditis – diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–80. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 10.Sinagra G, Anzini M, Pereira NL, et al. Myocarditis in clinical practice. Mayo Clin Proc. 2016;91:1256–66. doi: 10.1016/j.mayocp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Childs H, Friedrich MG. Cardiovascular magnetic resonance imaging in myocarditis. Prog Cardiovasc Dis. 2011;54:266–75. doi: 10.1016/j.pcad.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich MG, Strohm O, Schulz-Menger J, et al. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–9. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 14.Laissy JP, Messin B, Varenne O, et al. MRI of acute myocarditis: A comprehensive approach based on various imaging sequences. Chest. 2002;122:1638–48. doi: 10.1378/chest.122.5.1638. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Aty H, Boye P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: Comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 16.Yelgec NS, Dymarkowski S, Ganame J, et al. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol. 2007;17:2211–17. doi: 10.1007/s00330-007-0612-3. [DOI] [PubMed] [Google Scholar]
- 17.Goitein O, Matetzky S, Beinart R, et al. Acute myocarditis: Noninvasive evaluation with cardiac MRI and transthoracic echocardiography. Am J Roentgenol. 2009;192:254–58. doi: 10.2214/AJR.08.1281. [DOI] [PubMed] [Google Scholar]
- 18.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 19.Gutberlet M, Spors B, Thoma T, et al. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401–9. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz A, Mahrholdt H, Athanasiadis A, et al. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart. 2008;94:1456–63. doi: 10.1136/hrt.2007.131383. [DOI] [PubMed] [Google Scholar]
- 21.Sramko M, Kubanek M, Tintera J, et al. Utility of combination of cardiac magnetic resonance imaging and high-sensitivity cardiac troponin T assay in diagnosis of inflammatory cardiomyopathy. Am J Cardiol. 2013;111:258–64. doi: 10.1016/j.amjcard.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Lurz P, Eitel I, Klieme B, et al. The potential additional diagnostic value of assessing for pericardial effusion on cardiac magnetic resonance imaging in patients with suspected myocarditis. Eur Heart J Cardiovasc Imaging. 2014;15:643–50. doi: 10.1093/ehjci/jet267. [DOI] [PubMed] [Google Scholar]
- 23.Bohnen S, Radunski UK, Lund GK, et al. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging. 2015;8(6) doi: 10.1161/CIRCIMAGING.114.003073. pii: e003073. [DOI] [PubMed] [Google Scholar]
- 24.Lurz P, Luecke C, Eitel I, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: The MyoRacer-Trial. J Am Coll Cardiol. 2016;67:1800–11. doi: 10.1016/j.jacc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. www.cochranehandbook.org. [Google Scholar]
- 27.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 28.Takada T, Nishiwaki H, Yamamoto Y, et al. The role of digital rectal examination for diagnosis of acute appendicitis: A systematic review and meta-analysis. PLoS One. 2015;10:e0136996. doi: 10.1371/journal.pone.0136996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eitel I, Lucke C, Grothoff M, et al. Inflammation in takotsubo cardiomyopathy: Insights from cardiovascular magnetic resonance imaging. Eur Radiol. 2010;20:422–31. doi: 10.1007/s00330-009-1549-5. [DOI] [PubMed] [Google Scholar]
- 31.Greaves K, Oxford JS, Price CP, et al. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: Measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch Intern Med. 2003;163:165–68. doi: 10.1001/archinte.163.2.165. [DOI] [PubMed] [Google Scholar]
- 32.Shehab AM, Strohm O. Advanced tissue characterization in non-ischemic cardiomyopathies using contrast-enhanced cardiac magnetic resonance imaging. Saudi Med J. 2010;31:115–22. [PubMed] [Google Scholar]
- 33.Eitel I, Friedrich MG. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson. 2011;13:13. doi: 10.1186/1532-429X-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulten E, Agarwal V, Cahill M, et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: A systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9:e005001. doi: 10.1161/CIRCIMAGING.116.005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuruvilla S, Adenaw N, Katwal AB, et al. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: A systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250–58. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinkert S, Westermann D, Wang X, et al. Prevention of cardiac dysfunction in acute coxsackievirus B3 cardiomyopathy by inducible expression of a soluble coxsackievirus-adenovirus receptor. Circulation. 2009;120:2358–66. doi: 10.1161/CIRCULATIONAHA.108.845339. [DOI] [PubMed] [Google Scholar]
- 37.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: Implications for role of sampling error. Mayo Clin Proc. 1989;64:1235–45. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 38.Chow LH, Radio SJ, Sears TD, et al. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–20. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 39.Shanes JG, Ghali J, Billingham ME, et al. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation. 1987;75:401–5. doi: 10.1161/01.cir.75.2.401. [DOI] [PubMed] [Google Scholar]