Abstract
Introduction
Management of infections with carbapenem-resistant Enterobacteriaceae (CRE) is challenging. In recent times, agents such as colistin and fosfomycin have been used in combination with other antibiotics to treat such infections. In this study, we aim to seek frequency of colistin and fosfomycin resistance in CRE from Pakistan.
Methods
This study was conducted at clinical laboratories, Aga Khan University Hospital. In total, 251 CRE were included in the study. Colistin minimum inhibitory concentrations (MICs) were performed using broth microdilution (BMD) method and VITEK® 2 system, whereas fosfomycin susceptibility was performed using Kirby–Bauer method. MIC50 and MIC90 were calculated for colistin and agreement between VITEK and BMD was also calculated.
Results
Out of 251 strains colistin MIC of ≥4 µg/mL was seen in 40 (15.9%). Of these strains 20 (50%) were Klebsiella pneumoniae. Colistin MIC50 and MIC90 were found to be 0.5 and 16 µg/mL, respectively. BMD and VITEK 2 showed 100% categorical agreement. Essential agreement was 88.5% with kappa score 0.733 indicating strong agreement between VITEK and BMD. 31 out of 251 (12.3%) CREs were resistant to fosfomycin.
Conclusion
Study shows frequency of colistin and fosfomycin resistance to be 15.9% and 12.3%, respectively. In countries where rate of CREs is high, emerging resistance against these last resort antibiotics is alarming as it leaves clinicians with almost no options to manage such multidrug resistant and extensively drug resistant infections.
Keywords: emerging drug resistance, colistin resistance, fosfomycin resistance, carbapenam resistant enterobacteriaceae, salvage antibiotics
Video abstract
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) are increasingly being identified worldwide and pose a major threat to the treatment of infectious diseases and to infection control within heath care facilities.1 In 2014, a meta-analysis showed mortality rates attributable to CRE to be 26%–44%.2 Frequency of CRE is increasing in worldwide.3,4 In Pakistan, the rates have been increasing steadily, data from our hospital showed increase in carbapenem-resistant Escherichia coli, Enterobacter spp. and Klebsiella pneumoniae from 5%, 7% and 15% in 2013 to 6%, 23% and 22% by 2015, respectively.5
With increasing antimicrobial resistance and given the paucity of new drugs, the focus of management has shifted to older agents.6 Currently, such antibiotics in use in Pakistan include colistin and fosfomycin. It has earlier been shown that treatment of CRE with combination of carbapenem and colistin is associated with better outcomes7 leading to increased use of colistin in clinical practice.8 Fosfomycin has traditionally been used to treat urinary tract and gastrointestinal infections. A meta- analysis including 62 studies, showed utility of fosfomycin either alone or in combination with other antibiotics for the treatment of pneumonia, osteomyelitis, meningitis, ear, nose, throat infections and gynecological infections,9 hence, identifying fosfomycin as having potential for treatment of invasive infections. In February 2010, intravenous (IV) fosfomycin was prospectively evaluated for the treatment of carbapenem-resistant K. pneumoniae wherein all patients had bacteriological clearance with no adverse reactions.10
Given rising antimicrobial resistance and reports of CRE, this study was designed to evaluate the frequency of colistin and fosfomycin resistance in carbapenem-resistant Gram-negative bacterial infections in our population.
Materials and methods
Study setting
This was a prospective study conducted on archived bacterial isolates from clinical specimens received in the Clinical Microbiology Laboratory, Aga Khan University. The archived strains were stored at −80°C in glycerol phosphate broth and were revived on MacConkey agar before use.
Bacterial isolates
All CRE isolated from January 2015 to August 2016 were included. CREs were defined using CLSI 2015 cut-offs for ertapenem, imipenem or meropenem.11 Identification of strains included in the study was done using API 20E system. Strains with inherent resistance to colistin such as those belonging to genus Proteus, Providencia and Serratia were excluded from the study.
Susceptibility testing: Carbapenem susceptibilities were performed by disc diffusion (Kirby–Bauer) method on Mueller Hinton agar11 and by VITEK 2 system.12 Isolates were labeled as CREs by disc diffusion zone diameters and by VITEK minimum inhibitory concentrations (MICs). For interpretation CLSI 2015 breakpoints for imipenem, ertapenem and meropenem, that is, for ertapenem disc diameter ≤19 mm or MIC ≥1, for imipenem and meropenem disc diameter ≤21 mm or MIC ≥2 were used.11
Colistin susceptibility was performed as follows: broth microdilution using 96-well microtiter plates to perform MICs using cation adjusted Mueller Hinton broth as culture medium. Drug concentration ranging from 0.03 to 16 µg/mL was used. MICs were performed in batches of 10−20 strains per batch, 5 days a week from July 2016 till August 2016 on archived strains. Colistin sulfate powder was obtained from Sigma-Aldrich Co. (St Louis, MO, USA). ATCC E. coli 25922 and ATCC P. aeruginosa 27853 were used as quality control strains. Results were read at 24 hours and interpreted using colistin cut-offs for Enterobacteriaceae in EUCAST 2016, that is, MIC ≤2 as sensitive.13 Colistin MICs were also determined using automated VITEK 2 system. Results were compared with broth microdilution. Broth microdilution was used as the gold standard. Agreement between broth and VITEK was calculated.
Fosfomycin susceptibility was tested by disc diffusion method using fosfomycin trometamol disc (200 µg) (Thermo Fisher Scientific, Waltham, MA, USA) containing 50 µg G6PD on Muller Hinton agar. Results were interpreted using CLSI 2015 disc diffusion cut-offs for E. coli in urinary tract isolates, that is, ≥16 as sensitive, 13–15 intermediate and ≤12 as resistant.11
Ethical approval
As this study was conducted on archived bacterial isolates and not on patients’ samples, it was given exemption by the ethical review committee Aga Khan University Hospital (ERC# 3992-PAT-ERC-16).
Statistical analysis
Data entered in Microsoft Excel 2010® and imported to Stata SE (version 12) was used to calculate MIC50 and MIC90. MIC50 and MIC90 were defined as the lowest concentration of the antibiotic at which 50% and 90% of the tested strains were inhibited. Categorical and essential agreement between VITEK and broth microdilution was calculated using Kappa scores.
Results
The study included 251 strains. These included E. coli 39.04% (98/251) followed by K. pneumoniae 31% (78/251), Raoultella spp. 15.5% (Raoultella terrigena and Raoultella ornithinolytica) (39/251), Enterobacter spp. 7.5% (19/251), Klebsiella oxytoca 5.1% (13/251) and Citrobacter spp. 1.1% (3/251). Details regarding source and characteristics of patients from whom these isolates were obtained are provided in Table S1.
MIC50 and MIC90
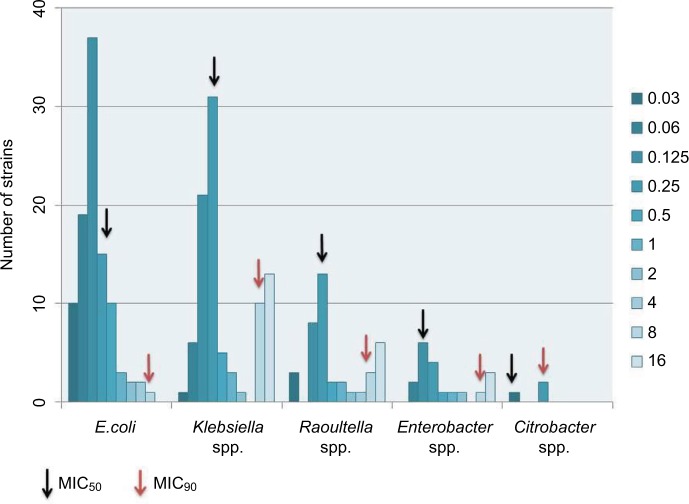
Colistin MICs by broth microdilution was performed on all strains. MIC50 value was calculated to be 0.5 µg/mL, whereas MIC90 was 16 µg/mL. MIC50 and MIC90 values among different bacterial species was separately calculated (Figure 1).
Figure 1.
Groups of Enterobacteriaceae with MIC ranges from 0.03 to 16 μg/mL.
Colistin MICs was ≥4 µg/mL in 40 out of 251 (15.9%) study strains which were identified as being colistin resistant. Out of these 40 strains 50% were K. pneumonia (n: 20), 22.5% R. terrigena (n:10), 10% Enterobacter spp. (n:4), 7.5% E. coli (n:3) and 7.5% K. oxytoca (n:3).
Agreement between VITEK and broth
VITEK MICs were available for 149 strains and agreement was calculated. Broth and VITEK showed 100% categorical agreement with kappa score of 1, CI (0.84–1.16). Essential agreement was calculated to be 88.59% with kappa score of 0.7334, CI (0.6–0.798).
Fosfomycin susceptibility
Among this CRE collection, rate of fosfomycin resistance was 12.3% (31/251). In the colistin-resistant CRE subgroup (n:40) 35 out of 40 remained susceptible to fosfomycin while 5 stains were found resistant to both colistin and fosfomycin simultaneously.
Discussion
Multidrug resistant (MDR) and extensively drug resistant (XDR) bacteria are a global threat. In countries with limited resources, over-stretched health systems make it difficult to manage such infections. Furthermore, inadequate implementation of infection control policies contribute toward dissemination of both nosocomial infections as well as of antimicrobial resistance.
Our study showed 15.9% colistin and 12.3% fosfomycin resistance among CREs. Both colistin and fosfomycin are salvage antibiotics for treating MDR and XDR infections. Hence emerging resistance against these agents significantly reduces available treatment options. Despite their potential side effects and high cost, in the absence of alternatives, clinicians have had no option but to use these antibiotics.
Aga Khan University hospital is a 680-bedded tertiary care hospital which caters to thousands of patients all year round. Our laboratory has more than 200 collection units and participates in external quality assurance surveys from College of American Pathologist on regular basis. Since rate of CRE in the hospital has been increasing over time, colistin or fosfomycin are only prescribed for patients on clinician’s advice and their use is closely monitored by the pharmacy service. As per Antibiotic policy of the hospital, IV fosfomycin and colistin have been labeled as restricted and controlled antibiotics which require mandatory approval from the infectious diseases pharmacist or ID physician for dispense.
Colistin resistance among CRE has been previously reported. Most of these studies have been case reports or outbreaks in hospital settings. An outbreak in three institutions in Detroit, MI, USA reported a cluster of colistin-resistant, carbapenem-resistant K. pneumoniae infection in 2009.14 Another study from Italy also reported emergence of colistin resistance in carbapenem-resistant K. pneumoniae strains collected from 21 hospital laboratories during November 2013 to April 2014.15 Goel et al reported emergence of a cluster of 24 cases of colistin resistant K. pneumoniae in oncology unit in India.16
Earlier, colistin resistance was known to be mediated by chromosomal mutations which altered the lipopolysaccharide of the bacterial outer membrane. These include mgrB, phoP/phoQ, pmrA, pmrB, pmrC, and crrABC.17 In 2015, a study from China reported emergence of plasmid mediated colistin resistance (mcr-1) in E. coli and K. pneumoniae from human and animals.18 A variant of mcr-1 (mcr-1.2) and mcr-2 were also detected in Italy and Belgium.19,20 Rapid rise of resistance in such a brief period is alarming and poses a major threat in countries with high CRE prevalence such as Pakistan, further minimizing available antibiotic options for treatment of CRE infections.
Due to excessive and inappropriate use of colistin, resistance has not only emerged among CRE but other bacterial species as well. In 2002, an outbreak of colistin-resistant Pseudomonas aeruginosa in a pulmonology unit in the UK created an infection control concern,21 over the years this was followed with different reports from Australia, India and Iran where colistin resistance was documented in Acinetobacter baumanii as well.22–24
With limited available options for treating CRE infections, fosfomycin held promise. An earlier study on 152 MDR (including 85 XDR) Enterobacteriaceae isolates, reported overall susceptibility against fosfomycin of 92.8% (141/152).25 These findings concurred with another study published in the same year, 2009, reporting 7% fosfomycin resistance in prospectively collected 68 K. pneumoniae isolates of which 23 were also nonsusceptible to tigecycline and/or colistin.26 A recent study from Germany in 2013 tested fosfomycin against CRE and reported 78% of the tested strain as fosfomycin sensitive.27 Hence, our data reporting 12.3% resistance in CRE suggests increasing resistance against this antibiotic.
Our study has few limitations. Due to financial constrain test strains were only identified on basis of biochemical identification using API 20E. Neither molecular identification of the isolates, nor resistance gene detection was performed for mcr-1, mrgB or other mutations. Hence additional studies are required to identify genetic mutations associated with colistin resistance in this part of the world. As we tested colistin and fosfomycin using two different methods (broth microdilution and disc diffusion) we could not look for synergism and, therefore, further studies are needed to evaluate synergy between different antibiotics combination for the treatment of CRE.
Conclusion
Our data suggest emerging in vitro resistance against colistin and fosfomycin in CREs from Pakistan. Our findings suggest a need for regular monitoring of antimicrobial resistance in the country including for colistin and fosfomycin. Our data further highlights the urgency for developing and implementing a national policy toward control of antimicrobial resistance in Pakistan.
Supplementary material
Table S1.
General characteristics of the bacterial isolates included in the study
| General characteristics of the study | N=251 |
|---|---|
| Mean, age | 50.9 years |
| Gender | |
| Male (%) | 56.8 |
| Female (%) | 43.13 |
| Site of organism recovery | |
| Urine | 143 |
| Blood | 61 |
| Cerebrospinal fluid | 4 |
| Pus | 11 |
| Tissue | 7 |
| Respiratory specimen | 14 |
| Other body fluids | 11 |
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant entero-bacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53(1):60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 5.Pakistan antimicrobial resistance network 2016. [Accessed February 10, 2017]. Available from http://www.parn.org.pk/index_files/Antimicrobial%20data.html.
- 6.Falagas ME, Grammatikos AP, Michalopoulos A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev Anti Infect Ther. 2008;6(5):593–600. doi: 10.1586/14787210.6.5.593. [DOI] [PubMed] [Google Scholar]
- 7.Van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75(2):115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanulík V, Suchánková H, Urbánek K, et al. Effect of colistin consumption and prevalence of colistin-resistant bacteria. Klin Mikrobiol Infekc. 2013;19(2):52–55. Czech with English Abstract. [PubMed] [Google Scholar]
- 9.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008;46(7):1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 10.Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011;37(5):415–419. doi: 10.1016/j.ijantimicag.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 11.CLSI Performance standards for Antimicrobial susceptibility testing. Approved Guideline. 2014;34(1):M100–S24. [Google Scholar]
- 12.Ling TK, Tam PC, Liu ZK, Cheng AF. Evaluation of VITEK 2 rapid identification and susceptibility testing system against gram-negative clinical isolates. J Clin Microbiol. 2001;39(8):2964–2966. doi: 10.1128/JCM.39.8.2964-2966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European committee of antimicrobial susceptibility testing (EUCAST) 2016. [Accessed october 22, 2016]. Available from: http://www.eucast.org/clinical_breakpoints.
- 14.Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother. 2011;55(2):593–599. doi: 10.1128/AAC.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monaco M, Giani T, Raffone M, et al. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 2014;19(42) doi: 10.2807/1560-7917.es2014.19.42.20939. pii20939. [DOI] [PubMed] [Google Scholar]
- 16.Goel G, Hmar L, De MS, Bhattacharya S, Chandy M. Colistin-resistant Klebsiella pneumoniae: report of a cluster of 24 cases from a new oncology center in eastern India. Infect Control Hosp Epidemiol. 2014;35(08):1076–1077. doi: 10.1086/677170. [DOI] [PubMed] [Google Scholar]
- 17.Cannatelli A, D’Andrea MM, Giani T, et al. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother. 2013;57(11):5521–5526. doi: 10.1128/AAC.01480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 19.Di Pilato V, Arena F, Tascini C, et al. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother. 2016;60(9):5612–5615. doi: 10.1128/AAC.01075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xavier BB, Lammens C, Ruhal R, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21(27) doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 21.Denton M, Kerr K, Mooney L, et al. Transmission of colistin-resistant Pseudomonas aeruginosa between patients attending a pediatric cystic fibrosis center. Pediatr Pulmonol. 2002;34(4):257–261. doi: 10.1002/ppul.10166. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50(9):2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taneja N, Singh G, Singh M, Sharma M. Emergence of tigecycline & colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India. Indian J Med Res. 2011;133(6):681. [PMC free article] [PubMed] [Google Scholar]
- 24.Bahador A, Taheri M, Pourakbari B, et al. Emergence of rifampicin, tigecycline, and colistin-resistant Acinetobacter baumannii in Iran; spreading of MDR strains of novel International Clone variants. Microb Drug Resist. 2013;19(5):397–406. doi: 10.1089/mdr.2012.0233. [DOI] [PubMed] [Google Scholar]
- 25.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents. 2010;35(3):240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother. 2010;54(1):526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaase M, Szabados F, Anders A, Gatermann SG. Fosfomycin susceptibility in carbapenem-resistant Enterobacteriaceae from Germany. J Clin Microbiol. 2014;52(6):1893–1897. doi: 10.1128/JCM.03484-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
General characteristics of the bacterial isolates included in the study
| General characteristics of the study | N=251 |
|---|---|
| Mean, age | 50.9 years |
| Gender | |
| Male (%) | 56.8 |
| Female (%) | 43.13 |
| Site of organism recovery | |
| Urine | 143 |
| Blood | 61 |
| Cerebrospinal fluid | 4 |
| Pus | 11 |
| Tissue | 7 |
| Respiratory specimen | 14 |
| Other body fluids | 11 |