Abstract
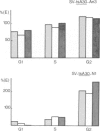
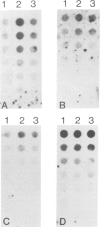
The p53 oncogene is thought to play a role in the proliferation of normal and transformed cells and its expression was postulated to be cell cycle dependent. Using flow cytofluorimetry sorting of populations of exponentially growing cells, coupled to a radioimmune assay, we have investigated the accumulation of p53 along the cell cycle in normal FR 3T3 rat cells as well as in two types of SV40-transformed derivatives, one of which only expresses the large-T protein during the G2 phase of the cell cycle. p53 was accumulated at a constant level throughout the cell cycle in FR 3T3 cells. Its level and stability increased to different extents in the two types of transformants. However, the formation of complexes between p53 and large-T was modulated by the G2-restricted accumulation of large-T, thus leading to a differential increase in the levels of p53 both in exponentially growing cells and along the cell cycle. This increase in the levels of p53 appeared to be regulated at a post-transcriptional level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benchimol S., Pim D., Crawford L. Radioimmunoassay of the cellular protein p53 in mouse and human cell lines. EMBO J. 1982;1(9):1055–1062. doi: 10.1002/j.1460-2075.1982.tb01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Lane D. P., Denhardt D. T., Harlow E. E., Nicklin P. M., Osborn K., Pim D. C. Characterization of the complex between SV40 large T antigen and the 53K host protein in transformed mouse cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):179–187. doi: 10.1101/sqb.1980.044.01.021. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Bulbrook R. D. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982 Oct 15;30(4):403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Freed M. I., Lubin I., Simmons D. T. Stoichiometry of large T antigen and pp53 in complexes isolated from simian virus 40-transformed rat cells. J Virol. 1983 Jun;46(3):1061–1065. doi: 10.1128/jvi.46.3.1061-1065.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert J., Clertant P., de Bovis B., Planche J., Birg F. Stabilization of the large T protein in temperature-independent (type A) FR 3T3 rat cells transformed with the simian virus 40 tsA30 mutant. J Virol. 1983 Sep;47(3):442–451. doi: 10.1128/jvi.47.3.442-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert J., Lawrence J. J., Birg F. Simian virus 40 T antigen is detected only in cells in the G2 phase of the cell cycle in one group of rat transformants. Virology. 1983 Apr 30;126(2):711–716. doi: 10.1016/s0042-6822(83)80028-2. [DOI] [PubMed] [Google Scholar]
- Imbert J., Lawrence J. J., Coulier F., Jeunet E., Billotey V., Birg F. Cell cycle-dependent expression of early viral genes in one group of simian virus 40-transformed rat cells. EMBO J. 1984 Nov;3(11):2587–2591. doi: 10.1002/j.1460-2075.1984.tb02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., DeLeo A. B., Appella E., Dubois G. C., Law L. W., Khoury G., Old L. J. A common transformation-related protein in murine sarcomas and leukemias. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):659–664. doi: 10.1101/sqb.1980.044.01.069. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Luka J., Klein G., Appella E. A 53-kilodalton protein common to chemically and virally transformed cells shows extensive sequence similarities between species. Proc Natl Acad Sci U S A. 1982 Jan;79(2):287–291. doi: 10.1073/pnas.79.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cell immortalization and transformation by the p53 gene. Nature. 1984 Dec 13;312(5995):596–597. doi: 10.1038/312596a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lowe J., Bird P., Hardie D., Jefferis R., Ling N. R. Monoclonal antibodies (McAbs) to determinants on human gamma chains: properties of antibodies showing subclass restriction or subclass specificity. Immunology. 1982 Oct;47(2):329–336. [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Chaudry F., Harvey R., Smith R., Rigby P. W., Paucha E., Smith A. E. T antigens of SV40-transformed cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):171–178. doi: 10.1101/sqb.1980.044.01.020. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Chandrasekaran K., Hoffman J. C., McFarland V. W. Quantitation of a 55K cellular protein: similar amount and instability in normal and malignant mouse cells. Mol Cell Biol. 1982 Jul;2(7):763–771. doi: 10.1128/mcb.2.7.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M., Bienz B., Givol D., Rechavi G., Zakut R. Analysis of recombinant DNA clones specific for the murine p53 cellular tumor antigen. EMBO J. 1983;2(10):1633–1639. doi: 10.1002/j.1460-2075.1983.tb01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Perbal B., Cuzin F. Growth control in simian virus 40-transformed rat cells: temperature-independent expression of the transformed phenotype in tsA transformants derived by agar selection. J Virol. 1978 Oct;28(1):1–5. doi: 10.1128/jvi.28.1.1-5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]
- Wade-Evans A., Jenkins J. R. Precise epitope mapping of the murine transformation-associated protein, p53. EMBO J. 1985 Mar;4(3):699–706. doi: 10.1002/j.1460-2075.1985.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]