Abstract
Objective
To determine the association of resistance exercise, independent of and combined with aerobic exercise, with the risk of developing metabolic syndrome (MetS).
Patients and Methods
This cohort includes adults (mean age, 46; SD, 9.5) who received comprehensive medical examinations at the Cooper Clinic in Dallas, Texas during January 1st, 1987 and December, 31st, 2006. Exercise was assessed by self-reported frequency and minutes per week of resistance and aerobic exercise, and meeting the US Physical Activity Guidelines (resistance exercise ≥2 days/week; aerobic exercise ≥500 MET-minutes/week) at baseline. The incidence of MetS was based on the National Cholesterol Education Program Adult Treatment Panel III criteria. We used Cox regression to generate hazard ratios (HRs) and 95% confidence intervals.
Results
Among 7418 participants, 15% (n=1147) developed MetS during a median follow-up of 4 years (max 19, min 0.1). Meeting the resistance exercise guidelines was associated with a 17% lower risk of MetS (HR, 0.83; 95% CI, 0.73–0.96; P=.009) after adjusting for potential confounders and aerobic exercise. Further, less than one hour of weekly resistance exercise was associated with 29% lower risk of developing MetS (HR, 0.71; 95% CI, 0.56–0.89; P=.003), compared to no resistance exercise. However, larger amounts of resistance exercise did not provide further benefits. Individuals meeting both recommended resistance and aerobic exercise guidelines had a 25% lower risk of developing MetS (HR, 0.75; 95% CI, 0.63–0.89; P<.001), compared to meeting neither guidelines.
Conclusions
Participating in resistance exercise, even less than one hour per week, was associated with a lower risk of developing MetS, independent of aerobic exercise. Health professionals should recommend patients to perform resistance exercise along with aerobic exercise to reduce MetS.
One third of US adults have metabolic syndrome (MetS)1. Cardiometabolic disorders, such as glucose intolerance, insulin resistance, central obesity, dyslipidemia, and hypertension are its key components2, 3. Therefore, MetS is an important risk factor for type 2 diabetes mellitus4, 5 and cardiovascular diseases (CVD)6, 7. Increasing physical activity (PA) is a cornerstone for preventing and treating MetS3, 8. Several intervention studies have shown the benefits of aerobic exercise for improving metabolic risk factors9, 10. Previous studies, mostly cross-sectional, have identified negative associations of muscular strength11–14 or resistance exercise15–17 with the prevalence of MetS. Furthermore, recent cohort studies have indicated that higher levels of resistance exercise were associated with lower risks of type 2 diabetes mellitus in men and women18–20, which suggests that increasing resistance exercise might be a potential target for preventing MetS. However, there is very little evidence from large epidemiological studies regarding the effects of resistance exercise on the development of MetS. Therefore, the aim of this study is to examine the association of resistance exercise, independent of/and combined with aerobic exercise, with the risk of developing MetS in relatively healthy middle-aged adults. We hypothesized that resistance exercise lowers the risk of developing MetS and the combination of resistance and aerobic exercise might be stronger associated with lower risk than either one independently.
METHODS
Study Population
The Aerobics Center Longitudinal Study is a cohort of men and women, who received extensive preventive medical examinations at the Cooper Clinic in Dallas, Texas during January 1st, 1987 and December 31st, 2006. Among 10 243 participants, we excluded 836 individuals with a history of myocardial infarction, stroke, or cancer and 1989 individuals with MetS at baseline. Our final sample included 7418 individuals (19% women). The participants were predominantly non-Hispanic whites (>95%), well educated, and employed in, or retired from, professional or executive positions21. The Cooper Institute institutional review board annually approved the study, and written informed consents were obtained from participants before data collection at baseline and during follow-up examinations.
Clinical examination
All participants performed comprehensive medical examinations at baseline, including body composition assessments, blood chemistry analyses, blood pressure measurements, electrocardiography, physical examination, and detailed medical history questionnaire. Body mass index (BMI) was calculated from measured weight and height squared (kg/m2). Waist circumference was measured with anthropometric tape at the umbilicus level. Blood chemistry analyses, measuring triglycerides, high-density lipoprotein (HDL) cholesterol and fasting glucose, were obtained with automated bioassays after 12-hour fasting. Resting systolic and diastolic blood pressure were measured by standard auscultatory methods after 5 minutes of seated rest, and calculated as the average of at least two readings separated by 2 minutes.
Age, gender, smoking status, alcohol consumption, personal history of physician-diagnosed CVD, cancer, and parental history of CVD, hypertension, and diabetes were assessed by a medical history questionnaire. Heavy alcohol drinking was defined as >14 and >7 alcoholic drinks per week for men and women, respectively22. The medical history questionnaire included a PA questionnaire containing self-reported leisure-time PA or recreational PA during the past 3 months. We classified aerobic exercise into four categories: “inactive (0 MET-minutes/week)”, “insufficient (1–499 MET-minutes/week)”, “medium (500–999 MET-minutes/ week)” and “high (≥1000 MET-minutes/week)” based on the 2008 US PA Guidelines23.
Assessment of resistance exercise
Self-reported resistance exercise was assessed in the medical history questionnaire. Participants were asked about the weekly frequency and average exercise duration (minutes) for each session of muscle-strengthening PA using either free weights or weight training machines over the past 3 months. We used frequency (0, 1, 2, 3, 4 or ≥5 times/week) and total amount (0, 1–59, 60–119, 120–179 and ≥180 minutes/week) of resistance exercise, as well as meeting the 2008 PA Guidelines for resistance exercise (≥2 times/week23), as our main exposures. The total amount of resistance exercise was calculated by multiplying frequency of exercise with the average minutes per session.
Ascertainment of MetS
Participants were classified as having MetS using the criteria of the National Cholesterol Education Program Adult Treatment Panel III3 at both baseline and follow-up. MetS was based on the presence of 3 or more of the following risk factors: 1) abdominal or central obesity (waist circumference >102 cm in men, >88 cm in women), 2) fasting hypertriglyceridemia (≥150 mg/dL), 3) low HDL cholesterol (<40 mg/dL in men, <50 mg/dL in women), 4) high blood pressure (≥130/85 mm Hg or history of physician-diagnosed hypertension) and 5) high fasting glucose (≥100 mg/dL or history of physician-diagnosed diabetes). Follow-up time was calculated from the baseline examination to the first event of MetS or the last follow-up examination through 2006 for individuals who did not develop MetS.
Statistical Analysis
Baseline characteristics were summarized as mean and standard deviation (SD) for continuous variables, and as number and percentage (%) for categorical variables. Baseline differences for participants with different amounts of resistance exercise were examined using analyses of variance (ANOVA) for continuous variables and chi-squared tests for categorical variables.
Cox proportional hazard regression was used to compute hazard ratios (HRs) and their 95% confidence intervals (CIs) of MetS across different amounts and frequencies of resistance exercise. Participants who reported no resistance exercise were used as reference category. The regression models were adjusted for age, gender, examination year, BMI, current smoking, heavy alcohol drinking, abnormal electrocardiography, parental history of CVD, hypertension, diabetes, and aerobic exercise (inactive, insufficient, medium, and high). In addition, we examined the independent and combined effects of meeting aerobic (≥500 MET/week23) and/or resistance exercise guidelines on the risk of developing MetS in the combined analyses.
To examine potential effect modification by sex in the association between resistance exercise and incident MetS, we tested interaction terms of sex and resistance exercise using Cox regression. In addition, we compared risk estimates in sex-stratified analyses. We did not find any significant interaction, and trends of developing MetS in men and women were similar. Therefore, we presented the results of pooled analyses. All statistical tests were 2-sided, and significance was set at P<.05. All analyses were conducted using SAS software, version 9.4.
RESULTS
Among 7418 participants, 15% (n=1147) developed MetS during a median follow-up of 4 years (maximum 19 years; Table 1). Among individuals who participated in resistance exercise (n=2785, 38%), resistance exercise was most frequently performed for 60–119 minutes per week (n=1061, 38%). Compared to individuals not performing resistance exercise, individuals with higher levels of resistance exercise were more likely to be younger, leaner (lower BMI and waist circumference), and aerobically active. However, the proportion of men decreased with higher levels of resistance exercise. Individuals who participated in resistance exercise were also less likely to smoke and had more favorable lipids profile (lower triglycerides and higher HDL cholesterol; all P<.05).
Table 1.
Baseline characteristics of the 7418 participants by the incidence of metabolic syndrome and weekly minutes of resistance exercise.
| Characteristics | Weekly minutes of resistance exercise (min/week) | P value | ||||
|---|---|---|---|---|---|---|
| 0 (n=4633) | 1–59 (n=670) | 60–119 (n=1061) | 120–179 (n=502) | ≥180 (n=552) | ||
| Age | 46.7 (9.7) | 45.9 (8.3) | 46.2 (9.0) | 45.1 (9.5) | 43.7 (10.1) | <.001 |
|
| ||||||
| Sex (male) | 3795 (82%) | 568 (85%) | 856 (81%) | 369 (74%) | 446 (81%) | <.001 |
|
| ||||||
| BMI (kg/m2) | 25.3 (3.2) | 24.9 (2.9) | 24.9 (3.0) | 24.8 (3.2) | 24.8 (3.1) | <.001 |
|
| ||||||
| Current smokers | 522 (11%) | 56 (8%) | 93 (9%) | 57 (11%) | 60 (11%) | .04 |
|
| ||||||
| Heavy alcohol drinking | 562 (12%) | 77 (11%) | 132 (12%) | 60 (12%) | 64 (12%) | .98 |
|
| ||||||
| Aerobic exercise (MET-min/week) | <.001 | |||||
| 0 | 1125 (24%) | 40 (6%) | 45 (4%) | 32 (6%) | 42 (8%) | |
| 1–499 | 708 (15%) | 89 (13%) | 117 (11%) | 65 (13%) | 73 (13%) | |
| 500–999 | 899 (19%) | 135 (20%) | 246 (23%) | 115 (23%) | 95 (17%) | |
| ≥ 1000 | 1901 (41%) | 406 (61%) | 653 (62%) | 290 (58%) | 342 (62%) | |
|
| ||||||
| Abnormal ECG | 387 (8%) | 53 (8%) | 69 (7%) | 29 (6%) | 34 (6%) | .05 |
|
| ||||||
| Parental history of cardiovascular disease | 1177 (25%) | 162 (24%) | 255 (24%) | 130 (26%) | 132 (24%) | .79 |
|
| ||||||
| Parental history of hypertension | 1602 (35%) | 251 (37%) | 379 (36%) | 191 (38%) | 184 (33%) | .28 |
|
| ||||||
| Parental history of diabetes | 632 (14%) | 91 (14%) | 134 (13%) | 61 (12%) | 67 (12%) | .71 |
|
| ||||||
| Metabolic syndrome | ||||||
|
| ||||||
| Waist circumference (cm) | 88.5 (11.0) | 86.8 (10.3) | 86.0 (10.7) | 84.5 (11.1) | 84.9 (10.3) | <.001 |
|
| ||||||
| Triglycerides (mg/dL) | 103.4 (59.6) | 92.9 (44.3) | 97.0 (52.4) | 96.0 (53.1) | 94.4 (59.6) | <.001 |
|
| ||||||
| HDL cholesterol (mg/dL) | 53.0 (14.5) | 55.0 (14.6) | 55.1 (14.4) | 56.6 (14.7) | 54.9(14.3) | <.001 |
|
| ||||||
| Systolic blood pressure (mm Hg) | 119 (13) | 119 (13) | 119 (13) | 119 (13) | 120 (13) | .67 |
|
| ||||||
| Diastolic blood pressure (mm Hg) | 79 (9) | 80 (9) | 79 (9) | 79 (10) | 80 (9) | .62 |
|
| ||||||
| Fasting glucose (mg/dL) | 96.2 (10.1) | 96.5 (13.0) | 96.2 (11.9) | 95.6 (11.4) | 96.2 (13.3) | .77 |
Data is presented in mean (SD) unless indicated as n (%).
Performing any resistance exercise was associated with a 17% lower risk of developing MetS (HR, 0.83; 95% CI, 0.72–0.95; P=.006) after adjusting for potential confounders, including aerobic exercise levels in the fully adjusted model 3 (Table 2). Meeting the resistance exercise guidelines had a similar 17% lower risk of MetS (HR, 0.83; 95% CI, 0.73–0.96; P=.009) in the full model (model 3). Furthermore, we found that resistance exercise at 1–59, 60–119, 120–179, and ≥180 minutes per week were all associated with lower HRs for MetS (all P<.05), compared to no resistance exercise; after adjusting for age, gender, and examination year (model 1). However, after further adjustment for other potential confounders and aerobic exercise levels (model 3), only 1–59 minutes per week of resistance exercise was associated with a 29% reduced risk of MetS (HR, 0.71; 95%CI, 0.56–0.89; P=.003). We also found that four days per week of resistance exercise was associated with a 38% lower risk of developing MetS (HR, 0.62; 95%CI, 0.44–0.89; P=.009), compared to no resistance exercise in the fully adjusted model (model 3). In additional analyses after further adjustment for the number of MetS risk factors (0, 1, or 2) at baseline, the results were virtually the same, in that the risk of developing MetS was 14% lower in individuals performing any resistance exercise (HR, 0.86; 95% CI, 0.75–0.98; P=.02), 14% lower in individuals meeting the recommended guidelines (HR, 0.86; 95% CI, 0.75–0.99; P=.03), 26% lower in individuals performing <1 hour per week (HR, 0.74; 95% CI, 0.58–0.93; P=.01), and 33% lower in individuals performing 4 times per week (HR, 0.67; 95% CI, 0.47–0.95; P=.03) resistance exercise.
Table 2.
Hazard ratios of metabolic syndrome by weekly frequency and minutes of resistance exercise.
| N (%) | No. of cases | Adjusted Hazard Ratio | |||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| Weekly minutes of resistance exercise (min/week) | |||||
| 0 | 4633 (62%) | 816 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
|
| |||||
| 1–59 | 670 (9%) | 80 | 0.62 (0.49–0.78) | 0.69 (0.55–0.86) | 0.71 (0.56–0.89) |
|
| |||||
| 60–119 | 1061 (14%) | 141 | 0.83 (0.69–0.99) | 0.93 (0.77–1.11) | 0.96 (0.80–1.16) |
|
| |||||
| 120–179 | 502 (7%) | 51 | 0.66 (0.50–0.88) | 0.78 (0.59–1.04) | 0.81 (0.61–1.07) |
|
| |||||
| ≥180 | 552 (7%) | 59 | 0.65 (0.50–0.85) | 0.76 (0.58–0.99) | 0.78 (0.60–1.02) |
|
| |||||
| P-trend | <.001 | .006 | .03 | ||
|
| |||||
| Any resistance exercise | |||||
|
| |||||
| No (0 min/week) | 4633 (62%) | 816 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
|
| |||||
| Yes (≥1 min/week) | 2785 (38%) | 331 | 0.71 (0.62–0.80) | 0.80 (0.71–0.91) | 0.83 (0.72–0.95) |
|
| |||||
| Weekly frequency of resistance exercise (frequency/week) | |||||
|
| |||||
| 0 | 4633 (62%) | 816 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
|
| |||||
| 1 | 206 (3%) | 22 | 0.80 (0.53–1.23) | 0.81 (0.53–1.24) | 0.83 (0.54–1.27) |
|
| |||||
| 2 | 766 (10%) | 83 | 0.72 (0.58–0.91) | 0.81 (0.65–1.02) | 0.84 (0.67–1.06) |
|
| |||||
| 3 | 1221 (16%) | 163 | 0.77 (0.65–0.91) | 0.86 (0.72–1.01) | 0.88 (0.74–1.05) |
|
| |||||
| 4 | 339 (5%) | 32 | 0.48 (0.34–0.68) | 0.60 (0.42–0.86) | 0.62 (0.44–0.89) |
|
| |||||
| ≥ 5 | 253 (3%) | 31 | 0.66 (0.46–0.95) | 0.79 (0.55–1.13) | 0.81 (0.56–1.16) |
|
| |||||
| P-trend | <.001 | .001 | .005 | ||
|
| |||||
| Recommended resistance exercise | |||||
|
| |||||
| No (<2 days/week) | 4839 (65%) | 838 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
|
| |||||
| Yes (≥2 days/week) | 2579 (35%) | 309 | 0.71 (0.62–0.80) | 0.81 (0.71–0.92) | 0.83 (0.73–0.96) |
Adjusted for age, gender and examination year.
Adjusted for model 1 plus body mass index, current smoking, heavy alcohol drinking, abnormal electrocardiography, parental history of cardiovascular disease, hypertension, and diabetes.
Adjusted for model 2 plus aerobic exercise (inactive, insufficient, medium and high).
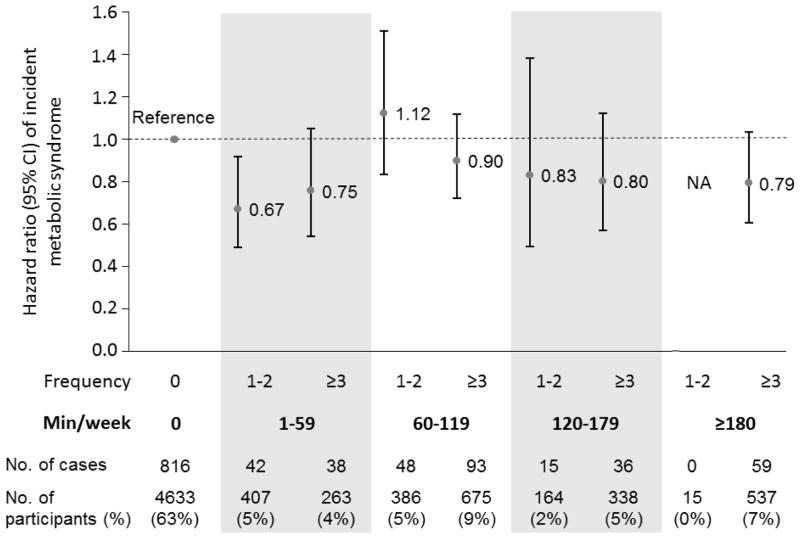
In addition, we examined the risk of MetS among individuals with the same total amount of weekly resistance exercise (minutes/week), but at different frequencies (1–2 vs ≥3 times/week). For example, some people may perform 2 hours of weekly resistance exercise in one or two sessions, especially during weekends (so-called “weekend warriors”), whereas others may perform the same 2 hours of weekly resistance exercise in more than 2 sessions. In the result, the joint analysis of frequency and the total amount of resistance exercise (Figure 1) did not show any significant differences in the risk of developing MetS between less frequent (1–2 times/week) and more frequent (≥3 times/week) exercisers among individuals with the same total amount of weekly resistance exercise. However, we observed a 33% lower risk of developing MetS (HR, 0.67; 95%CI, 0.49–0.91; P=.01) in individuals who performed resistance exercise 1–2 times per week with a total exercise amount of 1–59 minutes per week.
Figure 1.
Hazard ratios of metabolic syndrome by the combination of weekly frequency (1–2 vs ≥3 times/week) and minutes of resistance exercise (0, 1–59, 60–119, 120–179 and ≥180 min/week). The dots indicate hazard ratios and the lines present 95% confidence intervals. The model was adjusted for age (years), gender, examination year (year), body mass index (kg/m2), current smoking (yes/no), heavy alcohol drinking (yes/no), abnormal electrocardiography (yes/no), parental history of cardiovascular, hypertension, diabetes (yes/no for each), and aerobic exercise (inactive, insufficient, medium and high). Analysis in the category of ≥180 minutes in 1–2 sessions resistance exercise per week was not applicable (NA).
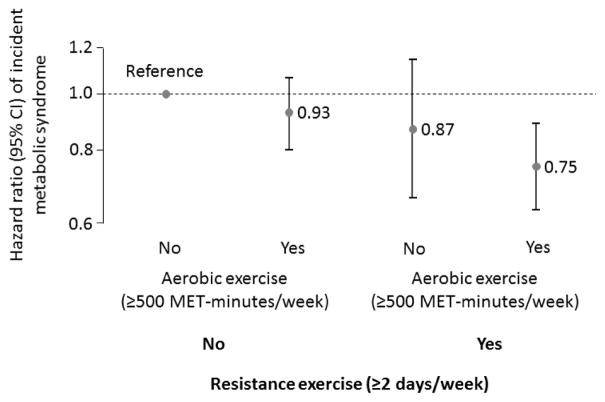
Figure 2 shows the independent and combined associations of meeting the resistance and/or aerobic exercise guidelines with incident MetS. We found that individuals meeting both recommended resistance and aerobic exercise guidelines had a 25% lower risk of developing MetS (HR, 0.75; 95% CI, 0.63–0. 89; P<.001), compared to individuals meeting neither guidelines.
Figure 2.
Hazard ratios of metabolic syndrome by meeting the 2008 US Physical Activity Guidelines for resistance (≥2 days/week) and aerobic activities (≥500 MET-minutes/week) at baseline. The bars present hazard ratios (95% confidence intervals). The model was adjusted for age (years), gender, examination year (year), body mass index (kg/m2), current smoking (yes/no), heavy alcohol drinking (yes/no), abnormal electrocardiography (yes/no), and parental history of cardiovascular disease, hypertension and diabetes (yes/no for each).
DISCUSSION
This large cohort study yielded three major study findings. First, we demonstrated that participating in resistance exercise, independent of aerobic exercise, significantly decreases the risk of developing MetS, compared to no resistance exercise in a middle-aged relatively healthy population. Specifically, less than one hour per week of resistance exercise resulted in significantly lower risk of MetS compared to no resistance exercise. However, higher volumes of resistance exercise did not provide further benefits (Table 2), suggesting against the “more is better” philosophy. Second, the combined analysis of weekly frequency and total amount of resistance exercise (Figure 1) showed no effect of exercise frequency in incident MetS at a given total volume of resistance exercise. Therefore, resistance exercise for less than one hour per week, regardless of training frequency, may be important in preventing MetS. Third, meeting both resistance and aerobic exercise guidelines was associated with 25% lower risk of developing MetS, compared to meeting neither of these guidelines (Figure 2). This suggests additional benefits of doing both resistance and aerobic exercise for the prevention of MetS.
Previous studies have indicated a negative association of muscular strength and MetS, which was still present after adjusting for aerobic fitness12. However, the protective effect of muscular strength against MetS might be explained by regular participation in resistance exercise, because resistance exercise is a major determinant of muscular strength24, 25. Cross-sectional studies of muscle-strengthening PA have also reported a negative association with the prevalence of MetS15–17, which is in line with our findings. Nevertheless, those prior studies only investigated the effect of participating in resistance exercise (yes/no) or meeting the resistance exercise guidelines (yes/no). On the other hand, our study further examined the dose-response relationship between resistance exercise and incident MetS across different weekly frequencies and total amounts of resistance exercise. In addition, we also examined the independent and combined effects of resistance and aerobic exercise on the development of MetS.
Several studies have investigated the associations between resistance exercise and type 2 diabetes mellitus, another common metabolic disease. Grontved et al. found a reduced risk of type 2 diabetes mellitus by performing less than one hour of resistance exercise per week in 32 000 men and 99 000 women 19, 20. In addition, they showed that a combination of aerobic and resistance exercise was superior in preventing type 2 diabetes mellitus. We found similar results for the prevention of MetS. Further, they found a linear dose-response relationship between the amount of resistance exercise and the risk of incident type 2 diabetes mellitus. In contrast, however, we did not observe a linear dose-response relationship between resistance exercise and the risk of developing MetS, suggesting against the “more is better” hypothesis regarding resistance exercise and development of MetS. However, this might be at least partially due to the smaller sample size and number of cases in our study. It is also possible that resistance exercise dose-response curves may be different between MetS and type 2 diabetes mellitus. These contradictory findings suggest that further investigations on dose-response relationships between resistance exercise and different health outcomes are clearly warranted. We also investigated the dose-response relationship between the frequency of resistance exercise and risk of MetS, demonstrating significant benefits of four times per week resistance exercise. However, this result is somewhat complicated since the frequency does not necessarily fully represent the total amount of resistance exercise. Therefore, the prescription of frequency in the current resistance exercise guidelines may lack sufficient detail, whereas a prescription of total minutes per week might be more appropriate.
The current study demonstrated that there is no significant difference in the risk of MetS between 1–59 and ≥180 minutes per week of resistance exercise, which suggests no additional benefits of higher levels of resistance exercise on the development MetS. In addition, the dose-response relationship between resistance exercise and MetS may not be linear, but reverse J-shaped, which has been found in studies regarding aerobic exercise and CVD health26–28. Although it is not clear why there are no further benefits on incident MetS by increasing the amount of resistance exercise, it may be related to no significant differences in blood pressure and fasting glucose across different amounts of resistance exercise, as shown in Table 1. However, more favorable lipid profiles (Triglycerides and HDL cholesterol) by increasing resistance exercise (Table 1) may partially explain the benefits of resistance exercise on the development of MetS since blood lipids are the components of MetS. Furthermore, additional analyses did not show significant differences in risk of MetS in individuals performing weekly 1–59 minutes resistance exercise for less than one year and more than one year (P>.05). A possible explanation could be the absence of training progression (no gradual increase in amount and/or intensity of resistance exercise) after a certain period, which results in a stabilization of the muscle mass and strength, and therefore no further health benefits. Future studies of long-term resistance exercise training with different doses and intensities are therefore needed to determine the protection against MetS as well as CVD.
In 2004, Lee et al.29 introduced the concept of ‘weekend warriors’, individuals who meet the aerobic exercise guidelines but performed their PA in 1–2 days per week, possibly during weekends. They demonstrated that ‘weekend warriors’ still had mortality benefits, compared to sedentary individuals, but their benefits were less, compared to individuals who were regularly physically active, especially in individuals with major CVD risk factors, such as smoking, overweight, and hypertension. In our study there was no effect of increased frequency with the same amount of resistance exercise (all P>.05). Nevertheless, only individuals performing 1–59 minutes of resistance exercise in 1–2 sessions per week had significantly lower risk of MetS, compared to no resistance exercise. This suggests that even a relatively small amount of resistance exercise once or twice per week may be enough to maximally reduce the risk of MetS, at least from the resistance exercise perspective. However, it should be mentioned that the sample sizes and number of cases were smaller in categories with higher levels of resistance exercise, which reduced the statistical power in these groups.
MetS is more prevalent in older and overweight individuals1. However, subgroup analyses in our study appear to show similar negative trends, although not significant, for resistance exercise and MetS in different BMI (<25 vs ≥25 kg/m2) and age (<50 vs ≥50 years old) groups (data not shown). The lack of statistically significance was probably due to the small number of participants and MetS cases across these strata. Nevertheless, the reduced risk of MetS by resistance exercise remained significant after adjusting for BMI and age, and shows consistency in our findings.
The strengths of this study include a large cohort with a relatively long follow-up time. Furthermore, we believe that this is the first prospective study that investigated the association between resistance exercise and incident MetS. However, limitations of our study include self-reported data on PA, which may cause measurement errors due to over-reporting of leisure-time PA30. Nevertheless, over-reporting generally causes an underestimation of the true effect of exercise on health outcomes31. Only baseline levels of PA were used for the analyses, therefore changes in PA patterns were not included in the study. Our study includes primarily well-educated non-Hispanic whites from middle-to-upper socioeconomic strata, which may limit the generalizability of the results, thus the findings may be different in other populations. On the other hand, homogeneity in ethnicity and socio-economic status reduces potential confounding by race/ethnicity, education, and income. Physiological characteristics of this cohort are also similar to other representative population samples21. Another limitation is that we had no information about medications to take into account in the analyses. Although we adjusted for potential confounders such as medical conditions (e.g., hypertension, diabetes, and abnormal electrocardiography) and lifestyle factors (e.g., smoking, alcohol intake, and body mass index), randomized controlled trials of resistance exercise are warranted to remove those confounding biases in the future.
CONCLUSION
Meeting the resistance exercise guidelines, independent of aerobic exercise, decreases the risk of developing MetS in a middle-aged adult population. Especially, relatively smaller amounts of resistance exercise, less than one hour in 1–2 sessions per week as could be seen in the “weekend warrior” profile, resulted in the highest reduction in the risk of developing MetS, compared to no resistance exercise. Also, meeting both resistance and aerobic exercise guidelines is superior in preventing MetS. Therefore, resistance exercise, independent of/and combined with aerobic exercise, should be included in one’s PA routine for the prevention of MetS. Clinicians should routinely recommend resistance exercise training, in addition to aerobic training, for the prevention of MetS and future CVD risk. Especially, individuals with CVD risk factors should consider more individualized, safe and effective exercise program under the direction of a qualified exercise professional.
Acknowledgments
Funding/Support: This study was supported by the National Institutes of Health grants (AG06945, HL62508, DK088195, and HL133069).
Additional Contributions: The authors thank the Cooper Clinic physicians and technicians for collecting the baseline data and staff at the Cooper Institute for data entry and data management.
List of abbreviations
- BMI
Body Mass Index
- CI
Confidence intervals
- CVD
Cardiovascular disease
- HDL
High-density lipoprotein cholesterol
- HR
Hazard ratios
- MetS
Metabolic Syndrome
- PA
Physical activity
- SD
Standard deviation
Footnotes
Role of the Funder/Sponsor: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Disclosures: Steven N. Blair has received unrestricted research grants from The Coca-Cola Company, but these grants were not used to support this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2(3):180–93. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 4.DeFina LF, Vega GL, Leonard D, Grundy SM. Fasting glucose, obesity, and metabolic syndrome as predictors of type 2 diabetes: the Cooper Center Longitudinal Study. J Investig Med. 2012;60(8):1164–8. doi: 10.2310/JIM.0b013e318275656a. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31(9):1898–904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–14. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training programs in metabolic syndrome and diabetes. J Cardiopulm Rehabil. 2005;25(2):59–66. doi: 10.1097/00008483-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Pattyn N, Cornelissen VA, Eshghi SR, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta-analysis of controlled trials. Sports Med. 2013;43(2):121–33. doi: 10.1007/s40279-012-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT) Am J Cardiol. 2011;108(6):838–44. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijndaele K, Duvigneaud N, Matton L, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39(2):233–40. doi: 10.1249/01.mss.0000247003.32589.a6. [DOI] [PubMed] [Google Scholar]
- 12.Jurca R, Lamonte MJ, Church TS, et al. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36(8):1301–7. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- 13.Jurca R, Lamonte MJ, Barlow CE, et al. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37(11):1849–55. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 14.Artero EG, Lee DC, Lavie CJ, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32(6):351–8. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magyari PM, Churilla JR. Association between lifting weights and metabolic syndrome among U.S. Adults: 1999–2004 National Health and Nutrition Examination Survey. J Strength Cond Res. 2012;26(11):3113–7. doi: 10.1519/JSC.0b013e3182472f95. [DOI] [PubMed] [Google Scholar]
- 16.Churilla JR, Magyari PM, Ford ES, Fitzhugh EC, Johnson TM. Muscular strengthening activity patterns and metabolic health risk among US adults. J Diabetes. 2012;4(1):77–84. doi: 10.1111/j.1753-0407.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churilla JR, Johnson TM, Magyari PM, Crouter SE. Descriptive analysis of resistance exercise and metabolic syndrome. Diabetes Metab Syndr. 2012;6(1):42–7. doi: 10.1016/j.dsx.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Crump C, Sundquist J, Winkleby MA, Sieh W, Sundquist K. Physical Fitness Among Swedish Military Conscripts and Long-Term Risk for Type 2 Diabetes Mellitus: A Cohort Study. Ann Intern Med. 2016 doi: 10.7326/M15-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grontved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med. 2012;172(17):1306–12. doi: 10.1001/archinternmed.2012.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grontved A, Pan A, Mekary RA, et al. Muscle-strengthening and conditioning activities and risk of type 2 diabetes: a prospective study in two cohorts of US women. PLoS Med. 2014;11(1):e1001587. doi: 10.1371/journal.pmed.1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness. Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129(6):1145–56. doi: 10.1093/oxfordjournals.aje.a115236. [DOI] [PubMed] [Google Scholar]
- 22.National Institute on Alcohol Abuse and Alcoholism. NIH Publication No. 05–5737. 2006. Alcohol use and alcohol use disorders in the United States: Main findings from the 2001–2002 National Epidemiologic survey on alcohol and related conditions (NESARC) [Google Scholar]
- 23.US Department of Health and Human Services. Physical Activity Guidelines for Americans. Washington (DC): US Department of Health and Human Services; 2008. [Accessed December 2, 2016]. http://www.health.gov/PAGuidelines. [Google Scholar]
- 24.Thomis MA, Beunen GP, Maes HH, et al. Strength training: importance of genetic factors. Med Sci Sports Exerc. 1998;30(5):724–31. doi: 10.1097/00005768-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116(5):572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 26.Eijsvogels TM, Molossi S, Lee DC, Emery MS, Thompson PD. Exercise at the Extremes: The Amount of Exercise to Reduce Cardiovascular Events. J Am Coll Cardiol. 2016;67(3):316–29. doi: 10.1016/j.jacc.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Lavie CJ, O'Keefe JH, Sallis RE. Exercise and the heart--the harm of too little and too much. Curr Sports Med Rep. 2015;14(2):104–9. doi: 10.1249/JSR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 28.Lavie CJ, Arena R, Swift DL, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117(2):207–19. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr The “weekend warrior” and risk of mortality. Am J Epidemiol. 2004;160(7):636–41. doi: 10.1093/aje/kwh274. [DOI] [PubMed] [Google Scholar]
- 30.Adams SA, Matthews CE, Ebbeling CB, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005;161(4):389–98. doi: 10.1093/aje/kwi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celis-Morales CA, Perez-Bravo F, Ibanez L, et al. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7(5):e36345. doi: 10.1371/journal.pone.0036345. [DOI] [PMC free article] [PubMed] [Google Scholar]