Abstract
Aim
This study aimed to explore the potential of detecting hepatocellular carcinoma (HCC)-associated DNA markers, TP53 249T mutations and aberrant methylation of RASSF1A and GSTP1 genes, for monitoring HCC recurrence. HCC remains a leading cause of death worldwide, with one of the fastest growing incidence rates in the US. While treatment options are available and new ones emerging, there remains a poor prognosis of this disease mostly due to its late diagnosis and high recurrence rate. Although there are no specific guidelines addressing how HCC recurrence should be monitored, recurrence is usually monitored by serum-alpha fetal protein and imaging methods such as magnetic resonance imaging (MRI). However, early detection of recurrent HCC remains limited, particularly at the site of treated lesion.
Methods
Here, the authors followed 10 patients that were treated for a primary HCC, and monitored for months or years later. At these follow-up visits, urine was collected and tested retrospectively for 3 DNA biomarkers that associate with HCC development.
Results
This 10-patient study compared detection of urine DNA markers with MRI for monitoring HCC recurrence. Five patients were confirmed by MRI for recurrence, and all 5 had detectable DNA biomarkers up to 9 months before recurrence confirmation by MRI.
Conclusion
Overall, this suggests that detection of HCC-associated DNA markers in urine could provide a promising tool to complement detection of recurrent HCC by imaging.
Keywords: Hepatocellular carcinoma, recurrence, circulating-tumor DNA, liver cancer, biomarker, urine tumor marker, hepatitis B virus
INTRODUCTION
Liver cancer is the sixth most common malignant neoplasm in the world and the second leading cause of cancer death worldwide, with an estimated 782,000 new liver cancer cases and 746,000 deaths during 2012.[1] Hepatocellular carcinoma (HCC) constitutes 70–85% of all types of liver cancer.[2] The high mortality rate of HCC (where 85% of patients die within 5 years) is mainly due to late detection and a high recurrence rate.[1–5] Rates of recurrence range from 15% for liver transplantation to nearly 100% for surgery or ablation.[6–10] Recurrence is most common within 2 years.[11]
Recently, a reduced recurrence rate has been reported for hepatitis B virus (HBV)-associated HCC with concomitant antiviral therapy following initial tumor ablation.[12–16] The high HCC recurrence rate can be attributed to (1) incomplete treatment; (2) micro-metastases within the liver; and (3) de novo lesions.[4,17] With improved assay, combination of alpha fetal protein (AFP), lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3%) and des-gamma-carboxyprothrombin (DCP) has been claimed sensitive for HCC surveillance.[18,19] Nonetheless, early detection of recurrent HCC has been difficult with the currently available diagnostic methods and serial imaging.[7–9, 20–22] Notably, there are no specific guidelines addressing how HCC recurrence should be monitored. Magnetic resonance imaging (MRI)/computed tomography (CT) imaging is the gold standard for diagnosis, although it is expensive and has limited utility in the detection of small tumors (< 2 cm), tumors in the presence of previously treated lesions (especially from local ablation), cirrhosis, obesity, and dysplastic nodules.[8,9,20] Thus, there is an urgent unmet medical need to have a sensitive test for monitoring HCC recurrence.
Cancer is a disease of the genome and epigenome, and detection of the underlying genetic mutations and epigenetic modifications in the periphery may allow us to detect cancer early.[23,24] Previously, Su et al.[25–29] demonstrated that fragmented cell-free DNA in urine contains DNA derived from the solid tumors including HCC and colon cancer, if such a tumor is present. They also demonstrated that cancer-related DNA (both mutated and methylated DNA), including HCC-derived DNA modifications, could be detected in the urine of patients with cancer.[27,29–31]
In this study, we demonstrate the feasibility of early detection of recurrent HCC by detecting three known HCC associated DNA modifications: TP53 249T mutation (shortened TP53m), and aberrant promoter methylation of Glutathione S-transferase pi 1 (mGSTP1) and Ras association domain family 1 isoform A (mRASSF1A) genes in urine as compared to the MRI imaging in a small (n = 10) blinded prospective study. These three DNA markers were chosen because of the availability of sensitive, cell-free DNA suitable PCR assays that target frequently altered genes in major pathways associated with hepatocarcinogenesis. They were previously demonstrated to be detectable in body fluids such as blood and urine of patients with HCC, regardless the level of serum AFP.[29,30,32,33] They therefore serve as potential biomarkers for HCC.
METHODS
Patient selection
To explore the potential of the three HCC markers for monitoring HCC recurrence in urine, 10 HCC patients with a history of HBV infection were studied at the Liver Disease Prevention Center, Division of Gastroenterology and Hepatology, Thomas Jefferson University Hospital, Philadelphia. After curative tumor ablation, patients were monitored for recurrence by MRI and serum AFP. Urine specimens were prospectively obtained when available. The urine was retrospectively examined for the presence of the three HCC DNA biomarkers. All patient samples were obtained with written informed consent and under institutional board approval from Thomas Jefferson University Hospital, Philadelphia, PA.
Urine DNA analysis
Urine collection, storage, and DNA isolation were carried out with written informed consent from patients as described previously.[34,35] DNA from specimens was isolated and fractionated to obtain low molecular weight (LMW) urine DNA (< 1 kb size). Bisulfite (BS) treatment of DNA was performed using the EZ DNA Methylation-Lightning™ Kit (Zymo Research, Irvine, CA) following manufacturer’s guidelines. Three DNA modifications, TP53 249T mutation (TP53m), aberrant promoter methylation of GSTP1 (mGSTP1), and aberrant promoter methylation of RASSF1A (mRASSF1A), were quantified in duplicate using assays kits, TP53 249T qPCR kit, mGSTP1 qPCR kit, and mRASSF1A qPCR kit (JBS Science Inc., Doylestown, PA), as per manufacturer specification.
RESULTS
To compare the detection of urine DNA markers to the currently available diagnostic methods (serum AFP and MRI imaging) for the diagnosis of HCC recurrence, urine DNA marker values were measured in a blinded fashion and plotted alongside serum AFP at the time of each collection (as shown in Figure 1 and described in “METHODS”). Briefly, urine samples were collected prospectively from HCC patients (when available) after curative treatment at follow-up visits. The samples were retrospectively analyzed for the HCC DNA biomarkers. For data analysis purposes, we plotted “positive (Pos)” as the time of confirmed recurrence by MRI, and “negative (Neg)” when MRI did not detect recurrence. Of the 10 patients with > 6 months of monitoring with urine DNA markers, cases 1–5 had recurrence of HCC confirmed by MRI.
Figure 1.
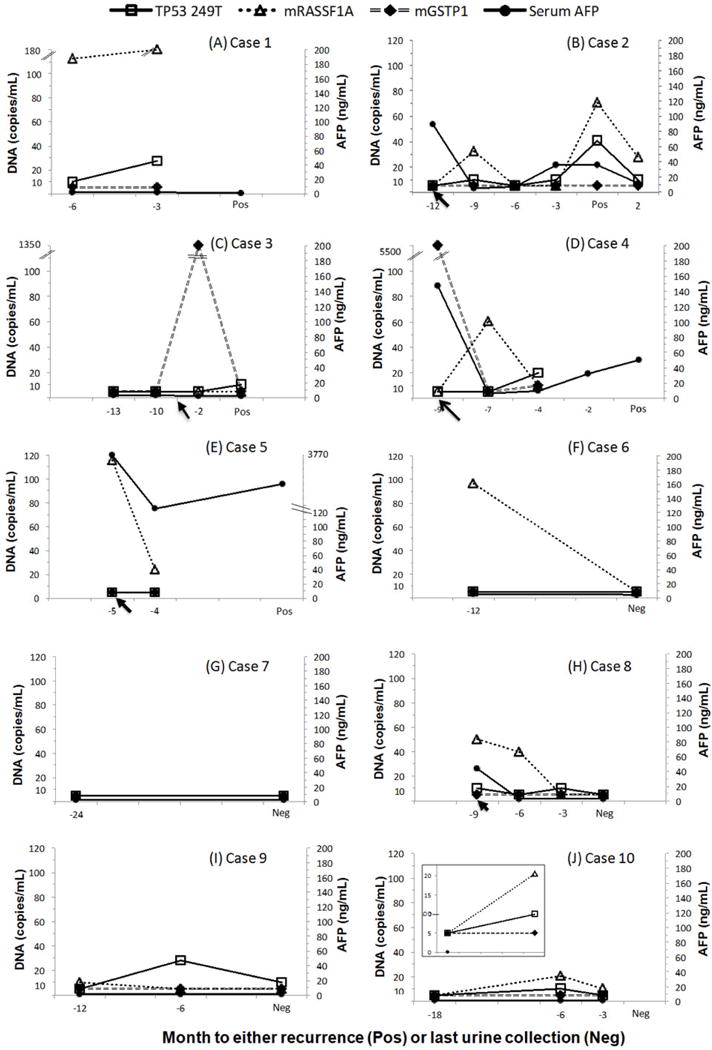
DNA biomarkers levels in serial urine samples from 10 patients. All patients were being monitored for HCC recurrence by MRI and serum AFP. The urine samples were collected prospectively from HCC patients (when available) after curative treatment (indicated by arrows) and at follow-up visits. Samples were retrospectively measured for HCC DNA biomarkers in a blinded fashion, with a follow-up MRI diagnosis of whether or not recurrence was detected. Three DNA biomarker values (copies/mL urine), TP53 249T mutation (TP53m), methylated RASSF1A (mRASSF1A) and methylated GSTP1 (mGSTP1), along with serum AFP (ng/mL serum), were plotted at office visits until the last available visit in which an MRI was performed. The “Pos” represents detection of HCC recurrence by MRI and the “Neg” represents no recurrence was detected by MRI at the time of the visit. HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; AFP: alpha fetal protein
Recurrent patients had one or more of the three DNA markers examined found in urine before or at the time of MRI diagnosis. One recurrent case (case 5) died of progressive HCC. Case 6 was lost for follow-up during the period of the study. Four patients (cases 7–10) had no recurrence confirmed by MRI. Their urine DNA markers were either not detected (case 7), fluctuated (case 8), or detected at low levels (cases 9 and 10).
Case 1
A 68-year-old male underwent transarterial chemoembolization (TACE) for HCC. Six years later, he showed tumor recurrence (Pos) by MRI. Urine specimens were obtained at 6 and 3 months prior to the MRI confirmation of recurrence (indicated as −6, −3 on the X-axis, Figure 1A). In the urine specimens, TP53m and mRASSF1A markers were detected at 6 months prior and increased at 3 months before MRI detection of recurrence. Unfortunately urine is missing at the time of MRI imaging. His serum AFP levels remained at 2 ng/mL throughout the study, indicating the tumor was AFP-negative.[36] He later received liver transplant.
Case 2
A 73-year-old male underwent TACE for HCC. Urine samples were collected after the treatment and during the follow-up period of 12 months when the tumor recurred [Figure 1B]. Three months after the initial TACE treatment (indicated by a black arrow on the X-axis), TP53m and mRASSF1A levels were elevated while serum AFP had returned to a baseline level of 5.3 ng/mL from 88.9 ng/mL at the time of TACE treatment. These two urine DNA markers dropped to baseline on the next visit 3 months later. The TP53m and serum AFP levels rose again about 3 months prior to the detection of recurrence by MRI. At the time of detection of the second recurrence (marked “Pos”), both TP53m and mRASSF1A levels were elevated. Serum AFP level was at 36.4 ng/mL, indicating a rise from the baseline. Two months after the second treatment, serum AFP, TP53 and mRASSF1A all decreased. The patient did not return after this visit.
Case 3
A 55-year-old male with a 4-cm HCC received TACE. The tumor recurred 5 years later, which was treated with microwave ablation (indicated by the black arrow on the X-axis; Figure 1C). The tumor recurred again during a follow up appointment 3 months later (marked “Pos”; Figure 1C). Urine DNA markers at two visits prior to the first recurrence were below the level of detection. However, mGSTP1 was elevated 1 month after microwave treatment. Interestingly, when the tumor recurred for a second time (1.6 cm) 3 months after treatment, the mGSTP1 was undetectable while TP53m was elevated. This may indicate the heterogeneity of HCC. Note, the serum AFP levels were below 20 ng/mL in the period of study.
Case 4
A 54-year-old male diagnosed with HCC and elevated AFP. Urine was collected at the time of diagnosis and treatment with microwave ablation [Figure 1D]. The DNA marker mGSTP1 was highly elevated in urine at the time of HCC diagnosis. Two months after treatment, both urine mGSTP1 and serum AFP levels decreased to the normal range while urine mRASSF1A was elevated. At the next visit 3 months later, mRASSF1A decreased but remained detectable while the two other DNA markers, TP53m and mGSTP1 increased. Four months later, an MRI detected a recurrent tumor (solid lesion). Unfortunately, the urine was not collected at “−2” and at the time of diagnosis “Pos”, hence there is no marker data available at these time points.
Case 5
A 56-year-old male underwent TACE for HCC. Urine was collected on the day of treatment and at a follow-up visit 1 month later [Figure 1E]. The mRASSF1A marker was detected in the urine on the day of TACE treatment, and the levels of mRASSF1A in the urine dropped one month following treatment. Similarly, serum AFP levels decreased nearly 10-fold from 3,770 ng/mL to 323 ng/mL. However, MRI 4 months later detected HCC recurrence and increased levels of serum AFP (1,522 ng/mL). No urine samples were collected at this time point or later. Despite receiving another TACE treatment, the patient passed away 8 months later.
Case 6
A 56-year-old male with HCC underwent TACE. Urine samples were collected at 3 and 4 years after TACE. mRASSF1A was found elevated at 3 years and negative at 4 years post TACE. The patient has had no recurrence [Figure 1F]. AFP was in normal range. The patient was lost for follow up.
Case 7
A 58-year-old male with HCC received TACE followed by radiofrequency ablation (RFA). Urine collection started 1 year after RFA. No biomarkers were detected 2 years post RFA, as the patient remained recurrence free [Figure 1G].
Case 8
A 62-year-old male with HCC received RFA. Urine samples were collected on the day of treatment and every three months after for 9 months [Figure 1H]. Serum AFP, TP53 mutation, and mRASSF1A levels were all elevated on the day of RFA, and decreased 3 and 6 months following the treatment to below the limit of detection. There has been no recurrence by MRI.
Case 9
A 27-year-old female was diagnosed with HCC at age 20 and the original tumor was treated 3 times with TACE in a 3-year period. Urine was collected every 6 months starting 4 years after the last TACE. TP53m mutation was detected in the urine collected on the second visit and decreased, but remained detectable in the third urine sample as indicated in Figure 1I. MRI suggested a mass in the liver, but the mass was not confirmed as recurrent HCC. The serum AFP levels were below 20 ng/mL in the period of study. The patient has been on antiviral treatment since the diagnosis of HCC.
Case 10
A 66-year-old male with HCC underwent RFA followed by resection. He has had no recurrence for the past 10 years. Two urine samples were collected at 8 years (−18) and 9 years (−6) after resection [Figure 1J]. Serum AFP is normal, and none of the DNA markers were detected until 6 months prior to the MRI, when the TP53m and mRASSF1A markers were elevated [Figure 1J]. TP53m reverted to baseline and mRASSF1A levels declined 3 months later (−3). At the time of MRI testing, there was no HCC recurrence detected from the visit.
DISCUSSION
This study demonstrates the potential applicability of using urine DNA markers in combination with serum AFP for the early detection of HCC recurrence in a small 10-case study. HCC recurrence is known to be the major factor for poor prognosis. In this small 10-case study, MRI identified recurrence in 5 out of 10 patients (cases 1–5). Encouragingly, for all 4 recurrent patients that remain in the study (cases 1–4), urine DNA markers were found to be elevated in urine samples as early as 9 months before MRI confirmation.
Although this is a small longitudinal 10 patient study, the potential of these urine DNA markers for management of HCC recurrence and important characteristics of HCC recurrence is demonstrated. First, for all remaining recurrent cases (cases 1–4), DNA markers were elevated before or at the time of diagnosis by MRI imaging. MRI/CT imaging is the gold standard for diagnosis of recurrent HCC, but has difficulty in detecting early recurrence in the previously treated areas (especially after local ablation). This may explain why the DNA markers were found in urine earlier than MRI diagnosis. Secondly, HCC, like other cancers, is a disease of the genome. Detection of genetic drivers of HCC may provide not only sensitive and earlier detection for monitoring HCC recurrence, but may also provide HCC genetic information to assist in patient management. Furthermore, since collection of urine can potentially be done at home and then shipped to certified laboratories for testing, the urine screening may result in better compliance while not requiring a doctor’s office visit. A larger longitudinal study will be needed to explore the application of urine DNA markers in monitoring HCC recurrence. Lastly, the levels of DNA biomarkers in urine may also be useful to measure effectiveness of cancer treatments that induces apoptosis of tumor cells. We have shown that circulating tumor DNA found in urine was mostly from apoptotic tumor cells.[28,34] The treatment that induce apoptosis should increase the amount of tumor derived DNA deposited in the blood and secreted into urine. This could be the circumstance for cases 2, 3 and 4 where an elevated mRASSF1A or mGSTP1 marker was detected after the treatment, suggesting the potential to use urine DNA markers to monitor effectiveness of therapy that induces tumor cell apoptosis.
Finally, HCC is often recognized as being multi-clonal. Interestingly, in recurrent case 3, mGSTP1 levels returned to not detectable in urine while TP53m was elevated in the urine collected 3 months later with the MRI report of a 1.6-cm lesion. We speculate that the rising of the TP53 mutated clone was different from the previously treated tumor nodule and was either not responding to the treatment or was derived from tumor evolution. Furthermore, it is possible that apoptosis of a small tumor nodule through immune system targeting could lead to a temporary rise in DNA markers associating with that tumor. For example, for case 6 the mRASSF1A was found elevated at 3 years and negative at 4 years post TACE, where recurrence was not detected.
It is important to note that the levels of urine DNA markers can fluctuate for several reasons including hydration of the patient at time of collection (which can result in diluted DNA in the urine). Therefore, the use of an internal control is important for appropriately setting cutoffs for the urine marker values. While urine protein creatinine is the most used internal control for urine concentration, our pilot study has suggested the concentration of LMW cell-free DNA in urine does not correlate with urine creatinine. Further studies are needed to identify a proper internal control for this work, which is currently in progress.
In conclusion, we have demonstrated that urine DNA biomarker testing may have potential for the early detection of HCC recurrence. A larger longitudinal study design to collect well-annotated serial patient samples is in progress, specifically to monitor for HCC recurrence, to test whether this urine DNA test can overcome the inherent limitations of imaging technology, and to provide a highly sensitive tool for monitoring HCC recurrence.
Acknowledgments
Financial support and sponsorship
This work has been supported by the National Institute of Health R44 CA165312, RO1 CA202769, and R43 CA192507.
Footnotes
Authors’ contributions
Concept/design: H.W. Hann, S. Jain, W. Song, Y.H. Su
Clinical sample preparation: H.W. Hann, G. Park
Experimental analysis: S. Jain, Y.H. Su
Manuscript preparation: H.W. Hann, S. Jain, W. Song, J. Steffen, Y.H. Su
Manuscript review: H.W. Hann, S. Jain, W. Song, J. Steffen, Y.H. Su
Conflicts of interest
W. Song is an employee and shareholder of JBS Science, Inc.; S. Jain and J. Steffen are employees of JBS Science, Inc; all other authors declare they have no other competing interests that exist.
Patient consent
In this prospective study, each patient was informed of this study and gave their consent.
Ethics approval
This study was conducted under the approval of the Institutional Review Board at Thomas Jefferson University Hospital, Philadelphia, PA.
References
- 1.Hung IF, Wong DK, Poon RT, Fong DY, Chui AH, Seto WK, Fung JY, Chan AC, Yuen JC, Tiu R, Choi O, Lai CL, Yuen MF. Risk factors and post-resection independent predictive score for the recurrence of hepatitis B-related hepatocellular carcinoma. PLoS One. 2016;11:e0148493. doi: 10.1371/journal.pone.0148493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong CC, Lee KF, Ip PC, Wong JS, Cheung SY, Wong J, Ho SC, Lai PB. Pre-operative predictors of post-hepatectomy recurrence of hepatocellular carcinoma: can we predict earlier? Surgeon. 2012;10:260–6. doi: 10.1016/j.surge.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Marrero JA, Pelletier S. Hepatocellular carcinoma. Clin Liver Dis. 2006;10:339–51. doi: 10.1016/j.cld.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045–7. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 5.Lok A, McMahon B. Chronic hepatitis B. Hepatology. 2001;34:1225–41. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 6.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–35. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Suzuki T, Shimamura T, Furukawa H, Matsushita M, Todo S. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol. 2009;16:1560–71. doi: 10.1245/s10434-009-0407-7. [DOI] [PubMed] [Google Scholar]
- 8.Minami Y, Nishida N, Kudo M. Therapeutic response assessment of RFA for HCC: contrast-enhanced US, CT and MRI. World J Gastroenterol. 2014;20:4160–6. doi: 10.3748/wjg.v20.i15.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minami Y, Kudo M. Imaging Modalities for assessment of treatment response to nonsurgical hepatocellular carcinoma therapy: contrast-enhanced US, CT, and MRI. Liver Cancer. 2015;4:106–14. doi: 10.1159/000367733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piao CY, Fujioka S, Iwasaki Y, Fujio K, Kaneyoshi T, Araki Y, Hashimoto K, Senoh T, Terada R, Nishida T, Kobashi H, Sakaguchi K, Shiratori Y. Lamivudine treatment in patients with HBV-related hepatocellular carcinoma – using an untreated, matched control cohort. Acta Med Okayama. 2005;59:217–24. doi: 10.18926/AMO/31969. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–7. [PubMed] [Google Scholar]
- 12.Hann HW, Coben R, Brown D, Needleman L, Rosato E, Min A, Hann RS, Park KB, Dunn S, DiMarino AJ. A long-term study of the effects of antiviral therapy on survival of patients with HBV-associated hepatocellular carcinoma (HCC) following local tumor ablation. Cancer Med. 2014;3:390–6. doi: 10.1002/cam4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BK, Park JY, Kim DY, Kim JK, Kim KS, Choi JS, Moon BS, Han KH, Chon CY, Moon YM, Ahn SH. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28:393–401. doi: 10.1111/j.1478-3231.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 14.Hann HW, Bergin D, Coben R, DiMarino AJ. Prevention of new hepatocellular carcinoma with concomitant antiviral therapy in chronic hepatitis B patients whose initial tumor was successfully ablated. Int J Cancer. 2011;128:739–42. doi: 10.1002/ijc.25382. [DOI] [PubMed] [Google Scholar]
- 15.Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–81. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- 16.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–14. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 17.Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T, Goto H. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929–35. doi: 10.1111/j.1440-1746.2006.04707.x. [DOI] [PubMed] [Google Scholar]
- 18.Hann HW, Li D, Yamada H, Satomura S, Coben R, DiMarino AJ. Usefulness of highly sensitive AFP-L3 and DCP in surveillance for hepatocellular carcinoma in patients with a normal Alpha-Fetoprotein. J Med Microbiol Diagn. 2014;3:1–6. [Google Scholar]
- 19.Carr BI, Kanke F, Wise M, Satomura S. Clinical evaluation of lens culinaris agglutinin-reactive α-fetoprotein and Des-γ-Carboxy prothrombin in histologically proven hepatocellular carcinoma in the United States. Dig Dis Sci. 2007;52:776–82. doi: 10.1007/s10620-006-9541-2. [DOI] [PubMed] [Google Scholar]
- 20.Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311–30. doi: 10.1148/radiol.2472061331. [DOI] [PubMed] [Google Scholar]
- 21.Yu JS. Hepatocellular carcinoma after transcatheter arterial chemoembolization: difficulties on imaging follow-up. Korean J Radiol. 2005;6:134–5. doi: 10.3348/kjr.2005.6.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol. 2013;1:593–8. doi: 10.3892/mco.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin CQ, Yuan CH, Qu Z, Guan Q, Chen H, Wang FB. Liquid biopsy of hepatocellular carcinoma: circulating tumor-derived biomarkers. Dis Markers. 2016;2016:1427849. doi: 10.1155/2016/1427849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan JC, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 25.Su YH, Wang M, Aiamkitsumrit B, Brenner DE, Block TM. Detection of K-ras mutation in urine of patients with colorectal cancer. Cancer Biomarker. 2005;1:177–82. doi: 10.3233/cbm-2005-12-305. [DOI] [PubMed] [Google Scholar]
- 26.Su YH, Wang M, Norton PA, Brenner DE, Block TM. Detection of mutated K-ras DNA in urine, plasma and serum from patients with colorectal carcinoma or adenomatous polyps. Ann N Y Acad Sci. 2008;1137:197–201. doi: 10.1196/annals.1448.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song BP, Jain S, Lin SY, Chen Q, Block TM, Song W, Brenner DE, Su YH. Detection of hypermethylated vimentin in urine of patients with colorectal cancer. J Mol Diagn. 2012;14:112–9. doi: 10.1016/j.jmoldx.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su YH, Wang M, Block TM, Landt O, Botezatu I, Serdyuk O, Lichtenstein A, Melkonyan H, Tomei D, Umansky S. Transrenal DNA as a diagnostic tool: important technical notes. Ann N Y Acad Sci. 2004;1022:81–9. doi: 10.1196/annals.1318.014. [DOI] [PubMed] [Google Scholar]
- 29.Lin SY, Dhillon V, Jain S, Chang TT, Hu CT, Lin YJ, Chen SH, Yu L, Block TM, Su YH. A locked nucleic acid clamp-mediated PCR assay for detection of a p53 codon 249 hotspot mutation in urine. J Mol Diagn. 2011;13:474–84. doi: 10.1016/j.jmoldx.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain S, Xie L, Boldbaatar B, Lin SY, Hamilton JP, Meltzer SJ, Chen SH, Hu CT, Block TM, Song W, Su YH. Differential methylation of the promoter and first exon of the RASSF1A gene in hepatocarcinogenesis. Hepatol Res. 2015;45:1110–23. doi: 10.1111/hepr.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SY, Jain S, Song W, Hu CT, Su YH. Strategic assay developments for detection of HBV 1762T/1764A double mutation in urine of patients with HBV-associated hepatocellular carcinomas. Hepatocellular Carcinoma - Clinical Research. 2012:139–54. [Google Scholar]
- 32.Jain S, Chen S, Chang KC, Lin YJ, Hu CT, Boldbaatar B, Hamilton JP, Lin SY, Chang TT, Chen SH, Song W, Meltzer SJ, Block TM, Su YH. Impact of the location of CpG methylation within the GSTP1 gene on its specificity as a DNA marker for hepatocellular carcinoma. PLoS One. 2012;7:e35789. doi: 10.1371/journal.pone.0035789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su YH, Lin SY, Song W, Jain S. DNA markers in molecular diagnostics for hepatocellular carcinoma. Expert Rev Mol Diagn. 2014;14:803–17. doi: 10.1586/14737159.2014.946908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su YH, Wang M, Brenner DE, Ng A, Melkonyan H, Umansky S, Syngal S, Block TM. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 2004;6:101–7. doi: 10.1016/S1525-1578(10)60497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su YH, Song J, Wang Z, Wang X, Wang M, Brenner DE, Block TM. Removal of high molecular weight DNA by carboxylated magnetic beads enhances the detection of mutated K-ras DNA in urine. Ann N Y Acad Sci. 2008;1137:82–91. doi: 10.1196/annals.1448.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]


