Abstract
Background
CD155, an immunoglobulin-like adhesion molecule, plays an important role in carcinoma such as cells migration, proliferation, metastasis, and tumor immune. The upregulation of CD155 has been found in several human malignancies, but its expression in cholangiocarcinoma (CCA) still remains unclear. The aim of this study is to investigate CD155 expression and its correlations with clinicopathologic data, angiogenesis, and prognosis in the patients with CCA.
Materials and methods
CD155 expression was investigated in 20 paired CCA tissues and corresponding paracancerous tissues by Western blotting and quantitative real-time polymerase chain reaction assays at protein and mRNA levels. Besides, this study evaluated the correlation between the tumor CD155 expression and the level of both vascular endothelial growth factor and intratumoral microvessel density by immunohistochemistry in 90 cases of CCA. Moreover, the clinical and prognostic significance of CD155 in CCA was assessed by immunohistochemistry.
Results
The protein and mRNA levels of CD155 were higher in CCA tumor tissues compared with corresponding paracancerous tissues (P<0.05). Immunohistochemical staining showed that CD155 was located in the cytoplasm of carcinoma cells and overexpressed in 61.2% (55/90) CCA tissues. Obviously, CD155 expression level was significantly correlated with tumor histological grade (P=0.002), lymph node metastasis (P<0.001), and tumor-node-metastasis (P=0.03). Additionally, Spearman rank correlation test demonstrated that CD155 expression was positively associated with vascular endothelial growth factor (r=0.481, P<0.001) and microvessel density (r=0.442, P<0.001) in CCA tissues. More importantly, CCA patients with high CD155 expression had a markedly shorter overall survival (P<0.001) and disease-free survival (P<0.001) after surgical resection, and multivariate analysis showed that high CD155 expression was an independent poor prognostic predictor of overall survival and disease-free survival (P<0.001).
Conclusion
Our results revealed that upregulated CD155 correlated with aggressive clinicopathologic characteristics, angiogenesis, and poor prognosis in CCA and may be a promising prognostic biomarker for the CCA patients.
Keywords: CD155, cholangiocarcinoma, angiogenesis, prognosis, immunohistochemistry
Introduction
Cholangiocarcinoma (CCA), which is the second most common subtype of primary hepatobiliary cancer and accounts for about 3% of gastrointestinal tumors, arises from bile duct epithelial cells.1 It is one of the most aggressive malignancies with an extremely poor prognosis. According to bile duct anatomical position, it can be subdivided into intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma, and distal cholangiocarcinoma.2 Since conventional chemotherapy and radiation therapy have a limited effect on improving long-term survival, currently, radical surgery is the only chance for cure or long-term survival of CCA patients.2–4 Unfortunately, owing to severe local invasion and distant metastases, many patients had missed the optimal timing for radical surgery by the time they are diagnosed with CCA.2–4 Therefore, it is urgently required to explore novel effective tumor markers and therapeutic strategies to improve the diagnosis and prognosis of CCA patients.
CD155, an immunoglobulin (Ig)-like adhesion molecule, is a domain structure composed of one transmembrane region, one extracellular region with three Ig-like loops, and one cytoplasmic region.5 Human CD155/Tage4/Necl-5/poliovirus receptor, first identified as poliovirus receptor in humans, is broadly distributed in a great variety of normal cells and tissues.6,7 Recently, some studies have shown that CD155 is expressed at a relatively high level in a variety of malignant tumors such as colon cancer cells,8 malignant glioma cells,9 pancreatic cancer cells,10 and lung cancer cells.11 As is well known, natural killer (NK) cells, the most essential natural immune cells, play a significant role in tumor immune in human body.12 Also some recent researches demonstrated that CD155 could be recognized by an active receptor called DNAM-1(CD226) on NK cells’ surface and it enhanced the cytotoxicity of immune cells to tumor cells.13,14 Additionally, CD155, apart from its immunological roles, has a vital influence on cell biological functions.5 It has been reported that the downregulation of CD155 in cell lines decreases their migration, proliferation, and metastasis.15–18 Finally, recent findings by Kinugasa et al showed that CD155 may control the interaction of integrin αvβ3 with VEGFR2 and regulate the vascular endothelial growth factor receptor (VEGFR)-induced angiogenesis.19
Against the above background, CD155 overexpression and its impact on cancer cells have been confirmed in different kinds of human tumors by previous studies. However, still there have been no report about CD155 in CCA. In the present study, we detected the expression of CD155 in CCA tumor tissues and surrounding nontumor tissues by immunohistochemistry, Western blotting, and quantitative real-time polymerase chain reaction (qRT-PCR) assays. Expression of vascular endothelial growth factor (VEGF) and CD31 in CCA tumor tissues was also assayed by immunostaining. Additionally, we analyzed the relationship of CD155 expression with VEGF, microvessel density (MVD), clinicopathologic features, and prognosis of the patients.
Materials and methods
Patients and tissue samples
In this study, 90 patients were registered from January 2008 to December 2015 at the Affiliated Provincial Hospital of Anhui Medical University. All patients had undergone curative resection and were pathologically diagnosed with CCA. Neither of them had accepted chemotherapy nor radiotherapy before surgery. Paraffin-embedded tissue samples including tumor tissue specimens and their corresponding paracancerous tissue specimens were obtained from the Department of Pathology and reviewed by two pathologists. CCA tissue beyond a tumor margin of 2 cm was defined as paracancerous tissue. The study was in accordance with the Helsinki Declaration and was approved by the Human Research Ethics Committee of the Affiliated Provincial Hospital of Anhui Medical University. Written informed consent was obtained from all patients for this study.
The clinicopathologic data acquired by retrospective medical records consisted of age, gender, tumor diameter, tumor location, histological grade, lymph node metastasis (LNM), perineural invasion (PNI), tumor-node-metastasis (TNM) stage, preoperative serum carcinoembryonic antigen (CEA) concentrations, and preoperative serum carbohydrate antigen19–9 (CA19–9) concentrations. The 90 patients consisted of 54 males and 36 females with a mean age of 60.3 years (range 40–78 years). Tumor histological grade was defined in accordance with the WHO classification and tumor stage was performed in the light of the American Joint Committee on Cancer (AJCC) TNM classification.20 Overall survival (OS) and disease-free survival (DFS) were investigated to evaluate CD155 influence upon patient prognosis. Follow-up data were collected and the median follow-up period was 19 months (range 4–62 months).
Immunohistochemistry for CD155, VEGF, and CD31
Immunohistochemistry was performed with a two-step protocol following the manufacturer instructions. Paraffin-embedded specimens were sectioned at 4 mm, mounted on slides, heated at 60°C for 20 minutes, dewaxed with xylene, rehydrated through in a graded series of alcohol (100%, 95%, 75%, respectively), and rinsed in PBS. Next, antigen retrieval was performed by heating tissue sections immersed in 0.01 mol/L sodium citrate buffer (pH 6.0) at 80°C for 20 minutes in a microwave oven. After endogenous peroxidase activity was blocked with 0.3% H2O2 for 10 minutes, the sections were incubated overnight (at least 10 hours) in a humid chamber at 4°C with primary polyclonal rabbit antibody against CD155 (LifeSpan BioSciences, Inc., Seattle, WA, USA), VEGF (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China), or CD31 (Zhongshan Golden Bridge Biotechnology Co., Ltd.). After washing with PBS, the sections were incubated with a biotinylated secondary antibody (mouse anti-rabbit-IgG; Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 15 minutes at 37°C. Then, immunoreactivity was visualized with 3,3-diaminobenzidine (DAB) substrate (Sigma-Aldrich Corp., St Louis, MO, USA). Eventually, the sections were counterstained with hematoxylin, dehydrated, and mounted. In addition, for negative control groups, the primary antibodies were replaced with PBS under the same conditions.
Semiquantitative assessment
Semiquantitative assessment was conducted according to the percentage of stained cells and intensity of immunostaining. The percentage of stained cells was classified as 0 (0%–10%), 1 point (11%–30%), 2 points (31%–50%), and 3 points (51%–100%). Staining intensity was defined as 0 (−), 1 point (+), 2 points (++), and 3 points (+++). The total immunoreactivity score was the product of the staining percentage and intensity scores.21 A final total score ≤3 was regarded as low expression, while a final total score >3 as high expression. Measurement of MVD was carried out after CD31 was stained. Microvessels were preliminarily evaluated at low magnification (×100) to confirm the most vascularized areas (hot spots) and then the highest MVD of each hot spot was calculated at high magnification (×200). The final MVD was the average value of measurements made for three hot spots in each carcinoma tissue.22 All sections were analyzed by two experienced pathologists who were blinded to both the clinicopathologic data and the stained results of each other.
Western blot analysis
In this research, radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) was used to lyse snap-frozen tumor tissues and adjacent nontumor tissue, and subsequently, the bicinchoninic acid protein assay was used to measure protein concentration. The same amount of protein samples were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). After being blocked with 5% nonfat milk, membranes were incubated overnight at 4°C with rabbit primary antibodies against human CD155 (LifeSpan BioSciences, Inc.) and human antibody of β-actin (Zhongshan Golden Bridge Biotechnology Co., Ltd.). After being washed with Tris-buffered saline/0.1% Tween three times, membranes were incubated with secondary antibodies for 1.5 hours at room temperature. The blots were captured and visualized with Alpha-EaseFC imaging system (Alpha Innotech, San Leandro, CA, USA). Using the Alpha-EaseFC software, the integrated density value (IDV) of each band was detected by drawing a rectangle outlining the band. A total IDV by summation of each band IDV was employed when a protein had double bands. Results were normalized to the internal control, β-actin.
Quantitative real-time PCR
Total RNA gathered from snap-frozen tissue samples was isolated using Trizol (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. The gene-specific primers for CD155 were designed as follows: forward, 5′-TATCTGGCTCCGAGTGCTTGCC-3′ and reverse, 5′-ACGACGGCTGCAAAAGTGGCG-3′. Glyceraldehyde 3-phosphate dehydrogenase was used as the internalized control, and the sequences were shown as follows: forward: 5′-CCTAGTTCGTCATGGGTGTGAACCA-3′ and reverse: 5′-GCCAGTAGAGGCAGGGATGATGTTC-3′. CD155 level was determined by SYBR Green-based RT-PCR performed on a PikoReal RT-PCR system (Thermo Fisher Scientific, Waltham, MA, USA) in the following conditions: an initial denaturation step for 10 minutes at 95°C, followed by 40 amplification cycles involving denaturation for 15 seconds at 95°C, then annealing for 30 seconds at 60°C, and finally elongation for 30 seconds at 72°C. We performed melting-curve analysis to monitor PCR product purity, and the data of relative gene expression were analyzed using the 2−ΔΔCt method.
Statistical analysis
All data were analyzed with SPSS 22.0 software (SPSS Inc, Chicago, IL, USA) and presented using mean ± SD. Significant statistical differences between groups were estimated applying the Student’s t-test and chi-square test. The chi-square test and Spearman’s correlation test were applied to assess the correlations of CD155 with VEGF and MVD. The relationship between the level of CD155 expression and patient survival time was analyzed with Kaplan–Meier survival analysis and the log-rank test. Multivariate survival analysis was implemented by Cox proportional hazards regression model to ascertain the independent prognostic factors that were significant in univariate analysis. P<0.05 was considered statistically significant.
Results
Expression of CD155 protein and mRNA in CCA tissues
To explore the CD155 expression in CCA, we first assessed the protein levels of CD155 in 20 paired tumor and adjacent nontumor samples by Western blotting method. The data showed that CD155 expression was significantly upregulated in tumor sample tissues than that in adjacent nontumor tissues (1.30±0.29 vs 1.03±0.27, P<0.05), and 80% of samples (16/20 paired) revealed that tumor tissues had a high expression (Figure 1). Meanwhile, qRT-PCR was used to detect CD155 expression at the protein level in all 20 CCA tumor samples and corresponding paracarcinomatous samples. As shown in Figure 2, CD155 mRNA expression level in tumor tissues was distinctly higher than adjacent nontumor tissues (4.09±1.24 vs 2.87±0.70, P<0.05) and the positive rate was 75.0% (15/20) in tumor tissues, which was consistent with the Western blotting result.
Figure 1.
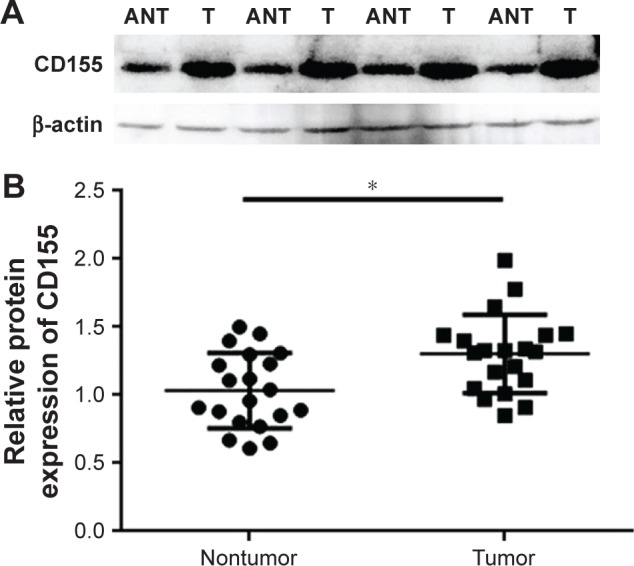
Western blotting analysis of CD155 protein expression in CCA.
Notes: (A) Representative results of CD155 protein expression in paired CCA T and the matched ANT from four patients. CD155 protein expression was normalized to β-actin. (B) All of the paired CCA tissues and the matched adjacent noncancerous tissues from 20 patients. *P<0.05.
Abbreviations: ANT, adjacent nontumorous tissues; CCA, cholangiocarcinoma; T, tumorous tissues.
Figure 2.
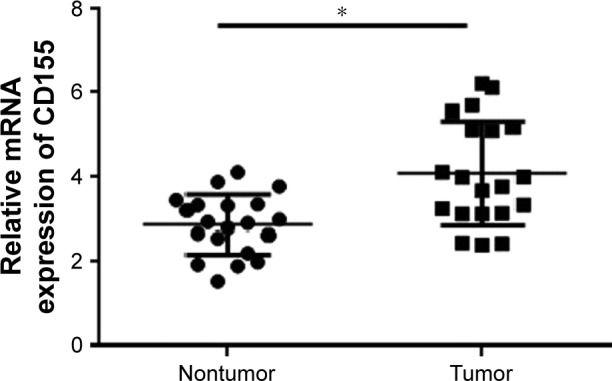
qRT-PCR analysis of CD155 mRNA expression in CCA.
Notes: Real-time polymerase chain reaction analysis of CD155 expression in 20 pairs of CCA tissues and matched adjacent nontumorous tissues. *P<0.05.
Abbreviations: CCA, cholangiocarcinoma; mRNA, messenger RNA; qRT-PCR, quantitative real-time polymerase chain reaction.
Immunohistochemical characteristics of CD155 and VEGF in CCA
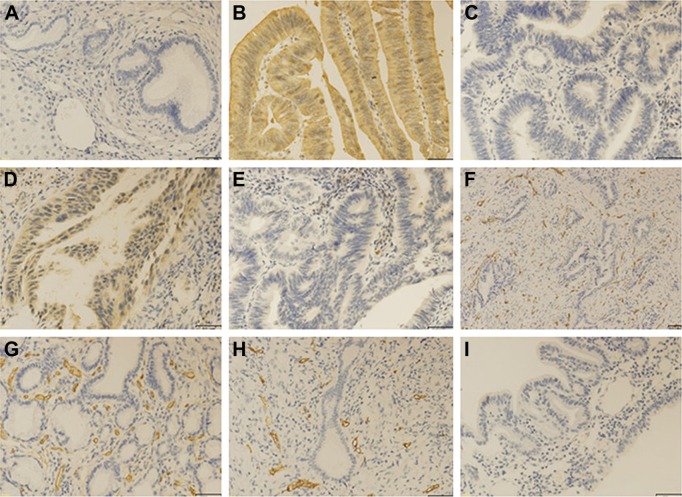
Immunohistochemistry showed that CD155 was mainly localized in cytoplasm of tumor cells with different staining intensity (Figure 3). Statistical analysis (Table 1) showed that CD155 expression was remarkably higher in CCA tumor tissue samples (55/90, 61.2%) when compared to adjacent nontumorous tissue samples (19/90, 21.1%; P<0.001). Likewise, we explored the expression of VEGF in cancerous tissues (Figure 3). The high expression of VEGF in cytoplasm of cancerous tissues was identified in 57 of 90 samples (63.3%; Table 2). Furthermore, Spearman rank correlation test revealed that CD155 expression was positively correlated with VEGF expression in CCA tissue (r=0.481, P<0.001; Table 2).
Figure 3.
Immunohistochemical staining for CD155, VEGF, and CD31 in CCA tissues and adjacent nontumor tissues.
Notes: The CD155 and VEGF were principally localized in cytoplasm of tumor cells with varying staining intensity. (A) Low CD155 expression in adjacent nontumor tissues. (B) High CD155 expression in CCA tissues. (C) Low CD155 expression in CCA tissues. (D) High VEGF expression in CCA tissues. (E) Low VEGF expression in CCA tissues. (F) MVD by CD31 staining in CCA tissues. (G) High MVD staining. (H) Low MVD staining. (I) Negative control. (A–E, G–I) Magnification 200×; (F) magnification 100×, bar =50 μm.
Abbreviations: CCA, cholangiocarcinoma; MVD, microvessel density; VEGF, vascular endothelial growth factor.
Table 1.
Differential expression of CD155 in CCA tissues and corresponding paracarcinoma tissues (n=90)
| Tissue | CD155 expression
|
P-value | |
|---|---|---|---|
| Low (%) | High (%) | ||
| Carcinoma tissues | 35 (38.8) | 55 (61.2) | <0.001 |
| Paracarcinoma tissues | 71 (78.9) | 19 (21.1) | |
Abbreviation: CCA, cholangiocarcinoma.
Table 2.
Correlations of CD155 expression with VEGF and MVD in CCA
| Immunoreactivity | CD155 expression
|
|||
|---|---|---|---|---|
| Low | High | r-value | P-value | |
| VEGF expression | <0.001 | |||
| Low | 23 | 10 | 0.481 | |
| High | 12 | 45 | ||
| MVD value | <0.001 | |||
| Low | 26 | 16 | 0.442 | |
| High | 9 | 39 | ||
Notes: Data presented as number of patients. The MVD was calculated after staining for CD31. The MVD was categorized as either low or high based on the median value of 31.
Abbreviations: CCA, cholangiocarcinoma; MVD, microvessel density; VEGF, vascular endothelial growth factor.
Relationship between CD155 expression and MVD in CCA tissues
CD31 staining was used to assess MVD in tumor tissues. The mean value of MVD was 30.9 per field (median, 31; range, 15–45). Tumor tissues with high CD155 expression had positively greater MVD values than those with low CD155 expression (33.5±5.8 vs 26.9±6.5; P<0.05; Figure 4). An MVD value ≥ the median value of 31 was considered to be high and an MVD value <31 was regarded as a low density. Spearman rank correlation test also revealed that CD155 expression was positively associated with MVD in carcinoma tissues (r=0.442, P<0.001; Table 2).
Figure 4.
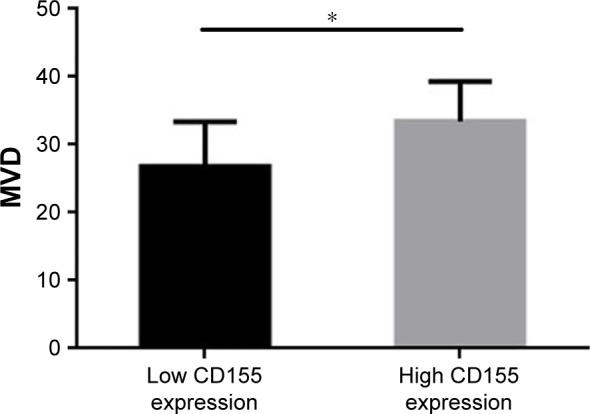
The association between MVD and CD155 intensity by immunohis-tochemistry.
Note: *P<0.001.
Abbreviation: MVD, microvessel density.
Relationship between CD155 expression and clinicopathological parameters in CCA
We investigated the relationship between CD155 expression and clinicopathologic characteristics in tumor tissues. As shown in Table 3, CD155 expression level was significantly related to histological grade (P=0.002), LNM (P<0.001), and TNM (P=0.03). However, immunohistochemistry results demonstrated that CD155 had no significant association with other parameters such as age, gender, tumor size, tumor location, PNI, serum CA19–9, and serum CEA.
Table 3.
Correlations between CD155 expression and clinicopathological characteristics in CCA
| Parameters | Total | CD155 expression
|
P-value | |
|---|---|---|---|---|
| Low | High | |||
| Age (years) | 0.864 | |||
| ≤60 | 37 | 14 | 23 | |
| >60 | 53 | 21 | 32 | |
| Gender | 0.377 | |||
| Male | 54 | 19 | 35 | |
| Female | 36 | 16 | 20 | |
| Tumor size, mm | 0.878 | |||
| ≤30 | 60 | 23 | 37 | |
| >30 | 30 | 12 | 18 | |
| Tumor location | 0.108 | |||
| Intrahepatic | 26 | 10 | 16 | |
| Perihilar | 33 | 17 | 16 | |
| Distal | 31 | 8 | 23 | |
| PNI | 0.361 | |||
| Absent | 46 | 20 | 26 | |
| Present | 44 | 15 | 29 | |
| LNM | <0.001 | |||
| Absent | 55 | 30 | 25 | |
| Present | 35 | 5 | 30 | |
| Histological grade | 0.002 | |||
| Moderate–poor | 36 | 7 | 29 | |
| Well | 54 | 28 | 26 | |
| TNM stage | 0.03 | |||
| I–II | 57 | 27 | 30 | |
| III–IV | 33 | 8 | 25 | |
| Serum CA19–9, U/mL | 0.561 | |||
| ≤37 | 13 | 6 | 7 | |
| >37 | 77 | 29 | 48 | |
| Serum CEA, U/mL | 0.147 | |||
| ≤5 | 69 | 24 | 45 | |
| >5 | 21 | 11 | 10 | |
Abbreviations: CA19–9, carbohydrate antigen19–9; CCA, cholangiocarcinoma; CEA, carcinoembryonic antigen; LNM, lymph node metastasis; PNI, perineural invasion; TNM, tumor-node-metastasis.
Relationship between CD155 expression and prognosis in CCA
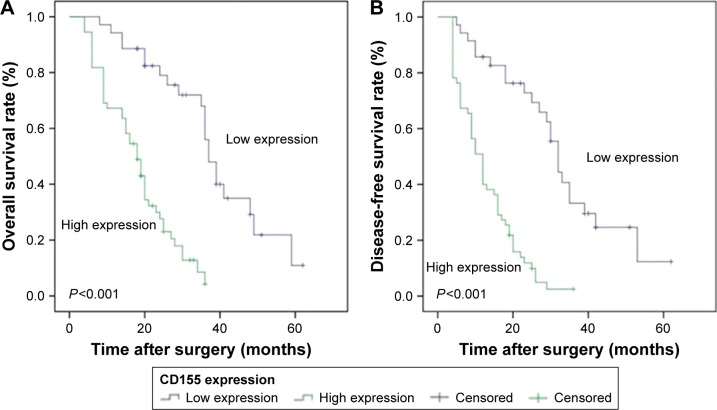
Kaplan–Meier method was applied to construct the survival curves in order to compare the DFS and OS between high CD155 expression and low CD155 expression in patients. As shown in Figure 5, patients with high CD155 expression (18.23±1.37 months) had a markedly shorter OS compared to patients with low CD155 expression (38.78±2.91 months, P<0.001). Meanwhile, the DFS of high CD155 expression (12.81±1.10 months) was also significantly lower than that of low CD155 expression (33.43±3.12 months, P<0.001) in patients.
Figure 5.
Kaplan–Meier analysis of OS and DFS curves of CCA patients based on CD155 expression as high or low.
Notes: (A) Patients with high CD155 expression had a shorter OS than those with low CD155 expression (n=90; P<0.001; log-rank test). (B) Patients with high CD155 expression had a shorter DFS than those with low CD155 expression (n=90; P<0.001; log-rank test).
Abbreviations: CCA, cholangiocarcinoma; DFS, disease-free survival; OS, overall survival.
Univariate analysis (Table 4) showed that CD155 expression, histological grade, LNM, and TNM staging had significant prognostic influences on OS and DFS. Multivariate survival analysis (Table 5) further revealed that CD155 expression served as an independent prognostic factor for OS (hazard ratio [HR] 3.995; 95% CI 1.985–8.039; P<0.001) and DFS (HR, 3.353; 95% CI, 1.752–6.416; P<0.001). Additionally, LNM and TNM staging were also independent predictors of OS or DFS.
Table 4.
Univariate analysis of factors related with DFS and OS
| Variables | OS
|
DFS
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age, years (≤60 vs >60) | 0.748 | 0.456–1.228 | 0.252 | 0.784 | 0.492–1.247 | 0.303 |
| Gender (male vs female) | 0.729 | 0.443–1.200 | 0.214 | 0.762 | 0.477–1.219 | 0.258 |
| Tumor size, cm (≤3 vs >3) | 1.023 | 0.613–1.706 | 0.931 | 1.086 | 0.671–1.758 | 0.737 |
| Tumor location (intrahepatic vs perihilar/distal) | 0.604 | 0.359–1.016 | 0.058 | 0.654 | 0.397–1.076 | 0.094 |
| Histological grade (moderate/poor vs well) | 0.589 | 0.363–0.955 | 0.032 | 0.574 | 0.363–0.908 | 0.018 |
| LNM (absent vs present) | 6.128 | 3.421–10.977 | <0.001 | 5.228 | 3.062–8.928 | <0.001 |
| PNI (absent vs present) | 1.316 | 0.811–2.137 | 0.266 | 1.288 | 0.817–2.032 | 0.276 |
| TNM stage (I–II vs III–IV) | 6.398 | 3.372–12.138 | <0.001 | 4.696 | 2.662–8.282 | <0.001 |
| Serum CEA, U/mL (≤5 vs >5) | 0.729 | 0.413–1.286 | 0.275 | 0.731 | 0.428–1.250 | 0.253 |
| Serum CA19–9, U/mL (≤37 vs >60) | 0.965 | 0.491–1.894 | 0.917 | 0.945 | 0.509–1.757 | 0.859 |
| CD155 expression (low vs high) | 5.443 | 2.822–10.498 | <0.001 | 5.068 | 2.836–9.054 | <0.001 |
Abbreviations: CA19–9, carbohydrate antigen19–9; CEA, carcinoembryonic antigen; DFS, disease-free survival; HR, hazard ratio; LNM, lymph node metastasis; OS, overall survival; PNI, perineural invasion; TNM, tumor-node-metastasis.
Table 5.
Multivariate analysis of factors associated with DFS and OS
| Variables | OS
|
DFS
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| CD155 expression (low vs high) | 3.995 | 1.985–8.039 | <0.001 | 3.353 | 1.752–6.416 | <0.001 |
| Histological grade (moderate–poor vs well) | 0.707 | 0.416–1.201 | 0.200 | 0.622 | 0.379–1.021 | 0.061 |
| TNM stage (I–II vs III–IV) | 4.448 | 2.212–8.941 | <0.001 | 3.284 | 1.750–6.164 | <0.001 |
| LNM (absent vs present) | 2.772 | 1.462–5.254 | 0.002 | 2.457 | 1.364–4.424 | 0.003 |
Abbreviations: DFS, disease-free survival; HR, hazard ratio; LNM, lymph node metastasis; OS, overall survival; TNM, tumor-node-metastasis.
Discussion
According to recent studies, upregulated CD155 expression has already been reported in human carcinoma such as colon cancer cells,8 malignant glioma cells,9 pancreatic cancer cells,10 and lung cancer cells.11 In addition, an increasing number of studies have demonstrated that the upregulation of CD155 expression in carcinoma cells strengthens migration, proliferation, and distant metastasis.15–18 For example, Morimoto et al concluded that the upregulated CD155 in cancer cells interacted with its counterreceptor in platelets (CD226), which affects metastasis of cancerous cells to the lungs.18 Sloan et al suggested that CD155 played a vitally important role in mediating tumor cell invasion and migration and that CD155 might contribute to tumorigenesis.15 Nevertheless, there has been a contrary conclusion. Qu et al showed that hepatocellular carcinoma tissues exhibited a significantly lower CD155 expression in comparison with adjacent non-tumorous tissues and that CD155 expression deficiency may be critical for the tumor immune escape of hepatocellular carcinoma cells.23 These conflicting findings showed that CD155 may serve dual functions owing to its immunological and nonimmunological mechanisms in different types of tumors. In the present study, our immunohistochemistry results initially showed CD155 expression and its clinical significances in CCA. Compared with paracarcinomatous tissues, the CD155 was overexpressed in CCA tissues. Statistical analysis demonstrated that CD155 expression was markedly higher in tumors with aggressive clinicopathological features like poor histological grade, LNM, and advanced TNM stage. In addition, we also investigated CD155 expression by Western blotting and qRT-PCR assays at protein and mRNA levels in 20 paired CCA samples, and the results were coincident with those in immunohistochemistry experiments.
Some researchers have indicated that tumor angiogenesis plays a significant part in tumor growth, invasion, metastasis, and prognosis.24,25 VEGF, as one of the most important angiogenesis facilitating factor, influences both physiological and pathological angiogenesis and regulates the tumor angiogenesis.26,27 MVD has been used to reflect the status of novo blood vessel formation and has been applied as an index for tumor angiogenesis.22 Other studies have showed that VEGF and MVD are significantly associated with many different tumor entities, including CCA.28,29 Mobius et al suggested that high tumor VEGF and MVD expression is related to decreased survival and poor prognosis in CCA.28 Likewise, Yao et al revealed that the VEGF and MVD were efficient predictors of vascular invasion and metastasis of hepatocellular carcinoma.29 Recently, the role of CD155 in tumor angiogenesis has drawn increasing attention. For instance, Kinugasa et al showed that CD155 interacted with VEGF receptor 2 and regulated VEGF-induced angiogenesis.19 Nishiwada et al demonstrated that CD155 expression was positively correlated with intratumoral MVD in human pancreatic cancer.10 Consistent with previous studies, our results suggested that tumors with high CD155 expression had higher MVD than those with low CD155. Meanwhile, Spearman’s rank correlation test showed that CD155 expression had a strong and positive correlation with VEGF and MVD in CCA. These results disclosed that CD155 might participate in facilitating angiogenesis of tumor and might be concerned with VEGF-mediated tumor angiogenesis in CCA. However, further studies are needed to unlock the specific mechanism of CD155 in tumor angiogenesis of CCA.
Several studies have demonstrated the prognostic value of CD155 expression in different tumors.10,11,30 For example, Nishiwada et al reported that CD155 expression had an independent prognostic value in pancreatic carcinoma, and patients with high CD155 expression had poorer postoperative prognosis than those with low CD155 expression.10 Meanwhile, Nakai et al demonstrated that the overexpression of CD155 by lung adenocarcinoma cells was evidently related to TNM staging, LNM, and the bronchioloalveolar carcinoma ratio of tumors and was also correlated with poor prognosis in patients.11 Consistent with previous studies, Kaplan–Meier survival analysis and log-rank test suggested that CCA patients with high CD155 expression had a significantly shorter DFS and OS than those with low CD155 expression. Univariate analysis revealed that high CD155 expression reduced survival time including OS and DFS. Moreover, multivariate survival analysis showed that high CD155 expression was an independent prognostic factor for unfavorable DFS and OS. Therefore, these results suggested that high CD155 expression had a negative influence on prognosis of CCA patients and may be used as a promising prognostic biomarker.
We have to acknowledge that our studies are somewhat limited. It is a typical single-institute retrospective study with a relatively small sample size, which was designed with a potential selection bias. At the same time, this study lacks some well-designed experiments such as cell invasion assay and cell migration assay. Therefore, we need to carry out more randomized, prospective, and multicentric researches with a larger sample size to support the present findings.
In summary, we, for the first time, revealed that CD155 is overexpressed in CCA tissues and that high CD155 expression is associated with aggressive clinicopathological features and angiogenesis. More importantly, high CD155 expression serves as an independent predictor of unfavorable prognosis in CCA patients after surgery. These findings may be of great value to discover a new strategy for the treatment of the CCA patients and thereby further prolong their survival time. However, further studies are needed to explore the precise mechanism of increased expression and biological function of CD155 in CCA.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No 81501354), Natural Science Foundation of Anhui Province (No 1608085QH197), and the Programs for Science and Technology Development of Anhui Province in 2016 (No 1606c08234).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990–2009. World J Gastroenterol. 2009;15(34):4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 3.Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17(8):691–699. doi: 10.1111/hpb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17(8):669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94(8):655–667. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koike S, Horie H, Ise I, et al. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990;9(10):3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 8.Masson D, Jarry A, Baury B, et al. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49(2):236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Inter-generic poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000;97(12):6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiwada S, Sho M, Yasuda S, et al. Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res. 2015;35(4):2287–2297. [PubMed] [Google Scholar]
- 11.Nakai R, Maniwa Y, Tanaka Y, et al. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010;101(5):1326–1330. doi: 10.1111/j.1349-7006.2010.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34(4):182–191. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Chan CJ, Andrews DM, McLaughlin NM, et al. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. 2010;184(2):902–911. doi: 10.4049/jimmunol.0903225. [DOI] [PubMed] [Google Scholar]
- 14.Tahara-Hanaoka S, Shibuya K, Kai H, et al. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107(4):1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- 15.Sloan KE, Eustace BK, Stewart JK, et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda W, Kakunaga S, Takekuni K, et al. Nectin-like molecule-5/Tage4 enhances cell migration in an integrin-dependent, Nectin-3-independent manner. J Biol Chem. 2004;279(17):18015–18025. doi: 10.1074/jbc.M312969200. [DOI] [PubMed] [Google Scholar]
- 17.Kakunaga S, Ikeda W, Shingai T, et al. Enhancement of serum- and platelet-derived growth factor-induced cell proliferation by Necl-5/Tage4/poliovirus receptor/CD155 through the Ras-Raf-MEK-ERK signaling. J Biol Chem. 2004;279(35):36419–36425. doi: 10.1074/jbc.M406340200. [DOI] [PubMed] [Google Scholar]
- 18.Morimoto K, Satoh-Yamaguchi K, Hamaguchi A, et al. Interaction of cancer cells with platelets mediated by Necl-5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene. 2008;27(3):264–273. doi: 10.1038/sj.onc.1210645. [DOI] [PubMed] [Google Scholar]
- 19.Kinugasa M, Amano H, Satomi-Kobayashi S, et al. Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angio-genesis. Circ Res. 2012;110(5):716–726. doi: 10.1161/CIRCRESAHA.111.256834. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhai LL, Wu Y, Huang DW, Tang ZG. Increased matrix metallopro-teinase-2 expression and reduced tissue factor pathway inhibitor-2 expression correlate with angiogenesis and early postoperative recurrence of pancreatic carcinoma. Am J Transl Res. 2015;7(11):2412–2422. [PMC free article] [PubMed] [Google Scholar]
- 22.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 23.Qu P, Huang X, Zhou X, et al. Loss of CD155 expression predicts poor prognosis in hepatocellular carcinoma. Histopathology. 2015;66(5):706–714. doi: 10.1111/his.12584. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 26.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 27.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60(2):203–212. [PubMed] [Google Scholar]
- 28.Mobius C, Demuth C, Aigner T, et al. Evaluation of VEGF A expression and microvascular density as prognostic factors in extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2007;33(8):1025–1029. doi: 10.1016/j.ejso.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Yao DF, Wu XH, Zhu Y, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4(2):220–226. [PubMed] [Google Scholar]
- 30.Atsumi S, Matsumine A, Toyoda H, Niimi R, Iino T, Sudo A. Prognostic significance of CD155 mRNA expression in soft tissue sarcomas. Oncol Lett. 2013;5(6):1771–1776. doi: 10.3892/ol.2013.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]