Abstract
Purpose
SHP2 has roles in a variety of signal transduction pathways and in many important cellular processes, including proliferation, differentiation, movement regulation, and apoptosis. In addition, SHP2 expression is closely associated with multiple types of malignancies. In this study, we examined the role of SHP2 in epithelial ovarian cancer.
Patients and methods
SHP2 expression in cancer and normal ovarian tissue specimens was evaluated by immunohistochemical staining and Western blot analyses. The correlation between the SHP2 expression level and clinicopathological features was analyzed. The role of SHP2 in epithelial ovarian cancer was evaluated by assessing SHP2 expression patterns in vitro and in vivo, and activation of the PI3K/AKT pathway was examined.
Results
SHP2 is expressed at higher levels in ovarian cancer tissues than in normal ovarian tissues and in an ovarian cancer cell line than in a normal ovarian cell line. On the basis of these findings, SHP2 is overexpressed in ovarian cancer both in vitro and in vivo. In addition, SHP2 overexpression is associated with tumor stage and differentiation, enhanced cell proliferation and invasion, and tumorigenesis and metastasis.
Conclusion
SHP2 overexpression enhances ovarian tumor proliferation and invasion by activating the PI3K-AKT axis, indicating that SHP2 potentially plays a direct role in the pathogenesis of ovarian epithelial cell cancer. These novel findings provide key insights that are applicable to basic cancer research and to the prevention and treatment of cancer.
Keywords: ovarian tumor, SHP2, overexpression, proliferation, invasion, metastasis
Introduction
Ovarian cancer is one of the most common gynecological malignancies, and its mortality rate has significantly increased in recent years.1 However, the precise mechanisms underlying the pathogenesis of ovarian cancer remain unclear. Ovarian epithelial carcinoma accounts for ~85%–90% of ovarian cancer cases; therefore, studies identifying the expression patterns and clinical significance of novel epithelial ovarian cancer markers and prognostic indicators might provide valuable knowledge.2 The balance between protein phosphorylation and dephosphorylation in vivo helps maintain normal biological functions, and a disruption of this balance contributes to the development of cancer.3 SHP2, a member of the protein tyrosine phosphatase (PTP) family of proteins, is encoded by the PTPN-11 gene. The SHP2 protein is a nonreceptor tyrosine phosphatase containing an SH2 domain and a PTP domain. SHP2 cooperates with cytokines and growth factors in signaling pathways that are activated by extracellular stimuli and contributes to multiple cellular processes, including cell proliferation, differentiation, cell movement, and the regulation of cell death.4–7 Abnormal expression of the SHP2 protein is associated with Noonan syndrome8–10 and multiple malignancies, including leukemia,1,2 breast,11–13 liver,14–16 lung17–19 and thyroid cancer.20 However, the expression pattern and clinical significance of SHP2 in ovarian cancer has not yet been reported. In the present study, we used immunohistochemistry assays to evaluate the expression of SHP2 in ovarian cancer tissue samples and 60 matched normal ovarian tissue samples from 60 patients with ovarian epithelial cancer, and we investigated the relationship between SHP2 expression and the pathological features and prognosis of ovarian epithelial cancer. Plasmids expressing SHP2 were transfected into ovarian cancer cell lines in vitro, and the impacts of SHP2 overexpression on cell invasion, apoptosis, proliferation, and the capacity to induce tumor formation in nude mice were subsequently investigated. The findings of this study might contribute to improvements in the diagnosis and prognosis of recurrent ovarian epithelial cancer.
Patients and methods
Clinical specimens
Ovarian cancer tissues and matched normal tissue samples from 60 patients with epithelial ovarian cancer were evaluated in this study. The age of the patients ranged from 23 to 70 years, with a mean age of 43 years. The ovarian cancer tissue samples were obtained from surgeries performed at Zhongda Hospital, Southeast University (Nanjing, Jiangsu, China). A postoperative pathological diagnosis of ovarian cancer was confirmed using paraffin-embedded specimens. The patients had no history of receiving preoperative chemotherapy, radiation, or other cancer treatments. All patients received 6–8 weeks of a standard postoperative chemotherapy treatment after providing informed consent. The study design was approved by the Institutional Review Board of Southeast University, and all patients provided written informed consent before participating in the study. The tissue specimens were staged according to the Federation of Gynecology and Obstetrics (FIGO) (2010, 7th edition) standard histological grading system as highly differentiated (42 cases) or poorly differentiated (18 cases) and either stages I–II (51 cases) or stages III–IV (9 cases). Fifteen patients were at least 50 years of age, and 45 patients were <50 years of age. Ascites was observed in 32 of the 60 patients and was absent in the remaining.21 Seventeen patients presented lymph node metastasis, and 43 did not exhibit lymph node metastasis. Distant metastasis and distant organ metastasis were observed in 6 and 54 cases, respectively.
Immunochemistry
The tissues were sectioned into 4 μm slices and preliminarily analyzed using previously reported immunohistochemical methods. The SHP2 polyclonal antibody (1:500 dilution; Cell Signaling Technology, Beverly, MA, USA) was used in conjunction with a rabbit anti-human secondary antibody. Samples incubated with a PBS solution lacking the primary antibody were used as negative controls. SHP2 staining was predominantly observed in the cytoplasm, and SHP2-positive cells were stained yellow or brown.
The sections were evaluated at a magnification of 400×. The average staining intensity and the percentage of positively stained cells were calculated based on 10 randomly selected fields of view, and 100 cells in each field were evaluated. The staining intensity was scored as follows: no staining, 0 points; weak staining, 1 point; moderate staining, 2 points or strong staining, 3 points. The percentage of positive cells was scored as follows: <5%, 0 points; 5%–25%, 1 point; >25%–50%, 2 points; >50%–75%, 3 points; and >75%, 4 points. In the second phase of the study, scores of 0 and 1 were classified as negative, and scores of 2 or greater were classified as positive.
Cell culture and cell transfection
The ovarian cancer A2780 (Chinese Academy of Sciences, Shanghai, China) cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). The SHP2-pcDNA3.1 vector and the empty pcDNA3.1 vector (laboratory stocks) were transfected into the A2780 human ovarian cancer cell line using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommended protocol, and stable cell lines were subsequently established. A2780 cells stably overexpressing SHP2 are referred to as the SHP2 group, A2780 cells stably transfected with the empty pcDNA3.1 vector are denoted as the vector group, and the nontransfected control A2780 cells are considered the A2780 group.
MTT assay
Cells from each of the 3 groups were plated in 96-well cell culture plates (1×104 cells/well), and the viability of the cells was measured after 0, 24, and 48 h using the MTT assay. All experiments were repeated 3 times. Briefly, 0.02 mL of MTT solution (5 mg/mL in PBS) was added to each well, and the reaction was incubated for 4 h at 37°C. Then, 0.15 mL of dimethyl sulfoxide was added to each well and incubated for 10 min.
Colony formation assay
Single-cell suspensions were seeded in 25 mm Petri dishes at a density of 1,000 cells/dish. The original medium was discarded when macroscopic colonies were first observed (~20 days), and the cells were gently washed twice with PBS. The cells were fixed for 15 min at room temperature and then incubated with a 0.1% crystal violet staining solution for 20 min. The cells were gently washed with water at room temperature and dehydrated before images were captured. The number of clones was counted using Carestream Molecular Imaging software, and the cloning efficiency was calculated using the following formula: cloning efficiency (%) = number of colonies counted/number of inoculated cells ×100%.
Soft agar colony formation assay
The cells were suspended in 0.7% agar medium in 6-well plates (1×103 cells/well) containing 1.2% agar underneath the medium. After the cells were cultured for 3 weeks, the clones were counted and imaged under a microscope.
Wound healing assay
The cells were cultured in 6-well plates in RPMI 1640 medium supplemented with 10% FBS. When the confluence of the cells at the bottom of the culture plate reached 90%, a “scar” was created in the center of each well using a 10 μL sterile micropipette tip. The cells were washed 3 times with PBS and subsequently cultured in RPMI 1640 medium supplemented with 2% FBS. The wound healing process was evaluated 0, 24, and 48 h after the creation of the wound using an inverted microscope.
Transwell invasion assay
RPMI 1640 medium was added to the upper and lower chambers of 24-well plates for 30 min, and RPMI 1640 medium supplemented with 5% FBS (500 μL) was added to the lower chamber. Cells in the logarithmic phase of growth were centrifuged and dissociated into single cells. Then, 1×105 cells resuspended in RPMI 1640 supplemented with 2% FBS were added to the upper chamber and incubated for 6 h at 37°C in a 5% CO2 incubator. The migration of randomly selected cells to the opposite side of the membrane was observed under a microscope, and a wet cotton swab was used to carefully remove the cells remaining on the membrane. The membrane was gently washed twice with PBS, placed in fixative, and incubated overnight with a 0.1% crystal violet staining solution. The membrane was subsequently washed with water to remove the excess dye, dried at room temperature, and imaged under a microscope. The dye was dissolved using 33% acetic acid, and the OD was measured at 570 nm using a microplate reader. Each experiment was repeated 3 times.
Detection of paclitaxel-induced apoptosis
The degree of apoptosis in the SHP2 group, the vector group, and the A2780 group was determined after the cells had been treated with paclitaxel. The cells were incubated in 6-well plates with RPMI 1640 medium containing 10% FBS overnight. Paclitaxel (3 μg/mL) was added to each well for 24 h before the cells were harvested and analyzed by flow cytometry. The levels of the SHP2 protein were determined by Western blot analysis, and apoptosis was assessed with a caspase-3 activity assay.
Western blot analysis
Western blot analyses were performed as previously described.21 Briefly, 15 μg of protein samples were separated by electrophoresis on a sodium dodecyl sulfate-polyacrylamide gel, and the separated proteins were transferred to nitrocellulose membranes. The membranes were then blocked in PBS Tween-20 (PBST; Invitrogen) for 1 h at room temperature and subsequently probed with the following primary antibodies overnight at 4°C: rabbit anti-SHP2 antibody (1:1,000 dilution; Cell Signaling Technology), rabbit anti-E-cadherin and anti-vimentin antibodies (1:500 dilution; Abcam, Cambridge, UK), rabbit anti-caspase-3 antibody (1:1,000 dilution; Cell Signaling Technology), anti-p-AKT antibody (1:1,000 dilution; Cell Signaling Technology), anti-AKT antibody (1:1,000 dilution; Cell Signaling Technology), and an anti-β-actin antibody (1:5,000 dilution; Abcam). After 3 washes with PBST, the membranes were incubated with secondary antibodies conjugated to horseradish peroxidase (GE Healthcare, Waukesha, WI, USA). The immunoreactive signals were detected using a chemiluminescence method (GE Healthcare). The membranes were then stripped with stripping buffer for 15 min at room temperature and immunoblotted with an anti-actin antibody. The film was digitalized using ImageJ 1.43 G software (NIH, Bethesda, MD, USA).
Xenograft model
All in vivo studies were performed according to a protocol, ethical and legal, approved by the Internal Review Board and by the animal care committee of the Southeast University (Nanjing, China). All experiments were performed in accordance with the named relevant institutional and national guidelines and regulations. SHP2 overexpression and control A2780 (1×106) cells were subcutaneously inoculated into 4-week-old female nude mice (4 mice per group) to evaluate whether SHP2 overexpression promoted the growth of ovarian tumors in vivo. The length and width of the tumors were measured once in every 3 days, and the mice were sacrificed 60 days after tumor cell inoculation. The tumor volume was calculated according to the following formula: tumor volume = (length × width2)/2.
Immunohistochemistry
Immunohistochemistry was performed using a previously described method.22 Tumor samples were fixed in 10% neutral-buffered formalin for at least 24 h and embedded in paraffin. The samples were sectioned into 3 μm-thick slices and stained with H&E.
Data and statistical analyses
All experiments were repeated for a minimum of 3 times. The data are presented as mean ± SD. All statistical analyses were conducted using SPSS 18 software, and P<0.05 was considered statistically significant. A paired Student’s t-test was performed to compare the differences in SHP2 expression between ovarian tissues and tumor tissues. The chi-square test was used to determine the correlations between the SHP2 levels and the clinical factors. Comparisons between groups were evaluated using 1-way analysis of variance (ANOVA), and Pearson’s correlation coefficients were calculated for bivariate correlations.
Results
SHP2 expression in ovarian cancer tissue specimens and ovarian cancer cell lines
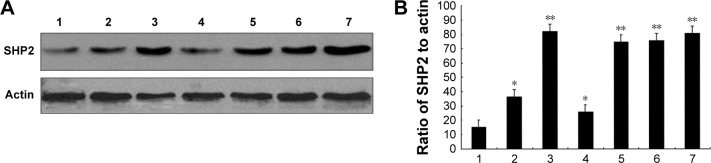
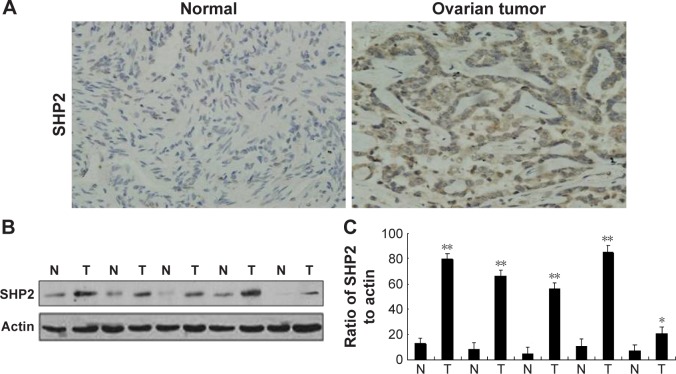
We evaluated SHP2 expression in the ovarian cancer cell lines SKOV3, Ho-8910PM, A2780, OVCA420, OVCA429, and OV-01063 and in the normal ovarian epithelial cell line IOSE80 using Western blot analysis. Compared with its expression in the normal ovarian epithelial cell line, SHP2 was expressed at higher levels in the ovarian cancer cell lines (Figure 1A and B). We also evaluated SHP2 expression in ovarian cancer tissues and matched normal ovarian tissue samples derived from 60 patients with ovarian cancer using immunohistochemistry assays. The SHP2 protein was detected as pale yellow to brown particles that were predominantly localized in the cytoplasm. The SHP2 protein was expressed at low levels in normal ovarian tissues and at relatively high levels in ovarian epithelial cancer tissues (Figure 2A). A Western blot analysis yielded similar results (Figure 2B and C). SHP2 expression was observed in 81.67% of the samples from the ovarian epithelial cancer group and 0% of the samples from the normal group, and this difference was statistically significant (P<0.05; Table 1). SHP2 expression was associated with the clinical stage, histological grade, lymph node metastasis, and distant metastasis, but it was not associated with age, histological subtype, or the presence of ascites (Table 2).
Figure 1.
SHP2 overexpression in human ovarian cancer cell lines.
Notes: (A) Expression of SHP2 in normal human ovarian cells and ovarian cancer cell lines. Lanes: 1, IOSE80; 2, SKOV3; 3, A2780; 4, Ho-8910PM; 5, OVCA420; 6, OVCA429; and 7, OV-01063 cells. (B) Quantification of the relative intensities of the bands obtained from the Western blot presented in (A). *P<0.05 and **P<0.01 compared with normal human ovarian cells (lane 1).
Figure 2.
SHP2 overexpression in human ovarian tumor tissues.
Notes: (A) Representative images of immunohistochemical staining of human ovarian tissues (magnification, 400×). Immunohistochemical staining was performed to evaluate the SHP2 expression levels in 60 human ovarian cancer tissues (3+) and 60 matched normal ovarian tissues (1+). (B) Western blot analysis of SHP2 protein expression in human ovarian tissue specimens. (C) A semi-quantitative Western blot analysis of human ovarian tissue samples revealed that the levels of the SHP2 protein were significantly elevated in tumor tissues compared with normal tissues. Actin was used as an internal control. **P<0.01 compared with normal tissues, *P<0.05.
Abbreviations: N, normal; T, tumor.
Table 1.
Expression of SHP2 in normal ovarian tissues and epithelial ovarian cancer
| Group | Cases | Expression of SHP2
|
P-value | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive rate (%) | |||
| Normal | 60 | 0 | 60 | 0 | |
| Cancer | 60 | 49 | 11 | 81.67 | <0.001 |
Table 2.
Correlation between SHP2 expression and clinicopathological factors in epithelial ovarian cancer
| Clinicopathological factors | Expression of the SHP2 protein
|
χ2 value | P-value | |
|---|---|---|---|---|
| Positive 1 | Negative 2 | |||
| Age (years) | 1.000 | |||
| <50 | 37 | 8 | 0.000 | |
| ≥50 | 12 | 3 | ||
| Pathological type | 1.000 | |||
| Serous | 17 | 3 | 0.329 | |
| Mucinous | 16 | 4 | ||
| Endometrioid | 16 | 4 | ||
| FIGO stage | 0.283 | |||
| Stages I–II | 40 | 11 | 1.155 | |
| Stages III–IV | 9 | 0 | ||
| Ascites | 0.929 | |||
| No | 23 | 5 | 0.008 | |
| Yes | 26 | 6 | ||
| Pathological differentiation | 0.190 | |||
| Well-middle | 32 | 10 | 1.717 | |
| Low | 17 | 1 | ||
| Lymph node metastasis | 0.053 | |||
| No | 32 | 11 | 3.754 | |
| Yes | 17 | 0 | ||
| Distant metastasis | 0.505 | |||
| No | 43 | 11 | 0.445 | |
| Yes | 6 | 0 | ||
SHP2 overexpression in A2780 cells enhances cell proliferation, migration, and invasion
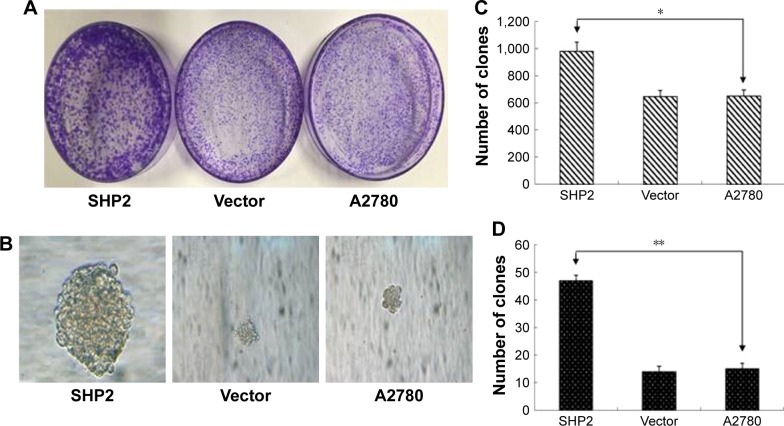
SHP2 expression has been shown to promote breast cancer cell proliferation and increases cancer cells’ resistance to chemotherapy.12 In the current study, we evaluated the effect of SHP2 overexpression on cell proliferation using colony and soft agar colony formation assays. The number and size of the colonies were increased in SHP2- overexpressing cells compared with A2780 cells and control cells transfected with the empty vector, indicating that the proliferation rate was increased in the SHP2 group (Figure 3A–D).
Figure 3.
SHP2 overexpression enhances the proliferation of A2780 cells.
Notes: (A) SHP2-positive foci in 3 wells from each cell line. (B) Anchorage-independent growth was assayed by evaluating colony growth in soft agar. Representative images are shown. The colonies formed by the SHP2-expressing cells are larger in size. Representative images of randomly selected cells from the experimental groups are shown. (C and D) The number of clones in each experimental group was counted. Each experiment was repeated a minimum of 3 times. The data shown here are representative of at least 3 independent experiments with similar results. Significant differences are indicated by asterisks (*P<0.05 and **P<0.01).
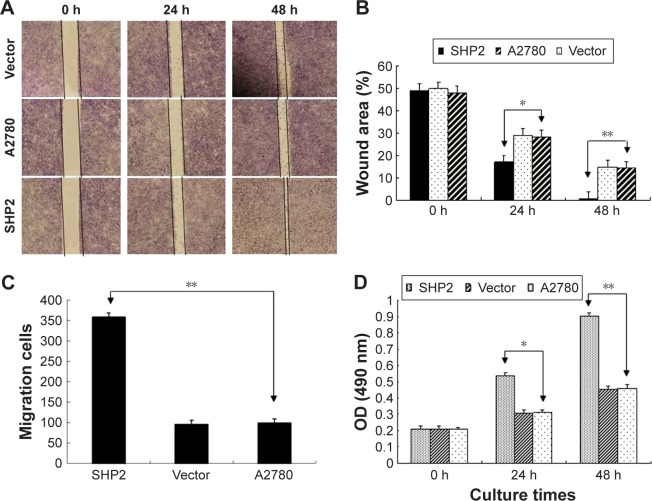
We performed transwell invasion and wound healing assays using 3 groups of A2780 cells to examine the impact of SHP2 overexpression on human ovarian cancer cell migration and invasion (Figure 4A–C). On the basis of the results of the wound healing experiments, cells in the SHP2 group migrated at a faster rate than cells in the A2780 group and the vector group (Figure 4A and B). According to the results of the transwell invasion assay, a significantly greater proportion of A2780 cells overexpressing SHP2 migrated through the basement membrane matrix (Matrigel) than the proportions observed for the other two groups (Figure 4C).
Figure 4.
SHP2 overexpression enhances the migration, invasion and proliferation of A2780 cells.
Notes: (A) Wound-healing assays were conducted to evaluate cell migration. The width of the scratch indicated the migratory ability of the tumor cells. (B) Crystal violet staining of the membrane used in the Boyden chamber migration assay. (C) The invasive cells were counted using high-power microscopy images at a magnification of 200×. **Invasive capacity of A2780 cells. (D) Proliferative capacity of A2780 cells (*P<0.05 and **P<0.01).
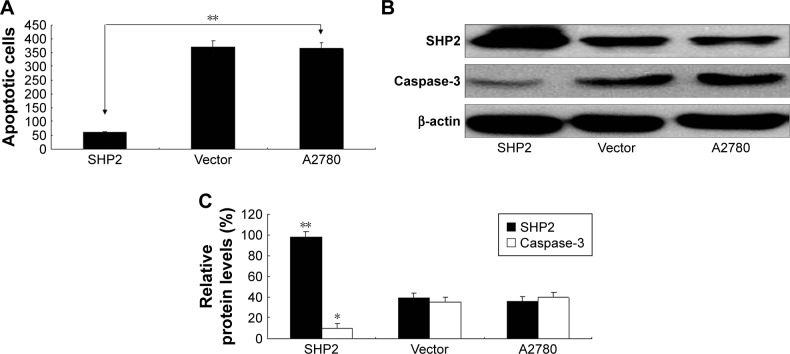
We used the MTT assay to evaluate the impact of SHP2 overexpression on the viability of A2780 cells (Figure 4D) and found that the viability of SHP2-overexpressing cells was significantly increased compared with control A2780 and empty vector-transfected cells. Similarly, as shown in the flow cytometry analysis, apoptosis was significantly decreased in paclitaxel-treated SHP2-overexpressing cells compared with paclitaxel-treated control cells (Figure 5A). In addition, caspase-3 activity was significantly increased in paclitaxel-treated SHP2-overexpressing cells compared with paclitaxel-treated control cells (Figure 5B and C).
Figure 5.
SHP2 overexpression is associated with increased resistance to the chemotherapeutic agent paclitaxel.
Notes: (A) Flow cytometry analysis of apoptosis in A2780 cells incubated with Taxol (3 μg/mL) for 24 h. The number of cells was calculated at the same time point, and the data are expressed as the growth rate of SHP2-overexpressing cells compared with the vehicle control group (**P<0.01). (B) SHP2 and caspase-3 expression. The expression levels were determined using Western blot assays. (C) Quantification of the relative intensities of the bands obtained from the Western blot assay presented in (B). *P<0.05 and **P<0.01 compared with the control group.
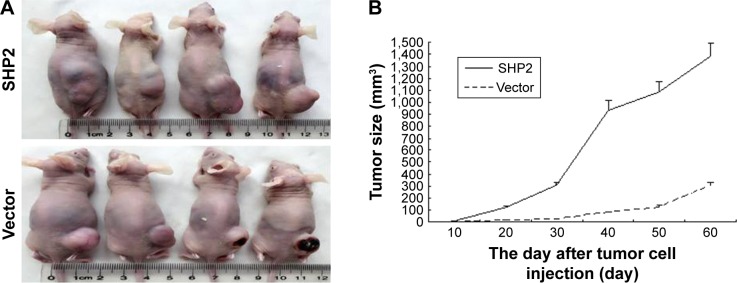
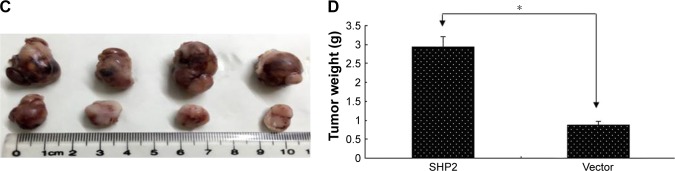
SHP2 overexpression promotes tumor growth in nude mice
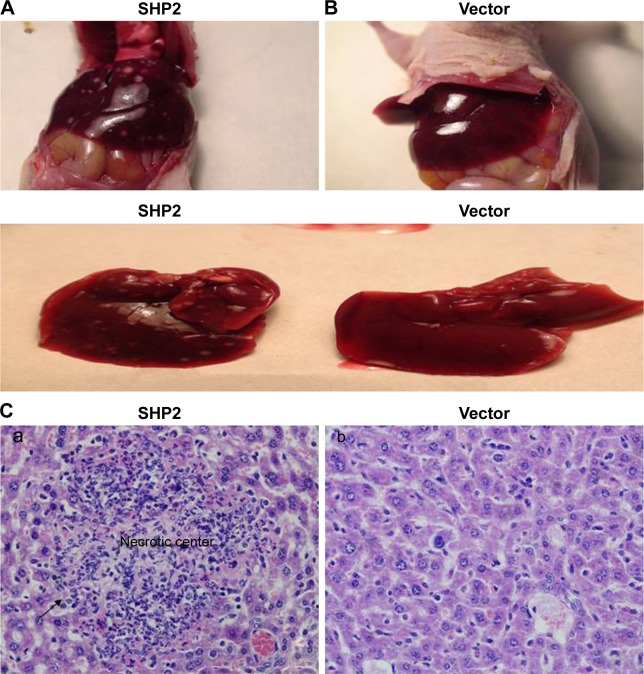
SHP2-overexpressing or control A2780 cells were subcutaneously injected into 4-week-old female nude mice to determine whether SHP2 overexpression promotes ovarian cancer cell tumorigenicity in nude mice. In mice injected with SHP2-overexpressing cells, tumor formation was observed ~7 days after inoculation, whereas tumor formation was observed ~15 days after inoculation in mice injected with the control A2780 cells. The mice were sacrificed 60 days after the tumor cell injection and the tumors were resected. A greater tumor weight was measured in the SHP2 group compared with the control group (Figure 6). Furthermore, liver metastases were visible in the SHP2 group (Figure 7A and B), and the pathological analysis of the resected tumors confirmed these findings (Figure 7C, black arrows indicate liver metastases). In contrast, metastases were not detected in the control nude mice (Table 3). On the basis of these data, SHP2 overexpression promotes ovarian cancer growth and metastasis in nude mice in vivo.
Figure 6.
SHP2 overexpression promotes tumor growth and tumor angiogenesis in a mouse xenograft model.
Notes: (A) Representative images of the ovarian tumors derived from the SHP2-overexpressing (lower row) and vector control (upper row) groups. The images were captured immediately after the mice were sacrificed. (B) The tumor size was monitored every 3 days after the cell injections. (C) The solid tumors were removed and (D) weighed after the mice were sacrificed. The data are presented as mean ± SEM (n=4 per group) (*P<0.05).
Figure 7.
Effect of SHP2 overexpression on tumor metastasis.
Notes: A2780 cells were injected into nude mice. The mice were sacrificed 50 days later and tumor metastasis to distant organs was subsequently assessed. Anatomical images of liver metastasis in mice injected with the SHP2-overexpressing (A) or vector control (B) cells. Lower panel: metastases in the resected livers. (C) Cell morphology was evaluated using H&E staining; a:SHP2 group; b:Vector group. Liver tissues were fixed, sectioned, and stained with H&E, as described in the “Materials and Methods” section. A representative image of metastatic nodes in the livers of mice injected with SHP2-overexpressing cells is shown (magnification, 400×). A minimum of six regions from each tumor were examined.
Table 3.
Effect of SHP2 on tumor (A2780) metastases in different organs of mice bearing xenografted tumors
| Group/organ | Mice with metastases | Liver | Kidney | Lung |
|---|---|---|---|---|
| Vector | 0/4 | 0 | 0 | 0 |
| SHP2 | 4/4 | 4 | 0 | 0 |
SHP2 overexpression promotes ovarian cancer cell invasion by activating AKT
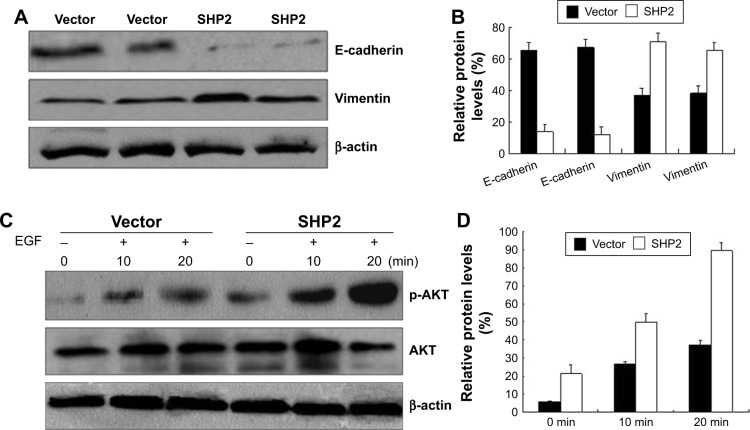
SHP2 activates Ras, at least in part, by promoting the phosphorylation of downstream ligands.23 SHP2 is required for the activation of signal transduction pathways that lead to the downstream activation of the PI3K/AKT pathway.24 Therefore, we examined the activation of PI3K/AKT-dependent kinases in SHP2-overexpressing and control A2780 and Ho-8910PM cells. We examined the levels of AKT and phosphorylated AKT using Western blot assays. AKT phosphorylation was observed in A2780 and Ho-8910PM cells that had been stimulated with EGF for 10 or 20 min, and AKT phosphorylation was significantly increased in SHP2-overexpressing cells compared with control cells (Figure 8C and D). Thus, SHP2 markedly enhances the EGF-mediated activation of the PI3K/AKT signaling pathway. This result is consistent with previous reports showing that SHP2 catalytic activity activates Ras-Raf signaling. However, the mechanisms underlying this effect have yet to be elucidated.
Figure 8.
SHP2 overexpression enhances cell migration and invasion by increasing phospho-AKT levels in ovarian cancer cells.
Notes: (A) Cultured cells were serum-starved for 24 h and stimulated with RPMI 1640 medium supplemented with 10% FBS for 2 h. The levels of the E-cadherin, vimentin, and β-actin proteins were evaluated by Western blotting. (B) Densitometry analysis of the bands from the Western blot using the ImageJ program. The levels of the E-cadherin and vimentin proteins were normalized to the levels of the β-actin protein. (C) A2780 cells were serum-starved overnight and subsequently incubated with EGF (5 ng/mL) for 10 or 20 min. The levels of the p-AKT, AKT, and β-actin proteins were evaluated by Western blotting. (D) Densitometry analysis of the bands from the Western blot assays using the ImageJ program. The levels of the p-AKT protein were normalized to the levels of the β-actin protein. The experiment was repeated 3 times.
Abbreviation: FBS, fetal bovine serum.
We further examined the expression of the pan-epithelial marker E-cadherin and the pan-mesenchymal marker vimentin in SHP2-overexpressing and control cells using Western blot assays. The results confirmed that the SHP2-overexpressing cells displayed significantly decreased E-cadherin expression levels and strongly increased vimentin expression levels compared with control cells. Furthermore, the SHP2-overexpressing cells formed mesenchymal-like intercellular junctions (Figure 8A and B).
Discussion
Tumor proliferation, migration, adhesion, and invasion are regulated by various signal transduction pathways in vivo, and the central link between these pathways is the phosphorylation and dephosphorylation of protein substrates. The balance between protein phosphorylation and dephosphorylation requires cooperation between protein tyrosine kinases and PTP, and increased levels of protein phosphorylation resulting from the disruption of this balance increase the risk of cancer.2,25,26 On the basis of a growing body of evidence, SHP2 is a key factor contributing to the development of pediatric myelomonocytic leukemia, myeloid leukemia, chronic myelomonocytic leukemia,2,9,10,27 and solid tumors, such as breast and lung cancer. In addition, the combination of SHP2 overexpression and Helicobacter pylori infection is closely associated with Cag-positive gastric cancer.24 The role of SHP2 in ovarian cancer has not been reported. Immunohistochemistry was used to evaluate the expression of the SHP2 protein in ovarian tumors and normal ovarian tissues, and the results revealed that SHP2 is expressed at higher levels in ovarian cancer tissues than in normal ovarian tissues. In summary, SHP2 expression is elevated in human ovarian cancer tissues, a finding that is consistent with results observed in other tumor types. Therefore, SHP2 might play an active role in the development of ovarian cancer. In addition, SHP2 overexpression promotes ovarian cancer in vivo. Furthermore, SHP2 overexpression promotes cell proliferation in ovarian cancer cells and tumor development in nude mice. Therefore, SHP2 might be a valuable biomarker for the diagnosis of ovarian epithelial cancer.
SHP2 expression levels were associated with the tumor differentiation status and closely associated with a low degree of tumor differentiation. Furthermore, SHP2 expression was associated with the FIGO stage. High levels of SHP2 expression were associated with more advanced stages of cancer (stages III and IV), and SHP2 was expressed at relatively lower levels in less advanced stages of ovarian cancer (stages I and II). Thus, SHP2 expression is associated with increased proliferation, tumor development, and tyrosine phosphorylation levels in ovarian cancer tissues. Changes in the SHP2 levels disrupted the PI3K/AKT signaling pathway, suggesting that these changes are associated with hyperplasia and ovarian tissue remodeling in patients with ovarian epithelial cancer. SHP2 overexpression was not significantly associated with the patient’s age or the presence of ascites.
According to the results of the transwell assays, cell invasion and migration were markedly increased in ovarian cancer cells overexpressing SHP2 compared with control cells. In addition, SHP2 overexpression enhanced cell proliferation and promoted colony formation in the MTT and colony formation assays, indicating that SHP2 stimulates the growth of ovarian cancer cells. We evaluated the effect of SHP2 overexpression on tumorigenesis using a nude mouse xenograft tumor model to further confirm the impact of SHP2 on ovarian cancer cell growth and found that SHP2 overexpression promoted tumor growth in nude mice. In conclusion, SHP2 overexpression inhibits apoptosis and promotes the proliferation, invasion, in vivo tumorigenicity, and metastasis of ovarian cancer; moreover, SHP2 expression is associated with ovarian tumor differentiation. In addition, the effects of SHP2 overexpression on ovarian cancer are mediated by the activation of the PI3K/AKT signaling pathway. On the basis of our results, SHP2 might be involved in the development of ovarian cancer, and these findings might facilitate the development of novel strategies for the treatment of drug-resistant ovarian cancer and provide novel insights into the role of SHP2 in cancer.
Conclusion
SHP2 overexpression promotes tumor cell migration and invasion, and SHP2 might mediate this effect by promoting the activation of the PI3K signaling pathway. These findings clearly establish a role for SHP2 in ovarian tumor growth. Further research should focus on identifying novel strategies for the prevention and treatment of this disease.
Acknowledgments
This work was supported by a grant from the National Natural Sciences Foundation of China (81501444).
Footnotes
Author contributions
BY contributed to the conception and designed the study. ZQH performed the in vitro and in vivo experiments and wrote the manuscript. JL and QG contributed to collecting, observing and analyzing the clinical data. SW contributed in analyzing the data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015;19(9):2075–2083. doi: 10.1111/jcmm.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. Protein-tyrosine phosphatase SHP-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem. 1998;273(33):21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 4.Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase SHP2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008;27(2):179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- 5.Bentires-Alj M, Paez JG, David FS, et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64(24):8816–8820. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- 6.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28(6):284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 7.Scherr M, Chaturvedi A, Battmer K, et al. Enhanced sensitivity to inhibition of SHP2, STAT5, and Gab2 expression in chronic myeloid leukemia (CML) Blood. 2006;107(8):3279–3287. doi: 10.1182/blood-2005-08-3087. [DOI] [PubMed] [Google Scholar]
- 8.Li SM. The biological function of SHP2 in human disease. Mol Biol (Mosk) 2016;50(1):27–33. doi: 10.7868/S0026898416010110. Russian. [DOI] [PubMed] [Google Scholar]
- 9.Xu R, Yu Y, Zheng S, et al. Overexpression of SHP2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia. Blood. 2005;106(9):3142–3149. doi: 10.1182/blood-2004-10-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D, Liu X, Yu WM, et al. Non-lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase PTPN11 (SHP2) on malignant transformation of hematopoietic cells. J Exp Med. 2011;208(10):1977–1988. doi: 10.1084/jem.20110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Z, Wang X, Fang H, et al. A tyrosine phosphatase SHP2 gain-of-function mutation enhances malignancy of breast carcinoma. Oncotarget. 2016;7(5):5664–5676. doi: 10.18632/oncotarget.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Z, Fang H, Wang X, Chen D, Chen Z, Wang S. Overexpression of SHP2 tyrosine phosphatase promotes the tumorigenesis of breast carcinoma. Oncol Rep. 2014;32(1):205–212. doi: 10.3892/or.2014.3201. [DOI] [PubMed] [Google Scholar]
- 13.Zhou XD, Agazie YM. Inhibition of SHP2 leads to mesenchymal to epithelial transition in breast cancer cells. Cell Death Differ. 2008;15(6):988–996. doi: 10.1038/cdd.2008.54. [DOI] [PubMed] [Google Scholar]
- 14.Han T, Xiang DM, Sun W, et al. PTPN11/SHP2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol. 2015;63(3):651–660. doi: 10.1016/j.jhep.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Deng R, Zhao X, Qu Y, et al. SHP2 SUMOylation promotes ERK activation and hepatocellular carcinoma development. Oncotarget. 2015;6(11):9355–9369. doi: 10.18632/oncotarget.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bard-Chapeau EA, Yuan J, Droin N, et al. Concerted functions of Gab1 and SHP2 in liver regeneration and hepatoprotection. Mol Cell Biol. 2006;26(12):4664–4674. doi: 10.1128/MCB.02253-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneeberger VE, Ren Y, Luetteke N, et al. Inhibition of SHP2 suppresses mutant EGFR-induced lung tumors in transgenic mouse model of lung adenocarcinoma. Oncotarget. 2015;6(8):6191–6202. doi: 10.18632/oncotarget.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furcht CM, Munoz Rojas AR, Nihalani D, Lazzara MJ. Diminished functional role and altered localization of SHP2 in non-small cell lung cancer cells with EGFR-activating mutations. Oncogene. 2013;32(18):2346–2355. 2355.e1–e10. doi: 10.1038/onc.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneeberger VE. Novel Roles of the Protein Tyrosine Phosphatase SHP2 in Non-Small Cell Lung Cancer [dissertations & theses] Florida: University of South Florida; 2014. [Google Scholar]
- 20.Hu ZQ, Ma RUI, Zhang CM, et al. Expression and clinical significance of tyrosine phosphatase SHP2 in thyroid carcinoma. Oncol Lett. 2015;10(3):1507–1512. doi: 10.3892/ol.2015.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartaglia M, Niemeyer CM, Fragale A, et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34(2):148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 22.Pagani MR, Oishi K, Gelb BD, Zhong Y. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell. 2009;139(1):186–198. doi: 10.1016/j.cell.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang WQ, Lin Q, Zhuang X, et al. Structure, function, and pathogenesis of SHP2 in developmental disorders and tumorigenesis. Curr Cancer Drug Targets. 2014;14(6):567–588. doi: 10.2174/1568009614666140717105001. [DOI] [PubMed] [Google Scholar]
- 24.Nagase L, Hayashi T, Senda T, Hatakeyama M. Dramatic increase in SHP2 binding activity of Helicobacter pylori Western CagA by EPIYA-C duplication: its implications in gastric carcinogenesis. Sci Rep. 2015;5:15749. doi: 10.1038/srep15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Zhang F, Niu R. Functions of SHP2 in cancer. J Cell Mol Med. 2015;19(9):2075–2083. doi: 10.1111/jcmm.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breitkopf SB, Yang X, Begley MJ, et al. A cross-species study of PI3K protein-protein interactions reveals the direct interaction of P85 and SHP2. Sci Rep. 2015;6(2):20471. doi: 10.1038/srep20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu R. SHP2, a novel oncogenic tyrosine phosphatase and potential therapeutic target for human leukemia. Cell Res. 2007;17(4):295–297. doi: 10.1038/cr.2007.18. [DOI] [PubMed] [Google Scholar]