Abstract
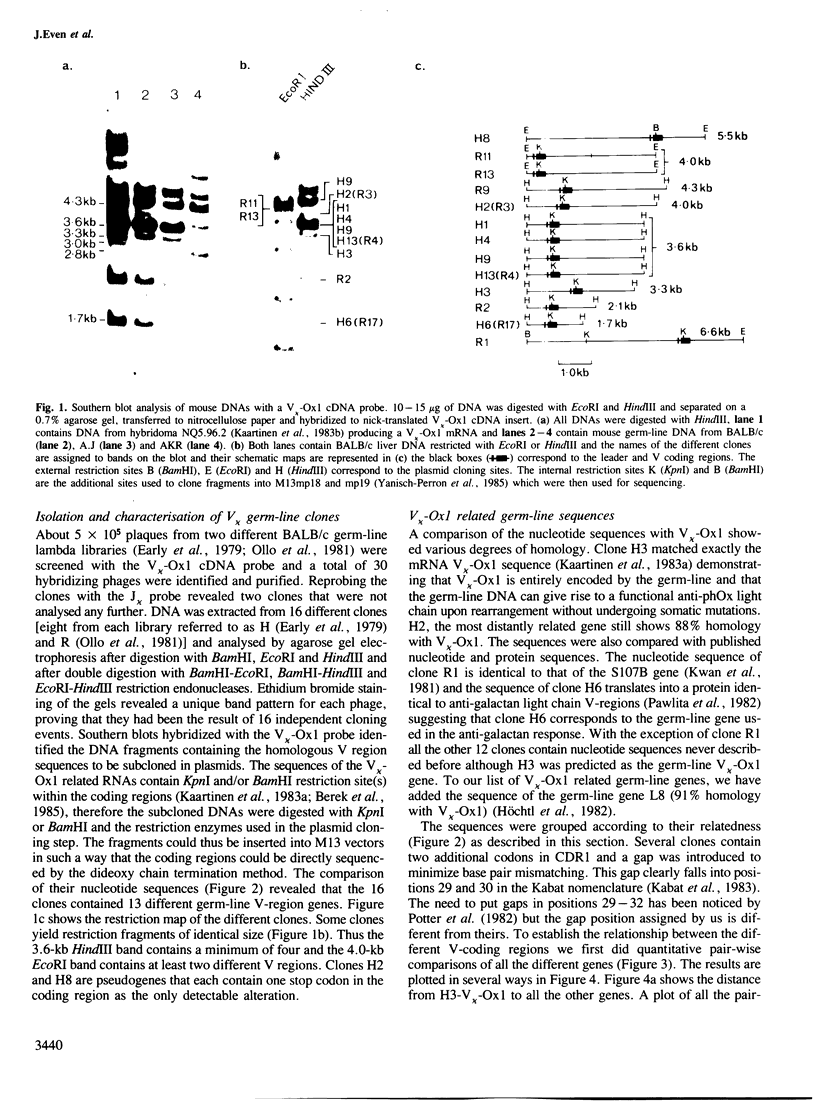
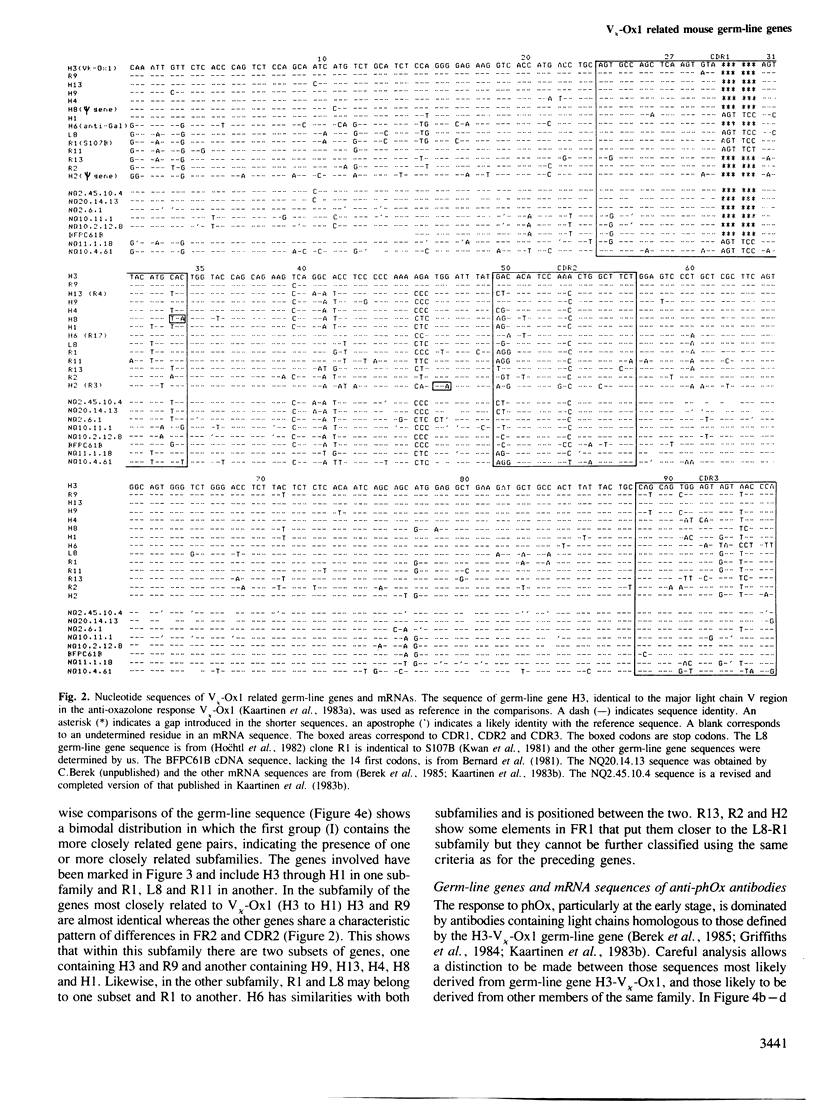
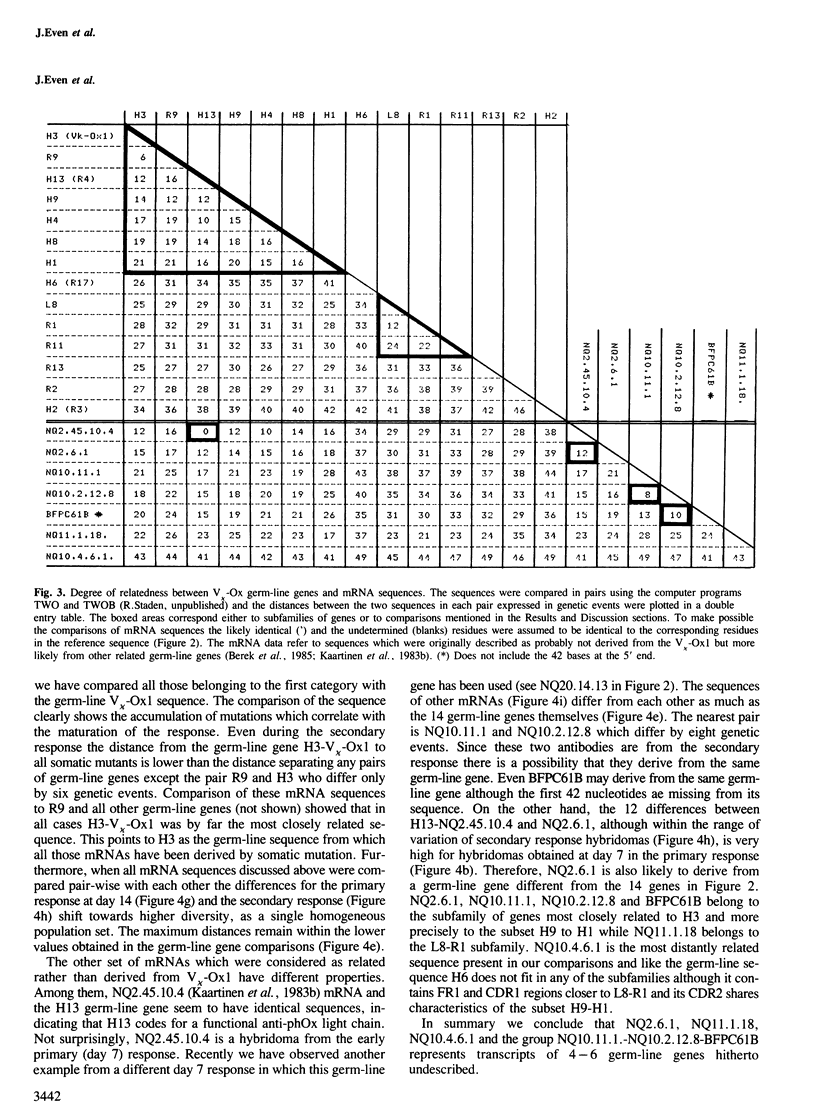
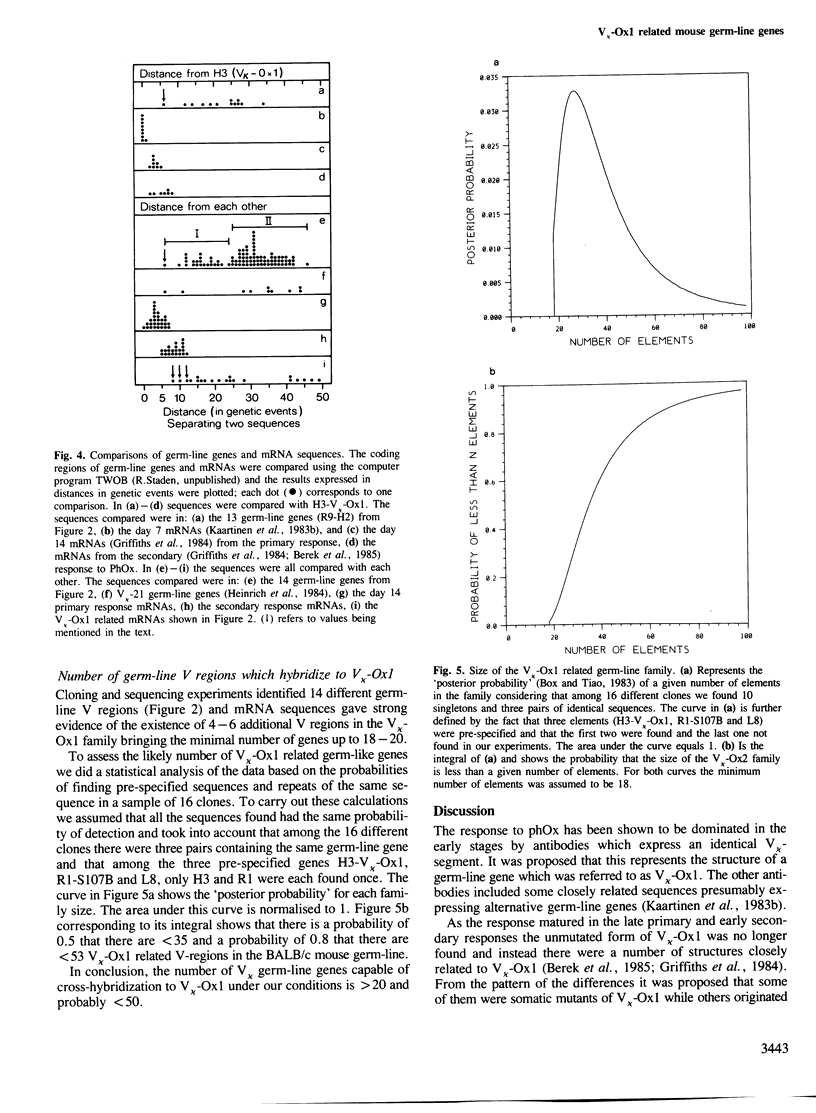
Direct sequencing of mRNA has shown that the early primary response of the BALB/c mouse to the hapten 2-phenyloxazolone is dominated by antibodies with a particular light chain, V kappa-Ox1. Although the V kappa-Ox1 sequence is still commonly expressed later in the response it now includes a number of nucleotide changes. From two independent BALB/c germ-line DNA libraries 13 different genes hybridizing to a V kappa-Ox1 probe were isolated and characterized. Two are identical to mRNA sequences found in the early primary response, one of which is the V kappa-Ox1 sequence. None of the germ-line clones show the characteristic nucleotide changes contained in the late anti-phenyloxazolone light chain mRNAs. These results demonstrate that the V kappa-Ox1 sequence used in the early primary response is entirely encoded by the germ-line and further substantiate the importance of somatic mutations in the maturation of the anti-phenyloxazolone response. The statistical analysis of the data shows that the V kappa-Ox1 related germ-line gene family contains greater than 20 and probably less than 50 genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berek C., Griffiths G. M., Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985 Aug 1;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Bernard O., Gough N. M., Adams J. M. Plasmacytomas with more than one immunoglobulin kappa mRNA: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5812–5816. doi: 10.1073/pnas.78.9.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Effron K., Rechavi G., Ben-Neriah Y., Zakut R., Givol D. Simple DNA sequences in homologous flanking regions near immunoglobulin VH genes: a role in gene interaction? Nucleic Acids Res. 1982 Jun 11;10(11):3353–3370. doi: 10.1093/nar/10.11.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Tyler B. M., Adams J. M. Sets of immunoglobulin V kappa genes homologous to ten cloned V kappa sequences: implications for the number of germline V kappa genes. J Mol Appl Genet. 1981;1(2):103–116. [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Traunecker A., Tonegawa S. Somatic mutation creates diversity in the major group of mouse immunoglobulin kappa light chains. J Exp Med. 1984 Feb 1;159(2):417–435. doi: 10.1084/jem.159.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höchtl J., Müller C. R., Zachau H. G. Recombined flanks of the variable and joining segments of immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1383–1387. doi: 10.1073/pnas.79.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R., Gershenfeld H., Weissman I. L. Evolution of a multigene family of V kappa germ line genes. EMBO J. 1984 Jan;3(1):185–191. doi: 10.1002/j.1460-2075.1984.tb01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Hamlyn P. H., Markham A. F., Karjalainen K., Pelkonen J. L., Mäkelä O., Milstein C. Anti-oxazolone hybridomas and the structure of the oxazolone idiotype. J Immunol. 1983 Feb;130(2):937–945. [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Max E. E., Seidman J. G., Leder P., Scharff M. D. Two kappa immunoglobulin genes are expressed in the myeloma S107. Cell. 1981 Oct;26(1 Pt 1):57–66. doi: 10.1016/0092-8674(81)90033-7. [DOI] [PubMed] [Google Scholar]
- Loh D. Y., Bothwell A. L., White-Scharf M. E., Imanishi-Kari T., Baltimore D. Molecular basis of a mouse strain-specific anti-hapten response. Cell. 1983 May;33(1):85–93. doi: 10.1016/0092-8674(83)90337-9. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ollo R., Auffray C., Sikorav J. L., Rougeon F. Mouse heavy chain variable regions: nucleotide sequence of a germ-line VH gene segment. Nucleic Acids Res. 1981 Aug 25;9(16):4099–4109. doi: 10.1093/nar/9.16.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlita M., Potter M., Rudikoff S. kappa-Chain restriction in anti-galactan antibodies. J Immunol. 1982 Aug;129(2):615–618. [PubMed] [Google Scholar]
- Pech M., Smola H., Pohlenz H. D., Straubinger B., Gerl R., Zachau H. G. A large section of the gene locus encoding human immunoglobulin variable regions of the kappa type is duplicated. J Mol Biol. 1985 Jun 5;183(3):291–299. doi: 10.1016/0022-2836(85)90001-4. [DOI] [PubMed] [Google Scholar]
- Potter M., Newell J. B., Rudikoff S., Haber E. Classification of mouse VK groups based on the partial amino acid sequence to the first invariant tryptophan: impact of 14 new sequences from IgG myeloma proteins. Mol Immunol. 1982 Dec;19(12):1619–1630. doi: 10.1016/0161-5890(82)90273-5. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Ram D., Glazer L., Zakut R., Givol D. Evolutionary aspects of immunoglobulin heavy chain variable region (VH) gene subgroups. Proc Natl Acad Sci U S A. 1983 Feb;80(3):855–859. doi: 10.1073/pnas.80.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff C., Milili M., Fougereau M. Functional and pseudogenes are similarly organized and may equally contribute to the extensive antibody diversity of the IgVHII family. EMBO J. 1985 May;4(5):1225–1230. doi: 10.1002/j.1460-2075.1985.tb03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]




