Abstract
Objective
To explore the abnormal intrinsic functional hubs in alcohol dependence using voxelwise degree centrality analysis approach, and their relationships with clinical features.
Materials and methods
Twenty-four male alcohol dependence subjects free of medicine (mean age, 50.21±9.62 years) and 24 age- and education-matched male healthy controls (mean age, 50.29±8.92 years) were recruited. The alcohol use disorders identification test and the severity of alcohol dependence questionnaire (SADQ) were administered to assess the severity of alcohol craving. Voxelwise degree centrality approach was used to assess the abnormal intrinsic functional hubs features in alcohol dependence. Simple linear regression analysis was performed to investigate the relationships between the clinical features and abnormal intrinsic functional hubs.
Results
Compared with healthy controls, alcohol dependence subjects exhibited significantly different degree centrality values in widespread left lateralization brain areas, including higher degree centrality values in the left precentral gyrus (BA 6), right hippocampus (BA 35, 36), and left orbitofrontal cortex (BA 11) and lower degree centrality values in the left cerebellum posterior lobe, bilateral secondary visual network (BA 18), and left precuneus (BA 7, 19). SADQ revealed a negative linear correlation with the degree centrality value in the left precentral gyrus (R2=0.296, P=0.006).
Conclusion
The specific abnormal intrinsic functional hubs appear to be disrupted by alcohol intoxication, which implicates at least three principal neural systems: including cerebellar, executive control, and visual cortex, which may further affect the normal motor behavior such as an explicit type of impaired driving behavior. These findings expand our understanding of the functional characteristics of alcohol dependence and may provide a new insight into the understanding of the dysfunction and pathophysiology of alcohol dependence.
Keywords: alcohol addiction, substance dependence, degree centrality, functional magnetic resonance imaging, functional connectivity driving behavior
Introduction
Alcohol dependence (AD), as a psychiatric disorder, is characterized by a morbid pattern of alcohol use, which often leads to adversely interpersonal, social and health consequences. The AD is associated with widespread brain function impairment, and recently knowledge about the AD on the underlying neurobiology has increased tremendously.1,2 Considering the high prevalence of AD and the known neurotoxic effects, it is essential to further expound its complex brain network impairments. However, the underlying mechanism of these neuropsychiatric alterations remains ambiguous.
Multiple lines of neurobiological researches have revealed brain function alterations associated with AD. Most of the studies focus their attentions on neuroimaging effects of alcohol cue exposure.3–5 In this process, neuroimaging methods were widely used to demonstrate task-specific or cue-specific alterations in regional brain activity. However, it may hamper us to insights into integrated global brain function alterations in resting-state or non-task condition when we solely focus on regional functional brain activity alterations under task-specific or cue-specific conditions because of the behavioral variability. Moreover, the integration between the task-specific or cue-specific related brain areas into a broader functional network in the brain remains unclear.6
It is proposed that resting-state functional magnetic resonance imaging (rfMRI), one of the hot areas in neuroim-aging and one that is suitable for the mechanism research of central nervous system, can detect the spontaneous neuronal activity of the human brain and provide new insights into the pathophysiology of disease, because of its advantages in not requiring exposure to radioactive tracers, accurate positioning, and ease of combining functional imaging with structural imaging.7 Functional connectivity is an important component of rfMRI, in which the seed-based functional connectivity measurement can be used to outline the functional connectome, but it only provides limited information of the relationships between the given seed point region and other brain regions in the whole-brain network. Recently, graph theory-based network analyses have been applied to explore the brain connectivity within whole-brain network.8–10 Specifically, degree centrality is a class of graph-theoretic measures assessing the importance of each node in brain network, in terms of its connectivity strength to every voxel.11–13 In contrast to traditional seed-based functional connectivity approach, the degree centrality approach provides an unbiased opportunity to search abnormalities within the entire connectivity matrix of full-brain functional connectome without priori hypothesis without a priori definition of regions of interest (ROIs). The degree centrality approach is a criterion to measure the importance of the individual node and reflects the functional brain network “hub” properties, that is, the communication ability of network information,14 and exhibits relatively high test–retest reliability.15 Recently, the degree centrality approach has been successfully used to disclose the neurobiological mechanism for several diseases, such as obsessive compulsive disorder,16 Alzheimer’s disease,17 and major depressive disorder.18 However, AD has not been studied.
Long-term alcohol abuse or AD is associated with the changes in behavior, brain function, and brain structure. However, the nature of these changes has not been well understood. To provide a new insight into the underlying neurobiological mechanism of AD, this study is the first to use degree centrality approach to identify altered intrinsic functional connectivity hubs based on voxel-based whole-brain correlation analysis from the entire connectivity matrix of full-brain functional connectome. Next, we conducted multiple linear regression analysis to evaluate the relationships between the clinical features and the degree centrality value of those significant alterations in intrinsic functional hubs.
Materials and methods
Subjects
A total of 24 male AD subjects (mean age, 50.21±9.62 years; mean education, 9.67±3.09 years) and 24 age- and education-matched male healthy controls (mean age, 50.29±8.92 years; mean education, 8.33±3.21 years) were recruited from the hospital and community. All AD subjects were the first-time visitors and previously had never taken benzodiazepine or chlormethiazole medications.
The AD subjects met the pertinent inclusion criteria: 1) met the diagnostic criteria of AD as defined by the Diagnostic and Statistical Manual of Mental Disorders, version 4 (DSM-IV); 2) had not received any treatment before; 3) had not any history of major psychiatric disorders and sleep disorders; 4) had not any other substance abuse or dependence (such as marihuana and tobacco addiction) as defined by DSM-IV; 5) right-handedness; 6) had not any family history of subjects with AD. The exclusion criteria comprised pathological brain MRI findings, inborn or other acquired diseases, and any foreign implants in the body.
Research design and process
An experienced psychiatrist evaluated the AD subjects with the DSM-IV for the life history of psychiatric disorders, as well as an unstructured clinical interview for the history of other psychiatric axis disorders. The alcohol use disorders identification test (AUDIT) and the severity of alcohol dependence questionnaire (SADQ) were administered to assess the severity of alcohol craving. The data of the mean years of drink and daily alcohol consumption were recorded.
All volunteers participated voluntarily and were informed of the purposes, methods, and potential risks. All volunteers signed an informed consent form. This study was approved by The Human Research Ethics Committee of The First Affiliated Hospital of Nanchang University.
MRI
The MRI scan was performed on 3-Tesla MR scanners (Trio; Siemens, Erlangen, Germany). High-resolution T1-weighted anatomical images were acquired with a three-dimensional spoiled gradient-recalled sequence in a sagittal orientation: 176 images (repetition time =1,900 ms, echo time =2.26 ms, thickness =1.0 mm, gap =0.5 mm, acquisition matrix =256×256, field of view =250×250 mm, flip angle =9°) were obtained. Next, an 8-min rs-fMRI scan was obtained with eyes closed. Total of 240 functional images (repetition time =2,000 ms, echo time =30 ms, thickness =4.0 mm, gap =1.2 mm, acquisition matrix =64×64, flip angle =90°, field of view =220×220 mm, 29 axial slices with Gradient-Recalled Echo-Planar Imaging pulse sequence) covering the whole brain were obtained. A simple questionnaire was administered immediately after the MRI scan to ensure whether the subjects were awake during the scan. The data of the subjects who were asleep during the scans will be excluded.
Data analysis
MRIcro software was used to ensure the data quality. The first 10 time points of the functional images were discarded because of the possible instability of the initial MRI signal and the participants’ adaptation to the scanning environment. On the basis of MATLAB2010a (Mathworks, Natick, MA, USA), the rest of the data preprocessing was performed by Data Processing and Analysis for Brain Imaging (DPABI 2.1) toolbox, including Digital Imaging and Communications in Medicine standards for form transformation, slice timing, head-motion correction, spatial normalization, and smooth with a Gaussian kernel of 6×6×6 mm3 full-width at half-maximum. The participants who had more than 1.5 mm maximum translation in x, y, or z and 1.5° degree of motion rotation were rejected. The Friston 24 head-motion parameters model, including six head-motion parameters, six head-motion parameters, and 12 corresponding squared items, was used to regress out the head-motion effects based on recent work showing that the higher-order models benefit from the removal of head-motion effects.19,20 Linear regression was applied to remove other sources of spurious covariates along with their temporal derivatives, including the global mean signal and white matter and cerebrospinal fluid signal. After the head-motion correction, the functional MRI images were spatially normalized to the Montreal Neurological Institute space and resampled at a resolution of 3×3×3 mm. After the preprocessing, the time series for each voxel were temporally bandpass filtered (0.01–0.1 Hz) and linearly detrended to reduce low-frequency drift and physiological high-frequency respiratory and cardiac noise.
Calculation of degree centrality maps
Degree centrality attributes a greater value to a voxel if it has strong connections with many other voxels in the brain. For the calculation of voxelwise degree centrality, the preprocessing fMRI data were used to compute the voxel-based whole-brain functional correlation analysis. The Pearson’s correlation coefficients (r) between each pair of brain gray matter voxels were computed. As a result, we acquired a matrix of Pearson correlation coefficients depicting whole-brain functional connectivity pattern. To obtain each subject’s graph, whole-brain functional network was then constructed by defining threshold of each correlation.11,12 Degree centrality was calculated by counting the number of significant suprathresholded correlations (or the degree of the binarized adjacency matrix) for each subject based on the individual voxelwise functional network. Finally, the voxelwise degree centrality map for each individual was converted into a z-score map using the following equation:
where i is the voxel index, degree centrality i is the degree centrality value for the ith voxel, std is the standard deviation, and Zi is the z-score for the ith voxel.
In this study, we repeated the network analysis using a range of correlation r thresholds (ie, r=0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, and 0.5) to determine that the between-group differences in degree centrality were not substantially affected by the selection of different r-value thresholds or nodes to construct the brain networks.
Statistical analysis
Behavioral data
The demographic factors (age, education, and years of education) and the questionnaire data were compared between groups using two-sample t-tests. Chi-square (χ2) test was used for categorical data. The statistical analysis was performed using IBM Statistical Package for the Social Sciences version 21.0 (SPSS 21.0). Data are presented as mean ± standard deviation. All the results were quoted as two-tailed, and P<0.05 was considered as statistically significant.
Voxelwise degree centrality data
For voxelwise degree centrality, two-sample t-tests were used to investigate the voxelwise degree centrality differences in brain regions between AD and healthy controls with age, and years of education as nuisance covariates of no interest. We analyzed group differences in two ways. First, we performed a threshold of P<0.05, corrected for multiple comparisons by false discovery rate (FDR) method. Second, we used a loose statistical threshold if correction for multiple comparison failed to detect any difference. We applied voxelwise analysis of variance with an uncorrected significance level of individual voxel threshold of P<0.01 with a minimum continuous cluster voxel volume (V) of 540 mm3 to determine the statistical significance.
Multiple linear regression analysis
Multiple linear regression analysis was performed to evaluate the relationships between the clinical features (dependent variable) and the degree centrality value of those different brain regions (independent variable). P<0.05 was considered to be the significant difference.
Results
Sample characteristics of alcohol-dependent subjects
The demographic characteristics of the AD subjects are presented in Table 1. There were no significant differences in mean age (t=−0.031, P=0.975) and mean education (t=1.466, P=0.149) between AD subjects and healthy controls. AD showed significantly higher AUDIT score than healthy controls (t=18.199, P<0.001). The mean years of drink, SADQ score, and daily alcohol consumption of AD subjects were (27.46±10.89) years, (20.21±7.09), and (237.5±115.39) mL, respectively.
Table 1.
Characteristics of AD subjects and healthy controls
| AD subjects | Healthy controls | t-value | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age, years | 50.21±9.62 | 50.29±8.92 | −0.031 | 0.975 |
| Sex (male, female) | 24 (24, 0) | 24 (24, 0) | N/A | N/A |
| Education, year | 9.67±3.09 | 8.33±3.21 | 1.466 | 0.149 |
| Years of drink, year | 27.46±10.89 | N/A | N/A | N/A |
| SADQ | 20.21±7.09 | N/A | N/A | N/A |
| AUDIT | 24.08±5.69 | 2.63±0.97 | 18.199 | <0.001 |
| Daily alcohol consumption, mL | 237.5±115.39 | N/A | N/A | N/A |
Note: Data are mean ± standard deviation values.
Abbreviations: AD, alcohol dependent; SADQ, severity of alcohol dependence questionnaire; AUDIT, alcohol use disorders identification test; N/A, not applicable.
Binarized degree centrality differences between groups
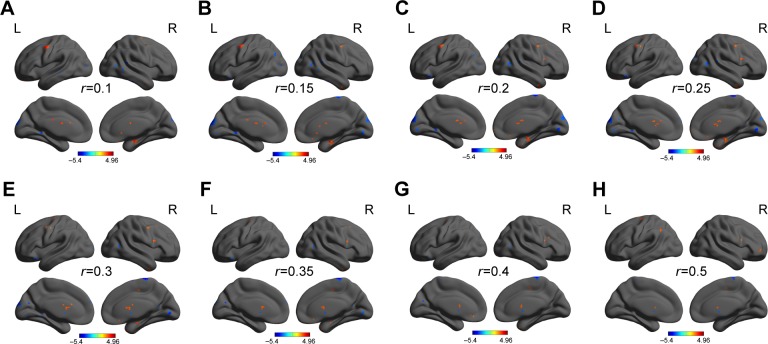
We analyzed binary voxelwise functional correlations and further investigated intra- and intergroup differences of binary voxelwise functional brain centrality. We observed highly similar intragroup differences of binary degree centrality, not dependent on different thresholding schemes, in several thresholds at r=0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, and 0.50 (Figure 1A–H). For this reason, in this study we only reported the results of binary degree centrality network with threshold at r=0.15.
Figure 1.
Binarized degree centrality maps with several thresholds between AD subjects and healthy controls.
Note: Between-group differences of binary network with different thresholds at r=0.10 (A), 0.15 (B), 0.20 (C), 0.25 (D), 0.30 (E), 0.35 (F), 0.40 (G), and 0.50 (H).
Abbreviations: AD, alcohol dependent; L, left; R, right.
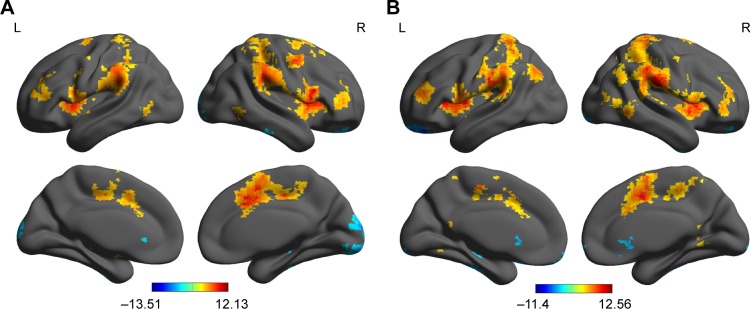
First, we reported within-group statistic map of degree centrality measurement for AD group (Figure 2A) and healthy controls group (Figure 2B) using one-sample t-test (P<0.01, continuous cluster voxel volume ≥540 mm3, FDR corrected). We found that both groups consistently showed significantly similar different binarized degree centrality values in brain areas, including the cerebellum posterior lobe, insula, secondary visual network, striatum, precentral gyrus, hippocampus and orbitofrontal cortex, precuneus, anterior cingulate, postcentral gyrus, and supplementary motor area (Figure 2A and B).
Figure 2.
Within-group statistic maps of AD subjects (A) and healthy controls (B) in binarized degree centrality network using one-sample t-test.
Note: Number (A) and voxel volume (B) of different DC values in brain areas.
Abbreviations: AD, alcohol dependent; DC, degree centrality; L, left; R, right.
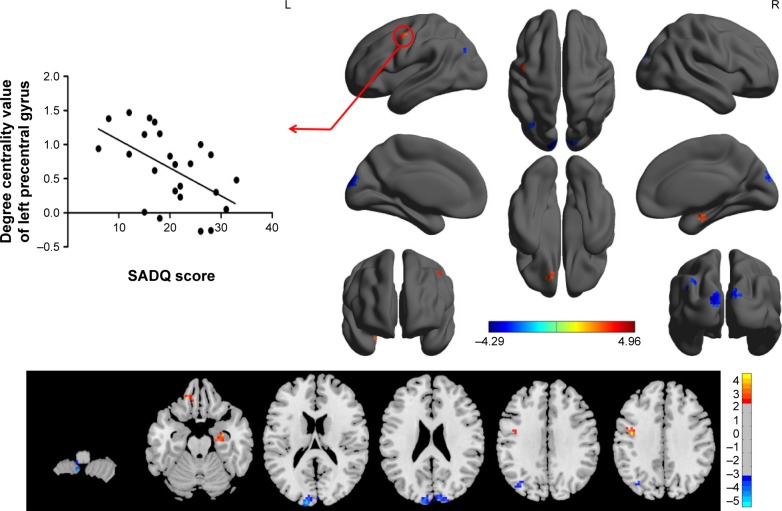
The binary degree centrality did not reveal any significant between-group differences after corrected FDR. Next, we compared the binary degree centrality differences between AD group and healthy controls group using a more liberal statistical approach (uncorrected P<0.01, V≥540 mm3), and several significant between-group differences were found (Figure 3; Table 2). Compared with the healthy controls, AD subjects exhibited significantly higher degree centrality values in the left precentral gyrus (BA 6), right hippocampus (BA 35, 36), and left orbitofrontal cortex (BA 11), and lower degree centrality values in the left cerebellum posterior lobe, bilateral secondary visual network (BA 18), and left precuneus (BA 7, 19). The mean degree centrality values in these different brain regions were extracted (Figure 4).
Figure 3.
Between-group differences in binarized degree centrality network with threshold at r=0.15, and their correlations with clinical features in AD subjects.
Abbreviations: AD, alcohol dependent; SADQ, severity of alcohol dependence questionnaire; L, left; R, right.
Table 2.
The binarized degree centrality differences between AD subjects and healthy controls
| Conditions | Brain regions of peak coordinates | R/L | BA | Voxel volume (mm3) | t-score of peak voxel | MNI coordinates
|
|---|---|---|---|---|---|---|
| X, Y, Z | ||||||
| AD > HCG | Precentral gyrus | L | 6 | 1,161 | 4.958 | −42, −6, 36 |
| AD > HCG | Hippocampus | R | 35, 36 | 567 | 4.3415 | 30, −9, −21 |
| AD > HCG | Inferior frontal gyrus | L | 11 | 540 | 4.4211 | −15, 39, −21 |
| AD < HCG | Cerebellum posterior lobe | L | N/A | 756 | −4.1576 | −9, −54, −57 |
| AD < HCG | Cuneus | L | 18 | 1,728 | −4.2934 | −12, −96, 18 |
| AD < HCG | Cuneus | R | 18 | 783 | −3.2916 | 12, −90, 24 |
| AD < HCG | Precuneus | L | 7, 19 | 1,026 | −3.7589 | −36, −72, 33 |
Notes: Between-group differences in binarized degree centrality thresholded at r=0.15. The statistical threshold was set at uncorrected voxel threshold of P<0.01 with a minimum voxel volume threshold of 540 mm3.
Abbreviations: AD, alcohol dependent; HCG, healthy control group; R, right; L, left; BA, Brodmann’s area; MNI, Montreal Neurological Institute; N/A, not applicable.
Figure 4.
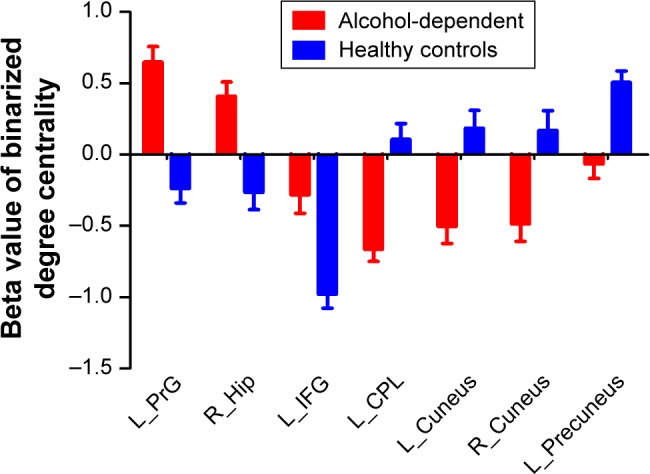
Binarized degree centrality values of between-group differences in different brain areas.
Note: Data are mean ± standard error values.
Abbreviations: L, left; R, right; PrG, precentral gyrus; Hip, hippocampus; IFG, inferior frontal gyrus; CPL, cerebellum posterior lobe.
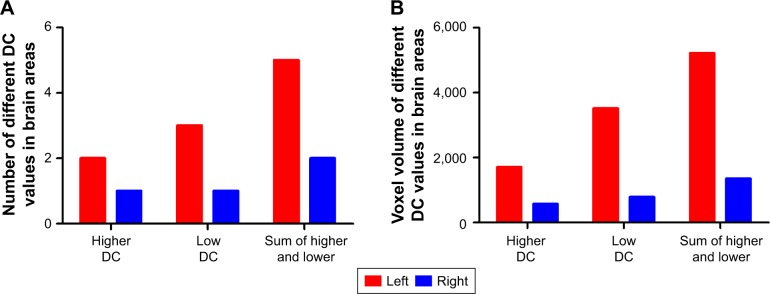
Interestingly, the higher degree centrality value, lower degree centrality value, and sum of both higher- and lower degree centrality values in brain areas between AD individuals and healthy controls consistently showed more numbers of different degree centrality values in brain areas in left than that of right (Figure 5A), and more total numbers of voxel volume of different degree centrality values in brain areas in left than that of right (Figure 5B).
Figure 5.
Unilateral lateralization in brain areas.
Note: Number (A) and voxel volume (B) of different DC values in brain areas.
Abbreviation: DC, degree centrality.
Regression analysis
As shown in Figure 2, SADQ in AD subjects revealed a negative linear correlation with degree centrality value in the left precentral gyrus (R2=0.296, β=−7.241, t=−3.041, P=0.006). No other significant linear correlations between degree centrality value in those different brain areas and clinical features were found (P>0.05).
Discussion
To the best of our knowledge, the current study is the first to apply resting-state degree centrality analysis approach to investigate the abnormal intrinsic functional hubs in male AD subjects, and their relationships with clinical features. Long-term alcohol abuse is associated with change in behavior, brain structure, and brain function. Specifically, we found that AD subjects were associated with a sequential pattern of widespread changes in resting-state degree centrality indices of intrinsic functional hubs, including significantly higher degree centrality values in the left precentral gyrus, right default mode network, and left orbitofrontal cortex, and lower degree centrality values in the left cerebellum posterior lobe, bilateral secondary visual network, and left executive control network. Furthermore, these intrinsic functional hubs revealed linear correlation with clinical features.
Left hemisphere and right hemisphere have the similar cerebral tissue structure, but their functional framework is different. Left hemisphere only receives offside stimulus, while the right hemisphere receives bilateral stimulus. Interestingly, in the present study we found a strong unilateral lateralization in male AD individuals, showing that higher, lower, and sum of both higher- and lower degree centrality values in brain areas between AD individuals and healthy controls consistently showed more numbers of different degree centrality value in brain areas in left than that of right, and this lateralization was also found in total numbers of voxel volume of different degree centrality values in brain areas. Similarly, in male smoking dependence subjects, right ear responses were selectively impaired while left ear responses increased.21 Li et al found lower fractional anisotropy only in the right hemisphere in insomnia patients.22 Recent studies show that execution attentional control is biased toward the right ear.23 The same is found for lateralized word processing in the visual modality.24 Our previous sleep deprivation study also found that both male and female healthy subjects, from rested wakeful status to sleep deprivation status, demonstrated a regular pattern of changes, that is, the unilateral tendency of the brain regional homogeneity regions started to disappear and the brain regional homogeneity regions spread to the opposite side or to bilateral sides.25 Therefore, the unilateral lateralization is not unique, and it may happen in ill condition and even in normal condition. To improve the efficiency of brain function in AD individuals, the human brain represented strong unilateral lateralization to conduct specialization and cooperation. Thus, the left lateralization may demonstrate that the left brain hemisphere displayed a facilitatory effect on production and transmission of brain activities in AD individuals.
The degree centrality reveals whole-brain network connectivity between particular brain voxels to other brain voxels, rather than within specific nodes or networks, which is relatively high in the functional hubs of the brain network. It may serve as an important hub for information integration, superior information propagation, and critical way stations for information processing, and may thus lead to effective information flow.12,26 Alcoholism-induced neuropathology does not typically eliminate a function; rather, it results in anatomically injured tissue or functionally disturbed networks that contribute to cognitive dysfunction, especially deficits of executive functions such as verbal and spatial working memory.27–28 In the present study we found significantly higher degree centrality values in the left orbitofrontal cortex, left precentral gyrus, and right hippocampus in AD subjects compared with healthy controls. There are two prevalent speculations. One explanation of this finding could be that this is a brain compensation mechanism that alcohol compromises the functionality of networks beyond self (within) compensation.29 The orbitofrontal cortex is a major area of motivation, drive, and salience evaluation, and is thought to play an important role in executive function, including self-control, adaptive responding in human addiction processing, as well as the output of compulsive drug-seeking behaviors.30 Therefore, the higher degree centrality in the cognitive function-related brain areas, such as orbitofrontal cortex and hippocampus, may utilize additional cognitive resources to help the individuals to achieve the same level of performance as before. Previous studies have shown long-term effects of alcohol on human brain structure both in gray and white matter.31,32 Another explanation of the higher degree centrality values in the left precentral gyrus, right hippocampus, and left orbitofrontal cortex could be that the hyperactivation in these areas may be interpreted as an enhanced neural effort to offset the brain structural damage. Therefore, the increased DC in those brain areas may reveal the widespread structural disturbances in AD.
Reduced degree centrality values in brain functional hubs represent fewer correlated activity and impaired roles of these hubs in facilitating neural network communication.33 In alcoholics, the concept of inefficiency includes difficulties in isolating irrelevant information,34 which is necessary for discriminating the targets from the distractors. Whether engaged in a task or during resting state, processing inefficiency in AD could be caused by the disruption of one or multiple functional brain networks. Thus, the inefficient processing may arise from or reveal disorganization of one or multiple functional brain networks, leading to inefficiency in information transmission from one place to other. In this framework, the reduced degree centrality value in the bilateral secondary visual network, cerebellar circuits, and left precuneus in the executive control network observed in our study may be an expression of inefficiency in information transmission and disorganization of these functional brain networks associated with AD.
The alcoholics were impaired on executive functions, visuospatial abilities, gaits, and balances.35 There was a decreased effective connectivity between the visuomotor system and the posterior parietal cortex after alcohol consumption.36 The application of alcohol resulted in a decreased activation amplitude across the entire visual cortex, and dose-dependent effects were found in such changes.37 The cerebellum posterior lobe(s) has been widely used for the adjusting nerve function, and adjusting the start and planning and the coordinating movement, and it also works together with the cerebrum to complete the functions such as the cognition, language, and emotion.25,38 The cerebellum has been shown to be particularly vulnerable to alcoholism-related damage.35 Functional network connectivity between the frontal–temporal–basal ganglia and the cerebellar circuits, responsible in part for maintaining normal motor behavior by integrating their overlapping motor control functions, appears to be disrupted by alcohol intoxication, in turn associated with an explicit type of impaired driving behavior.39 Precuneus is associated with some basic cognitive activities of human brain under resting-state condition, such as collection and evaluation of information, self-referential mental activity, extraction of episodic memory, emotion, and anxiety. Acute exposure to cue of ethanol was found to significantly impact the functional hubs in the precuneus in nonhuman primate brain,40 and which was also found in human brain in heavy drinkers and individuals with alcohol use disorder.41 In humans, a functional connectivity study of changes in chronic alcohol users found increased connectivity between the precuneus and the cerebellum.42 Reduced degree centrality value in the left precuneus in the executive control network was observed in the present study, which was consistent with the previous study that individuals with problematic alcohol use had significantly lower network connectivity strength than controls in the left executive control and primary visual networks, and the connectivity strength of the executive control network is negatively correlated with alcohol disorder severity.43 The executive control function might be viewed as particularly pertinent to the field of substance dependence because it is thought to be involved in goal-directed behavior and cognitive control. These findings suggested that long-term alcoholism had significantly impaired the functional connectivity of the neural network hubs in the cerebellar cortex, executive control function, and visual cortex, because of which the negative functional deficits implicated the visuomotor system and further affected the maintenance of normal motor behavior, which appears to be disrupted by alcohol intoxication, in turn associated with an explicit type of impaired driving behavior.39
Conclusion
Our study provides new insight into understanding the dysfunction and pathophysiology of AD using graph-theoretic whole-brain network analysis with unbiased opportunity to search abnormalities within the entire connectivity matrix of full-brain functional connectome without priori hypothesis and a priori definition of ROIs. The present study showed that the pattern of negative functional deficits appears to be disrupted by alcohol intoxication, which implicates at least three principal neural systems: cerebellar-executive control-visual cortex, which further affected normal visuomotor behaviors such as an explicit type of impaired driving behavior. These findings highlight the role of functional connectivity in the pathophysiology of AD and expand our understanding of the functional characteristics of AD.
Acknowledgments
This study was supported by Zhejiang Provincial Medical and health technology program of health department (grant no 2017KY632), and the National Natural Science Foundation of China (grant No 8170071333).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsycho-pharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltz AM, Gates KM, Engels AS, et al. Changes in alcohol-related brain networks across the first year of college: a prospective pilot study using fMRI effective connectivity mapping. Addict Behav. 2013;38(4):2052–2059. doi: 10.1016/j.addbeh.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donald KA, Eastman E, Howells FM, et al. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatr. 2015;27(5):251–269. doi: 10.1017/neu.2015.12. [DOI] [PubMed] [Google Scholar]
- 5.Lv W, Wu Q, Liu X, et al. Cue reactivity in nicotine and alcohol addiction: a cross-cultural view. Front Psychol. 2016;7:1335. doi: 10.3389/fpsyg.2016.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjoerds Z, Stufflebeam SM, Veltman DJ, Van den Brink W, Penninx BW, Douw L. Loss of brain graph network efficiency in alcohol dependence. Addict Biol. 2017;22(2):523–534. doi: 10.1111/adb.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai XJ, Liu CL, Zhou RL, et al. Long-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2015;11:761–772. doi: 10.2147/NDT.S78335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 9.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo XN, Ehmke R, Mennes M, et al. Network centrality in the human functional connectome. Cereb Cortex. 2012;22(8):1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
- 13.Telesford QK, Simpson SL, Burdette JH, Hayasaka S, Laurienti PJ. The brain as a complex system: using network science as a tool for understanding the brain. Brain Connect. 2011;1(4):295–308. doi: 10.1089/brain.2011.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Martino A, Zuo X, Kelly C, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo X, Xing X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci Biobehav Rev. 2014;45:100–118. doi: 10.1016/j.neubiorev.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Gottlich M, Kramer UM, Kordon A, Hohagen F, Zurowski B. Resting-state connectivity of the amygdala predicts response to cognitive behavioral therapy in obsessive compulsive disorder. Biol Psychol. 2015;111:100–109. doi: 10.1016/j.biopsycho.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Guo Z, Liu X, Hou H, Wei F, Liu J, Chen X. Abnormal degree centrality in Alzheimer’s disease patients with depression: a resting-state functional magnetic resonance imaging study. Exp Gerontol. 2016;79:61–66. doi: 10.1016/j.exger.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, Yao J, Jiang X, et al. Sub-hubs of baseline functional brain networks are related to early improvement following two-week pharmacological therapy for major depressive disorder. Hum Brain Mapp. 2015;36(8):2915–2927. doi: 10.1002/hbm.22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan CG, Cheung B, Kelly C, et al. A Comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn C, Pogun S, Gunturkun O. Smoking modulates language lateralization in a sex-specific way. Neuropsychologia. 2010;48(14):3993–4002. doi: 10.1016/j.neuropsychologia.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Tian J, Bauer A, Huang R, et al. Reduced integrity of right lateralized white matter in patients with primary insomnia: a diffusion-tensor imaging study. Radiology. 2016;280(2):520–528. doi: 10.1148/radiol.2016152038. [DOI] [PubMed] [Google Scholar]
- 23.Hugdahl K, Westerhausen R, Alho K, Medvedev S, Laine M, Hämäläinen H. Attention and cognitive control: unfolding the dichotic listening story. Scand J Psychol. 2009;50(1):11–22. doi: 10.1111/j.1467-9450.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls ME, Wood AG, Hayes L. Cerebral asymmetries in the level of attention required for word recognition. Laterality. 2001;6(2):97–110. doi: 10.1080/713754408. [DOI] [PubMed] [Google Scholar]
- 25.Dai XJ, Gong HH, Wang YX, et al. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep Med. 2012;13(6):720–727. doi: 10.1016/j.sleep.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Sato JR, Salum GA, Gadelha A, et al. Decreased centrality of subcortical regions during the transition to adolescence: a functional connectivity study. Neuroimage. 2015;104:44–51. doi: 10.1016/j.neuroimage.2014.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180(4):583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- 29.Jansen JM, van Holst RJ, van den Brink W, Veltman DJ, Caan MW, Goudriaan AE. Brain function during cognitive flexibility and white matter integrity in alcohol-dependent patients, problematic drinkers and healthy controls. Addict Biol. 2015;20(5):979–989. doi: 10.1111/adb.12199. [DOI] [PubMed] [Google Scholar]
- 30.Yucel M, Lubman DI. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: Implications for diagnosis, treatment and prevention. Drug Alcohol Rev. 2007;26(1):33–39. doi: 10.1080/09595230601036978. [DOI] [PubMed] [Google Scholar]
- 31.Durkee CA, Sarlls JE, Hommer DW, Momenan R. White matter microstructure alterations: a study of alcoholics with and without post-traumatic stress disorder. PLoS One. 2013;8(11):e80952. doi: 10.1371/journal.pone.0080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grodin EN, Lin H, Durkee CA, Hommer DW, Momenan R. Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: effects of co-morbid substance abuse. Neuroimage Clin. 2013;2:469–476. doi: 10.1016/j.nicl.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beucke JC, Sepulcre J, Talukdar T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70(6):619–629. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 34.Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuropsycho-pharmacol Biol Psychiatry. 2002;26(5):919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24(5):611–621. [PubMed] [Google Scholar]
- 36.Luchtmann M, Jachau K, Adolf D, et al. Decreased effective connectivity in the visuomotor system after alcohol consumption. Alcohol. 2013;47(3):195–202. doi: 10.1016/j.alcohol.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Calhoun VD, Altschul D, McGinty V, et al. Alcohol intoxication effects on visual perception: an fMRI study. Hum Brain Mapp. 2004;21(1):15–26. doi: 10.1002/hbm.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai XJ, Nie X, Liu X, et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J Clin Sleep Med. 2016;12(3):363–374. doi: 10.5664/jcsm.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rzepecki-Smith CI, Meda SA, Calhoun VD, et al. Disruptions in functional network connectivity during alcohol intoxicated driving. Alcohol Clin Exp Res. 2010;34(3):479–487. doi: 10.1111/j.1530-0277.2009.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Telesford QK, Laurienti PJ, Friedman DP, Kraft RA, Daunais JB. The effects of alcohol on the nonhuman primate brain: a network science approach to neuroimaging. Alcohol Clin Exp Res. 2013;37(11):1891–1900. doi: 10.1111/acer.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18(1):121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21(10):2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiland BJ, Sabbineni A, Calhoun VD, et al. Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(9):2445–2453. doi: 10.1111/acer.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]