Abstract
PURPOSE
To report the long-term control and toxicity outcomes of patients with clinically localized prostate cancer, who underwent low-dose-rate prostate brachytherapy with magnetic resonance spectroscopic image (MRSI)–directed dose escalation to intraprostatic regions.
METHODS AND MATERIALS
Forty-seven consecutive patients between May 2000 and December 2003 were analyzed retrospectively. Each patient underwent a preprocedural MRSI, and MRS-positive voxels suspicious for malignancy were identified. Intraoperative planning was used to determine the optimal seed distribution to deliver a standard prescription dose to the entire prostate, while escalating the dose to MRS-positive voxels to 150% of prescription. Each patient underwent transperineal implantation of radioactive seeds followed by same-day CT for postimplant dosimetry.
RESULTS
The median prostate D90 (minimum dose received by 90% of the prostate) was 125.7% (interquartile range [IQR], 110.3–136.5%) of prescription. The median value for the MRS-positive mean dose was 229.9% (IQR, 200.0–251.9%). Median urethra D30 and rectal D30 values were 142.2% (137.5–168.2%) and 56.1% (40.1–63.4%), respectively. Median followup was 86.4 months (IQR, 49.8–117.6). The 10-year actuarial prostate-specific antigen relapse–free survival was 98% (95% confidence interval, 93–100%). Five patients (11%) experienced late Grade 3 urinary toxicity (e.g., urethral stricture), which improved after operative intervention. Four of these patients had dose-escalated voxels less than 1.0 cm from the urethra.
CONCLUSIONS
Low-dose-rate brachytherapy with MRSI-directed dose escalation to suspicious intraprostatic regions exhibits excellent long-term biochemical control. Patients with dose-escalated voxels close to the urethra were at higher risk of late urinary stricture.
Keywords: MR spectroscopy, Prostate, Brachytherapy, Dose escalation
Introduction
Dose escalation is a critical component in radiation therapy (RT) for prostate cancer. Randomized controlled trials (1, 2) and institutional series (3) have demonstrated a benefit in prostate-specific antigen (PSA) relapse–free survival for patients who underwent dose escalation during external beam RT. Institutional series have also suggested the existence of a dose–response relationship for low-dose-rate (LDR) brachytherapy (4–6). In addition, higher radiation doses have been associated with lower positive biopsy rates along with improved clinical outcomes for patients with negative biopsies after both external beam RT (7) and brachytherapy (8). However, dose escalation to the entire prostate gland can result in increased gastrointestinal and genitourinary toxicity (1).
Studies have shown that prostate cancer often recurs at the original site after RT (9, 10). As a result, investigators have been interested in developing methods for escalating radiation dose to intraprostatic regions, while both maintaining coverage of the entire prostate gland and respecting normal tissue dose constraints. This form of dose escalation would in theory lead to improved clinical outcomes without higher toxicity rates. However, intraprostatic dose escalation requires advanced imaging capabilities, which can detect intraprostatic tumor deposits with acceptable sensitivity and specificity. Furthermore, highly conformal RT techniques are needed for safe and effective treatment delivery.
In this report, we summarize our institutional experience with intraprostatic dose escalation using LDR brachytherapy and magnetic resonance spectroscopic imaging (MRSI). We used LDR brachytherapy with real-time ultrasound-based intraoperative treatment planning (11, 12) because this modality allows for highly conformal dose escalation to intraprostatic regions. We incorporated preoperative MRSI into treatment planning because this modality can identify regions suspicious for intraprostatic tumor deposits based on elevated choline plus creatine-to-citrate ratios (13, 14). We had previously explored the feasibility of generating MRS-optimized dose distributions for permanent prostate implants (15). We now present our long-term clinical outcomes for patients treated with this technique.
Methods and materials
Patient population
Forty-seven patients with clinically localized prostate cancer, who underwent LDR brachytherapy with MRSI-directed dose escalation between May 2000 and December 2003, were included in this retrospective analysis. As shown in Table 1, 35 patients (74%) had National Cancer Center Network low-risk disease (T1-T2a, Gleason 6, and PSA <10 ng/mL). The remaining 12 patients (26%) had intermediate-risk disease (T2b-T2c, Gleason 7, or PSA 10–20 ng/mL). Clinical T-stage was defined by digital rectal examination. The numbers of patients with suspicious and definite radiographic extracapsular extension, as assessed by MRI, were 7 (15%) and 1, respectively. The median International Prostate Symptom Score, which was available for 24 patients, was 6. The median pretreatment prostate volume, as assessed by MRI, was 26.0 cc among all patients. Eight patients received neoadjuvant androgen-deprivation therapy (ADT), primarily for cytore-duction, and MRI was obtained before ADT in six of these patients. With respect to brachytherapy, 45 patients received 125I monotherapy with a prescription dose of 144 Gy. One patient underwent an 125I boost to 110 Gy followed by 50.4 Gy external beam RT due to concern for suspicious extracapsular extension on MRI. This patient had also received neoadjuvant ADT. Another patient received 103Pd monotherapy to 140 Gy.
Table 1.
Baseline clinical characteristics
| Characteristics | Total, n (%) |
|---|---|
| Age | |
| Median (IQR) | 66 (59–69) |
| Clinical stage | |
| T1c | 33 (70) |
| T2a | 12 (26) |
| T2b | 2 (4) |
| Gleason score | |
| 6 (3 + 3) | 39 (83) |
| 7 (3 + 4) | 7 (15) |
| 7 (4 + 3) | 1 (2) |
| Pretreatment PSA | |
| Median (IQR) | 5.1 (3.8–7.0) |
| NCCN risk | |
| Low | 35 (74) |
| Intermediate | 12 (26) |
| Pretreatment MRI | |
| Organ confined | 39 (83) |
| Suspicious ECE | 7 (15) |
| Definite ECE | 1 (2) |
| Pretreatment MRI prostate volume (cc) | |
| Median (IQR) | 26.0 (21.0–36.5) |
| Baseline IPSS (n = 24) | |
| Median (IQR) | 6 (4–9) |
| Neoadjuvant ADT | 8 (17) |
| Isotope used | |
| 125I (definitive, 144 Gy) | 45 (96) |
| 125I (boost, 110 Gy) + EBRT (50.4 Gy) | 1 (2) |
| 103Pd (definitive, 140 Gy) | 1 (2) |
| Followup, mo | |
| Median (IQR) | 86.4 (49.8–117.6) |
ADT = androgen-deprivation therapy; ECE = extracapsular extension; EBRT = external beam radiation therapy; IQR = interquartile range; NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen.
MRSI imaging
Before the procedure, all patients were scanned on a General Electric Signa 1.5 T MR scanner with an endorectal radiofrequency probe inflated with 100 cc of air. Acquired sequences included T1-weighted images and T2-weighted axial images. Spectroscopic analysis was conducted on T2-weighted sequences using 0.12 cc (0.625 cm × 0.625 cm × 0.30 cm) voxels over a 50-mm field of view. Peak areas of choline, creatine, and citrate were then calculated on an offline workstation, and MRS-positive voxels that were suspicious for malignancy based on choline plus creatine-to-citrate ratios were identified based on previously established criteria (14, 16).
LDR brachytherapy with MRSI-directed dose escalation
The LDR brachytherapy and intraoperative planning technique have been previously described (15). Briefly, the patients were intubated under general anesthesia and placed in an extended lithotomy position. Interstitial needles were inserted into the peripheral substance of the prostate gland through a perineal template under transrectal ultrasound image guidance. Axial ultrasound images of the entire prostate were subsequently acquired with aerated urethral gel contrast at 5-mm increments. After the images were transferred to the brachytherapy treatment-planning system, contours of the target volume and organs at risk were drawn on each axial image. Needle positions were also identified on each axial image.
MRS-positive voxels were then mapped onto the ultrasound image using a previously validated registration algorithm with an average positional error of 2.2 mm (17). A computerized intraoperative treatment-planning system, which used a genetic optimization algorithm, determined the optimal seed distribution to deliver a standard prescription dose to the entire prostate, while escalating the dose to MRS-positive voxels with corresponding abnormalities on T2-weighted sequences (16). Dose-volume constraints included a prostate V100 (percent volume receiving at least 100% of the prescription dose) >90%, a minimum MRS-positive region dose of 150% of the prescription dose, a maximal urethral dose of 130%, and a maximum rectal dose of 120% (15). Radioactive seeds were then placed with a Mick applicator under ultrasound and fluoroscopic guidance. Figure 1 depicts an MRSI dose-escalated treatment plan.
Fig. 1.
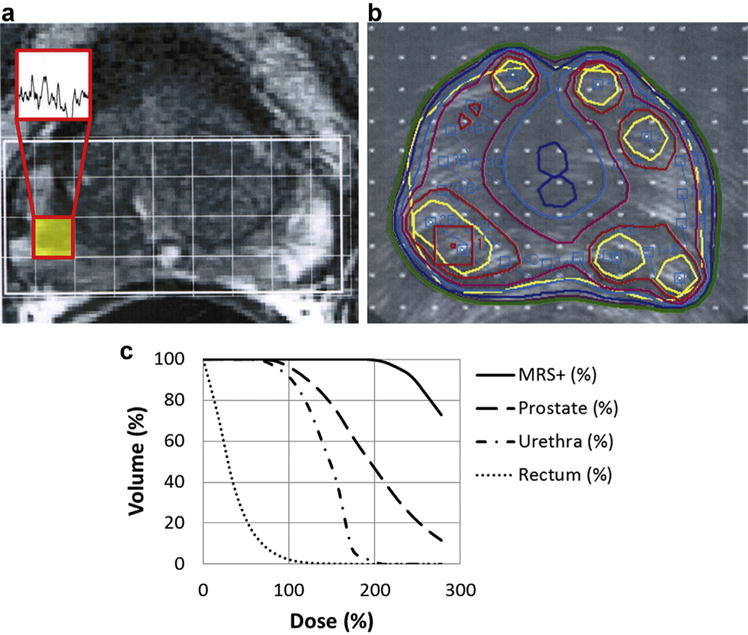
Magnetic resonance spectroscopic image (MRSI) dose-escalated treatment planning for a patient with low-risk disease. (a) Endorectal MRI shows T2 hypointensity in the right peripheral zone. MRS-positive voxel suspicious for cancer is highlighted, and associated spectrum is shown. (b) Intraoperative prostate ultrasound treatment plan, with dose escalation directed to MRS-positive voxel (red square). Prostate and urethral contours are blue. Isodose lines (% prescription): yellow, 208%; red, 167%; magenta, 139%; light blue, 125%; dark blue, 111%; purple, 104%; and green, 100%. (c) Dose-volume histograms for the MRS-positive (+) regions, prostate, urethra, and rectum, from a Day 0 postimplantation CT scan. All doses are represented in terms of percent prescription (144 Gy). MRS = magnetic resonance spectroscopic. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Postimplantation CT scans with 3-mm-thick slices were obtained on the same day of the procedure with a Foley catheter in place. Dosimetric parameters included the prostate D90 (minimum dose received by 90% of the prostate gland), prostate V100, prostate V150, prostate V200, and the prostate V (MRS-positive mean dose) (percent volume receiving at least the mean dose of the MRS-positive voxels). For the MRS-positive voxels, extracted parameters included the volume, number of separate regions, minimum dose, mean dose, V120, V150, and V200. Normal tissue parameters for the urethra included the D5, D20, D30, and V150cc (volume [cc] receiving at least 150% of the prescription dose). Parameters extracted for the rectum included the D1cc (minimum dose received by 1 cc), D30, and V100cc (volume [cc] receiving at least 100% of the prescription dose).
Followup
Patients were evaluated every 3 months for the first year, every 6 months for the next 5 years, and yearly thereafter. At baseline and at each subsequent visit, PSA values were recorded. PSA relapse was defined according to the Phoenix definition (nadir PSA + 2 ng/mL dated at call). A PSA bounce of >2 ng/mL was not counted as a relapse if subsequent values fell below 0.5 ng/mL without intervention. Urinary and rectal toxicities were assessed according to the Common Terminology Criteria for Adverse Events, version 4. Acute toxicity was defined as toxicity occurring within 12 months of implantation, and late toxicity was defined as toxicity occurring more than 12 months after implantation. The Kaplan–Meier method was used to evaluate the PSA relapse–free survival and the time to urinary-symptom resolution. Urinary-symptom resolution was defined as the return of the total International Prostate Symptom Score to within 5 points of the baseline score, if available, and cessation of postimplant medications for lower urinary tract symptoms. All statistical analysis was performed using the R statistical software v3.0 (The R foundation for Statistical Computing).
Results
Postimplantation dosimetry
Table 2 shows dosimetric outcomes based on postim-plantation CT. The median D90 was 125.7% of the prescription dose, and only 1 patient had a D90 less than prescription dose. The median V100 was 98.0%. The median number of MRS-positive regions was 1 (range, 1–6), and the median volume of the combined MRS-positive regions on a per patient basis was 0.29 cc. This volume exceeded 1% of the entire prostate gland in 19 patients (40%). Median values for the MRS-positive V120%, V150%, and V200% were 100%, 100%, and 68.0%. Only 3 patients had an MRS-positive V120% <90%. The median value for the MRS-positive mean dose was 229.9% (interquartile range [IQR], 200.0–251.9%), and 74% of patients had a mean dose greater than 200%. The MRS-positive mean dose did not differ between patients with low-risk and intermediate-risk disease (p = 0.95 on the Wilcoxon rank sum test). The median volume of the prostate receiving a dose greater than the MRS-positive mean dose was 26.0%. The median rectum D30 was 56.1% (IQR, 40.1–63.4) of prescription. Median values for the urethra D5 and D30 were 164.9% (151.4–197.0) and 142.2% (137.5–168.3) of the prescription dose, respectively.
Table 2.
Dosimetric outcomes based on Day 0 postimplantation CT
| Dosimetric parameters | Median (IQR) |
|---|---|
| Prostate ultrasound volume (cc) | 34.5 (27.2–44.4) |
| Prostate seeds implanted | 77 (67–87) |
| Prostate D90 (% prescription dose) | 125.7 (110.3–136.5) |
| Prostate V100 (%) | 98.0 (94.0–98.5) |
| Prostate V150 (%) | 74.0 (61.5–84.0) |
| Prostate V200 (%) | 41.0 (30.5–47.5) |
| Prostate V (MRS-positive mean dose) (%) | 26.0 (15.5–37.6) |
| MRS-positive volume (cc) | 0.29 (0.16–0.49) |
| MRS-positive separate regions (number) | 1 (1–2) |
| MRS-positive minimum dose (% prescription dose) | 154.2 (128.2–174.3) |
| MRS-positive mean dose (% prescription dose) | 229.9 (200.0–251.9) |
| MRS-positive V120 (%) | 100 (100) |
| MRS-positive V150 (%) | 100 (90.9–100) |
| MRS-positive V200 (%) | 68.0 (46.3–87.3) |
| Urethra D5 (% prescription dose) | 164.9 (151.4–197.0) |
| Urethra D20 (% prescription dose) | 151.4 (141.7–173.6) |
| Urethra D30 (% prescription dose) | 142.2 (137.5–168.2) |
| Urethra V150 (cc) | 0.30 (0.05–0.64) |
| Urethra V200 (cc) | 0.00 (0.00–0.06) |
| Rectum D1cc (% prescription dose) | 112.5 (98.3–138.5) |
| Rectum D30 (% prescription dose) | 56.1 (40.1–63.4) |
| Rectum V100 (cc) | 1.7 (0.9–2.7) |
IQR = interquartile range; MRS = magnetic resonance spectroscopic.
A comparative analysis of urethra D5 values for postimplant CT vs. intraoperative ultrasound was then performed. Twenty-six patients had intraoperative dose-volume histograms readily available for analysis. For this patient subset, the intraoperative ultrasound urethra D5 of 125% (IQR, 118–134) was less than the postimplant CT urethra D5 of 167% (IQR, 154–198) (p < 0.01 based on the Wilcoxon signed-rank test).
Clinical outcomes
The median followup was 86.4 months (IQR, 49.8–117.6). Among the 39 patients who did not receive ADT, the median PSA nadir was 0.05 (IQR, 0.05–0.08) and the median time to PSA nadir was 49.2 months (IQR, 38.4–69.0). The 10-year PSA relapse–free survival estimate was 98% (95% confidence interval, 93–100%), as shown in the Kaplan–Meier curve in Fig. 2a. When subdivided by National Cancer Center Network risk categories, 10-year PSA relapse–free survival estimates were 100% and 89% (71–100%) for low- and intermediate-risk patients, respectively. One patient with unfavorable intermediate-risk disease (clinical T1c, Gleason 4 + 3, initial PSA of 5.1) experienced a PSA relapse. Preoperative MRI showed two lesions: one in the peripheral zone of the left midgland with definite extracapsular extension and another in the right posterior midgland. Both findings were concordant on MRSI. He received 125I monotherapy to 144 Gy, along with MRSI-directed dose escalation to both lesions. The minimum and mean MRS-positive doses were 185.4% and 334.7% of prescription, respectively. He did not receive ADT. At 33.6 months, the PSA had increased from a nadir of 0.68 to 5.1. MRI was obtained at 36.0 months. Posttreatment multiplanar T2-weighted images demonstrated the brachytherapy seed distribution in addition to contraction of the left midgland and right posterior midgland peripheral zone tumors. In addition, there was resolution of the previously noted MR spectroscopy metabolite abnormalities, consistent with posttreatment changes. A suspicious 5.0-mm right external iliac lymph node chain was noted. Bone scan was negative. Biopsy was not performed. A 18F-fluorodeoxyglucose PET scan at 51.6 months showed progressive right pelvic lymphadenopathy that corresponded to further PSA rises to 18. The patient was then lost to followup. Among the entire cohort, there were eight recorded patient deaths, although none were attributed to prostate cancer. The 10-year overall survival estimate based on Kaplan–Meier analysis was 84% (81–99%).
Fig. 2.
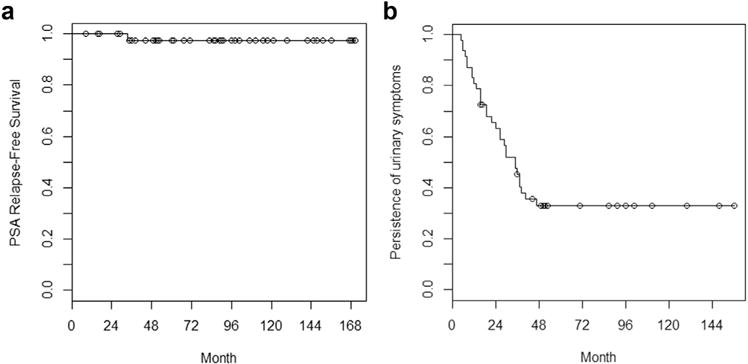
Kaplan–Meier curves of (a) PSA relapse–free survival per the Phoenix definition and (b) time to urinary-symptom resolution. PSA = prostate-specific antigen.
Table 3 shows the incidences of the maximal recorded acute and late urinary and rectal toxicities. Twenty-eight patients (60%) experienced Grade 2 acute urinary toxicity, including 3 patients who were catheterized within 90 days of the procedure. Ten patients (21%) with late Grade 2 urinary toxicity had persistent urinary symptoms requiring medications (e.g., alpha antagonists, anticholinergics, nonselective anti-inflammatory drugs) or pads for urinary incontinence. Five patients (11%) had late Grade 3 urinary toxicity requiring either stricture dilation or transurethral resection of the prostate at a median time of 51.6 months. After surgical correction, urinary symptoms improved for all patients.
Table 3.
Incidences of acute and late toxicities
| Location | Grade | Acute (<1 year) (%) | Late (>1 year) (%) |
|---|---|---|---|
| Genitourinary | 0 | 3 (6) | 14 (30) |
| 1 | 16 (34) | 18 (38) | |
| 2 | 28 (60) | 10 (21) | |
| 3 | 0 | 5 (11) | |
| Gastrointestinal | 0 | 32 (68) | 28 (60) |
| 1 | 13 (28) | 12 (25) | |
| 2 | 2 (4) | 6 (13) | |
| 3 | 0 | 1 (2) |
The urethra D5 doses among patients with late Grade 3 (median, 177.1%; IQR, 163.9–262.5) vs. late Grade 0–2 (median, 164.8%; IQR, 151.0–194.4) urinary toxicities were not statistically different based on the Wilcoxon rank sum test (p = 0.30). However, there was an inverse relationship (Pearson’s correlation coefficient −0.35; p = 0.03) between the urethra D5 and the minimum distance of the center of a dose-escalated voxel (0.625 cm width) to the urethra among the 38 patients with imaging readily available for analysis, as shown in Fig. 3. Furthermore, 4 of the 5 patients who experienced Grade 3 toxicity had a dose-escalated voxel less than 1.0 cm from the urethra. Among the 29 patients (62%) with complete resolution of urinary symptoms after implant, the median time for urinary-symptom resolution was 21.6 months. The Kaplan–Meier curve for this metric is shown in Fig. 2b.
Fig. 3.
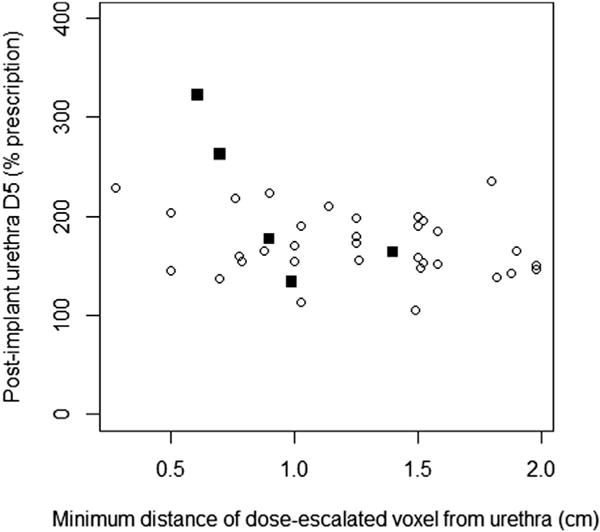
Graph showing relationship between urethra D5 on postimplant dosimetry and the minimum distance of the center of a dose-escalated voxel to the urethra for 38 patients with available imaging. Circles represent patients with late Grade 0–2 urinary toxicity. Squares represent patients with late Grade 3 urinary toxicity.
The numbers of patients with late Grade 2 and 3 rectal toxicities were 6 (13%) and 1 (2%), respectively. All patients with Grade 2 toxicities had moderately symptomatic proctitis, primarily rectal bleeding, that necessitated steroid suppositories or pads. The 1 patient with Grade 3 rectal toxicity required a blood transfusion due to severe rectal bleeding at 34.8 months after brachytherapy.
Discussion
In this report, we summarize our institutional experience using MRSI-directed dose escalation to intraprostatic regions suspicious for malignancy in a moderately sized patient cohort. Most patients achieved MRS-positive mean doses greater than 200% of the prescription dose, while maintaining excellent dosimetric coverage of the entire prostate. We only captured one PSA relapse, in a patient with unfavorable intermediate-risk disease and radiographic extracapsular extension. Although this patient experienced a nodal failure based on imaging, there was no evidence of local recurrence based on subsequent MRSI. Our PSA relapse–free survival curve, with its 10-year estimate of 98%, was comparable with that from a contemporary patient cohort treated without dose escalation (18).
However, patients were subject to higher urethra and rectal doses. The median urethra D30 of 142.2% of the prescription dose was greater than the reported value of 111% from an institutional series of patients treated without dose escalation within the same period (18). The greater urethral doses possibly translated into the higher than expected late Grade 3 urinary toxicity incidence of 11%, especially compared with the 4% incidence in the prior series. On the other hand, the late Grade 2 urinary toxicity incidence of 21% was similar to the 19% incidence in the prior series. Of note, the late Grade 2 and 3 urinary toxicity incidences from a more recent patient cohort were 17% and 3%, respectively (19). The median rectum D30 of 56.1% in this study was also greater than the 37% value cited in the prior series (18). The late Grade 2 rectal toxicity incidence appeared higher (13% vs. 7%), although late Grade 3 rectal toxicity incidence was similar (2% vs. 1%).
Previous investigators have reported their experience with MRSI-directed dose escalation for prostate brachytherapy. Using LDR brachytherapy, DiBiase et al. boosted MRSI-defined volumes to 130% of the prescription dose, with median maximal urethral and rectal doses to 160% and 110% of prescription, respectively. No Grade 3 toxicities according to the Radiation Therapy Oncology Group modified scale were reported among 15 patients, although followup time was not reported (20). Pouliot et al. performed an inverse planning study on MRSI-directed dose escalation to a dominant intraprostatic lesion (DIL) with high-dose-rate (HDR) brachytherapy in 10 patients. The authors concluded that DIL dose could be escalated to 120% of the prescription dose without increasing doses to surrounding structures (21). A subsequent study involving a class solution during inverse planning showed that dose escalation of up to 150% was feasible in 13 of 15 patients without exceeding RTOG 0321 constraints (22). More recently, authors from the same institution developed a novel algorithm for aligning endorectal MRSI with treatment-planning CT/MRI for planning HDR brachytherapy boost to the DIL (23). Kazi et al. (24) also evaluated the feasibility of MRSI-directed dose escalation to the DIL in a patient receiving combination external beam and HDR brachytherapy.
Prostate brachytherapy with intraprostatic dose escalation has also been performed with modalities other than MRSI. Ellis et al. published multiple series on radioimmu-noguided (111In capromab pendetide) dose-escalated LDR brachytherapy to 150% of the prescription dose (25–27). Gaudet et al. performed sextant biopsy-directed dose escalation to a DIL with LDR brachytherapy to 150% of the prescription dose. Imaging was not incorporated for defining the DIL. The 50 patients treated with dose escalation had a median intraprostatic lesion V150 of 95.7% intraoperatively and a median urethra D5 of 167.1% of the prescription dose on a Day 30 postimplant CT scan. The authors found no differences in acute or late toxicities between this dose-escalated group and a reference group treated without dose escalation within a 36-month followup period (28). Crook et al. more recently reported on the dosimetry of multiparametric MRI-guided DIL dose escalation using HDR brachytherapy in a series of 26 patients. Dose escalation of 125% was successfully achieved, and there were no significant differences in rectal or urethral doses when compared with a reference cohort treated without dose escalation (29). Mason et al. also published an institutional series for multiparametric MRI-guided focal tumor dose escalation with HDR brachytherapy in 15 patients (30). Other investigators have reported on intra-prostatic dose escalation for tomotherapy using MRI (31) as well as intensity-modulated RT using MRI alone (32) or in combination with MRSI (33).
A key strength of our study is that we performed intra-prostatic dose escalation in a moderately sized patient cohort with a relatively long median followup time of 86.4 months. To the best of our knowledge, our study has the longest followup of any RT series involving intraprostatic dose escalation. As a result, we were able to obtain a meaningful correlation between dosimetric data and toxicity end points, especially for late Grade 3 urinary symptoms. Furthermore, the incorporation of LDR brachytherapy allowed us to deliver profound dose escalation, with 74% of patients achieving an MRS-positive mean dose above 200 Gy.
A key limitation of this study is that postimplant dosimetry did reveal higher than expected urethral and rectal doses, despite considerable effort to balance intraprostatic dose escalation with surrounding normal tissue constraints. In our study, we were able to demonstrate a negative relationship between the minimum distance of a dose-escalated voxel to the urethra and urethra D5 on postimplant dosimetry. Furthermore, 4 of the 5 patients with urinary stricture did have a dose-escalated voxel located within 1.0 cm of the urethra. Fortunately, urethral strictures were surgically corrected in all cases. However, practitioners must recognize that dose escalation may not be feasible for regions close to the urethra. In addition, urethra constraints must be maintained for all intraprostatic dose escalation strategies. Newer techniques such as real-time intraoperative planning CT assessment may provide a better indication of postimplant dosimetry during seed placement, especially for intraprostatic dose escalation(34).
In addition, known limitations in the sensitivity, specificity, and spatial resolution of MRSI preclude us from confirming that all MRS-positive voxels contained cancer (14), although investigators have correlated MRSI-findings with the probability that a lesion harbors Gleason score ≥4 + 3 disease (35). Biopsy, clinical examination, and/or current standard of care multiparametric MRI (which includes functional techniques including diffusion-weighted and dynamic contrast-enhanced MRI that were not in routine use at the time that our patient population was treated) may provide additional assurance that cancerous regions actually receive the escalated dose. Finally, the clinical benefit of intraprostatic dose escalation remains in question. Given the similar PSA relapse–free survival curves for this study and institutional cohorts treated without dose escalation (5, 18), our results do not suggest a local control benefit for intraprostatic dose escalation in patients with low- and possibly favorable intermediate-risk disease. The prospective study by Crook et al. (29) is currently evaluating the role of intraprostatic dose escalation to DIL for intermediate- and high-risk patients. Intraprostatic dose de-escalation for low-risk patients could be an alternative strategy for decreasing normal tissue toxicity while preserving the clinical efficacy of prostate brachytherapy.
Acknowledgments
Financial disclosure: The authors acknowledge generous support from the National Institutes of Health grants 1R21CA78626-01 and R21CA84258 and funds from Jan Calloway and Wayne Calloway.
Footnotes
Conflicts of interest: None.
References
- 1.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Yamada Y, Fuks Z, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys. 2008;71:1028–1033. doi: 10.1016/j.ijrobp.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Stock RG, Stone NN, Tabert A, et al. A dose-response study for I-125 prostate implants. Int J Radiat Oncol Biol Phys. 1998;41:101–108. doi: 10.1016/s0360-3016(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Chou JF, Pei X, et al. Predicting biochemical tumor control after brachytherapy for clinically localized prostate cancer: the Memorial Sloan-Kettering Cancer Center experience. Brachytherapy. 2012;11:245–249. doi: 10.1016/j.brachy.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi Y, Yorozu A, Ohashi T, et al. A dose-response analysis of biochemical control outcomes after (125)I monotherapy for patients with favorable-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2014;90:1069–1075. doi: 10.1016/j.ijrobp.2014.08.340. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Reuter VE, Fuks Z, et al. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–1373. doi: 10.1016/j.juro.2007.11.063. discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone NN, Stone MM, Rosenstein BS, et al. Influence of pretreatment and treatment factors on intermediate to long-term outcome after prostate brachytherapy. J Urol. 2011;185:495–500. doi: 10.1016/j.juro.2010.09.099. [DOI] [PubMed] [Google Scholar]
- 9.Cellini N, Morganti AG, Mattiucci GC, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53:595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 10.Pucar D, Hricak H, Shukla-Dave A, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69:62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Yamada Y, Cohen G, et al. Postimplantation dosimetric analysis of permanent transperineal prostate implantation: improved dose distributions with an intraoperative computer-optimized conformal planning technique. Int J Radiat Oncol Biol Phys. 2000;48:601–608. doi: 10.1016/s0360-3016(00)00655-6. [DOI] [PubMed] [Google Scholar]
- 12.Zelefsky MJ, Yamada Y, Marion C, et al. Improved conformality and decreased toxicity with intraoperative computer-optimized transperineal ultrasound-guided prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;55:956–963. doi: 10.1016/s0360-3016(02)04142-1. [DOI] [PubMed] [Google Scholar]
- 13.Kurhanewicz J, Vigneron DB, Hricak H, et al. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24-0.7-cm3) spatial resolution. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 14.Scheidler J, Hricak H, Vigneron DB, et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging–clinicopathologic study. Radiology. 1999;213:473–480. doi: 10.1148/radiology.213.2.r99nv23473. [DOI] [PubMed] [Google Scholar]
- 15.Zelefsky MJ, Cohen G, Zakian KL, et al. Intraoperative conformal optimization for transperineal prostate implantation using magnetic resonance spectroscopic imaging. Cancer J. 2000;6:249–255. [PubMed] [Google Scholar]
- 16.Zaider M, Zelefsky MJ, Lee EK, et al. Treatment planning for prostate implants using magnetic-resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2000;47:1085–1096. doi: 10.1016/s0360-3016(00)00557-5. [DOI] [PubMed] [Google Scholar]
- 17.Mizowaki T, Cohen GN, Fung AY, et al. Towards integrating functional imaging in the treatment of prostate cancer with radiation: the registration of the MR spectroscopy imaging to ultrasound/CT images and its implementation in treatment planning. Int J Radiat Oncol Biol Phys. 2002;54:1558–1564. doi: 10.1016/s0360-3016(02)03805-1. [DOI] [PubMed] [Google Scholar]
- 18.Zelefsky MJ, Yamada Y, Cohen GN, et al. Five-year outcome of intraoperative conformal permanent I-125 interstitial implantation for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:65–70. doi: 10.1016/j.ijrobp.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Hathout L, Folkert MR, Kollmeier MA, et al. Dose to the bladder neck is the most important predictor for acute and late toxicity after low-dose-rate prostate brachytherapy: implications for establishing new dose constraints for treatment planning. Int J Radiat Oncol Biol Phys. 2014;90:312–319. doi: 10.1016/j.ijrobp.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiBiase SJ, Hosseinzadeh K, Gullapalli RP, et al. Magnetic resonance spectroscopic imaging-guided brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52:429–438. doi: 10.1016/s0360-3016(01)02609-8. [DOI] [PubMed] [Google Scholar]
- 21.Pouliot J, Kim Y, Lessard E, et al. Inverse planning for HDR prostate brachytherapy used to boost dominant intraprostatic lesions defined by magnetic resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2004;59:1196–1207. doi: 10.1016/j.ijrobp.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Hsu IC, Lessard E, et al. Class solution in inverse planned HDR prostate brachytherapy for dose escalation of DIL defined by combined MRI/MRSI. Radiother Oncol. 2008;88:148–155. doi: 10.1016/j.radonc.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed G, Cunha JA, Noworolski S, et al. Interactive, multi-modality image registrations for combined MRI/MRSI-planned HDR prostate brachytherapy. J Contemp Brachytherapy. 2011;1:26–31. doi: 10.5114/jcb.2011.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazi A, Godwin G, Simpson J, et al. MRS-guided HDR brachytherapy boost to the dominant intraprostatic lesion in high risk localised prostate cancer. BMC Cancer. 2010;10:472. doi: 10.1186/1471-2407-10-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis RJ, Sodee DB, Spirnak JP, et al. Feasibility and acute toxicities of radioimmunoguided prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2000;48:683–687. doi: 10.1016/s0360-3016(00)00646-5. [DOI] [PubMed] [Google Scholar]
- 26.Ellis RJ, Vertocnik A, Kim E, et al. Four-year biochemical outcome after radioimmunoguided transperineal brachytherapy for patients with prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2003;57:362–370. doi: 10.1016/s0360-3016(03)00588-1. [DOI] [PubMed] [Google Scholar]
- 27.Ellis RJ, Zhou H, Kaminsky DA, et al. Rectal morbidity after permanent prostate brachytherapy with dose escalation to biologic target volumes identified by SPECT/CT fusion. Brachytherapy. 2007;6:149–156. doi: 10.1016/j.brachy.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Gaudet M, Vigneault E, Aubin S, et al. Dose escalation to the dominant intraprostatic lesion defined by sextant biopsy in a permanent prostate I-125 implant: a prospective comparative toxicity analysis. Int J Radiat Oncol Biol Phys. 2010;77:153–159. doi: 10.1016/j.ijrobp.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 29.Crook J, Ots A, Gaztanaga M, et al. Ultrasound-planned high-dose-rate prostate brachytherapy: dose painting to the dominant intraprostatic lesion. Brachytherapy. 2014;13:433–441. doi: 10.1016/j.brachy.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Mason J, Al-Qaisieh B, Bownes P, et al. Multi-parametric MRI-guided focal tumor boost using HDR prostate brachytherapy: a feasibility study. Brachytherapy. 2014;13:137–145. doi: 10.1016/j.brachy.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Maggio A, Fiorino C, Mangili P, et al. Feasibility of safe ultra-high (EQD(2)>100 Gy) dose escalation on dominant intra-prostatic lesions (DILs) by Helical Tomotheraphy. Acta Oncol. 2011;50:25–34. doi: 10.3109/0284186X.2010.530688. [DOI] [PubMed] [Google Scholar]
- 32.Ippolito E, Mantini G, Morganti AG, et al. Intensity-modulated radiotherapy with simultaneous integrated boost to dominant intra-prostatic lesion: preliminary report on toxicity. Am J Clin Oncol. 2012;35:158–162. doi: 10.1097/COC.0b013e318209cd8f. [DOI] [PubMed] [Google Scholar]
- 33.van Lin EN, Futterer JJ, Heijmink SW, et al. IMRT boost dose planning on dominant intraprostatic lesions: gold marker-based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int J Radiat Oncol Biol Phys. 2006;65:291–303. doi: 10.1016/j.ijrobp.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 34.Zelefsky MJ, Worman M, Cohen GN, et al. Real-time intraoperative computed tomography assessment of quality of permanent interstitial seed implantation for prostate cancer. Urology. 2010;76:1138–1142. doi: 10.1016/j.urology.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brame RS, Zaider M, Zakian KL, et al. Regarding the focal treatment of prostate cancer: inference of the Gleason grade from magnetic resonance spectroscopic imaging. Int J Radiat Oncol Biol Phys. 2009;74:110–114. doi: 10.1016/j.ijrobp.2008.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


