Summary
Background
Lenalidomide plus dexamethasone is a reference treatment for patients with newly diagnosed myeloma. The combination of the proteasome inhibitor bortezomib with lenalidomide and dexamethasone has shown significant efficacy in the setting of newly diagnosed myeloma. We aimed to study whether the addition of bortezomib to lenalidomide and dexamethasone would improve progression-free survival and provide better response rates in patients with previously untreated multiple myeloma who were not planned for immediate autologous stem-cell transplant.
Methods
In this randomised, open-label, phase 3 trial, we recruited patients with newly diagnosed multiple myeloma aged 18 years and older from participating Southwest Oncology Group (SWOG) and National Clinical Trial Network (NCTN) institutions (both inpatient and outpatient settings). Key inclusion criteria were presence of CRAB (C=calcium elevation; R=renal impairment; A=anaemia; B=bone involvement) criteria with measurable disease (measured by assessment of free light chains), Eastern Cooperative Oncology Group (ECOG) performance status of 0–3, haemoglobin concentration 9 g/dL or higher, absolute neutrophil count 1 × 103 cells per mm3; or higher, and a platelet count of 80 000/mm3 or higher. We randomly assigned (1:1) patients to receive either an initial treatment of bortezomib with lenalidomide and dexamethasone (VRd group) or lenalidomide and dexamethasone alone (Rd group). Randomisation was stratified based on International Staging System stage (I, II, or III) and intent to transplant (yes vs no). The VRd regimen was given as eight 21-day cycles. Bortezomib was given at 1·3 mg/m2 intravenously on days 1, 4, 8, and 11, combined with oral lenalidomide 25 mg daily on days 1–14 plus oral dexamethasone 20 mg daily on days 1, 2, 4, 5, 8, 9, 11, and 12. The Rd regimen was given as six 28-day cycles. The standard Rd regimen consisted of 25 mg oral lenalidomide once a day for days 1–21 plus 40 mg oral dexamethasone once a day on days 1, 8, 15, and 22. The primary endpoint was progression-free survival using a prespecified one-sided stratified log rank test at a significance level of 0·02. Analyses were intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00644228.
Findings
Between April, 2008, and February, 2012, we randomly assigned 525 patients at 139 participating institutions (264 to VRd and 261 to Rd). In the randomly assigned patients, 21 patients in the VRd group and 31 in the Rd group were deemed ineligible based mainly on missing, insufficient, or early or late baseline laboratory data. Median progression-free survival was significantly improved in the VRd group (43 months vs 30 months in the Rd group; stratified hazard ratio [HR] 0·712, 96% CI 0·56–0·906; one-sided p value 0·0018). The median overall survival was also significantly improved in the VRd group (75 months vs 64 months in the Rd group, HR 0·709, 95% CI 0·524–0·959; two-sided p value 0·025). The rates of overall response (partial response or better) were 82% (176/216) in the VRd group and 72% (153/214) in the Rd group, and 16% (34/216) and 8% (18/214) of patients who were assessable for response in these respective groups had a complete response or better. Adverse events of grade 3 or higher were reported in 198 (82%) of 241 patients in the VRd group and 169 (75%) of 226 patients in the Rd group; 55 (23%) and 22 (10%) patients discontinued induction treatment because of adverse events, respectively. There were no treatment-related deaths in the Rd group, and two in the VRd group.
Interpretation
In patients with newly diagnosed myeloma, the addition of bortezomib to lenalidomide and dexamethasone resulted in significantly improved progression-free and overall survival and had an acceptable risk-benefit profile.
Funding
NIH, NCI, NCTN, Millennium Pharmaceuticals, Takeda Oncology Company, and Celgene Corporation.
Introduction
Front-line or initial therapy for multiple myeloma is designed to achieve the maximum response in the largest number of patients with associated sustained remission duration and prolonged survival.1,2 The use of the immunomodulatory drugs thalidomide and lenalidomide, and the proteasome inhibitor bortezomib, have been associated with improved survival.3,4 Combinations of lenalidomide or bortezomib with conventional anti-multiple myeloma drugs have produced high overall response rates and excellent outcomes in both the relapsed and front-line setting as reviewed recently.5-7 Both lenalidomide and bortezomib are approved for use in patients with previously untreated multiple myeloma.
Lenalidomide and bortezomib have different but synergistic mechanisms of action. Shared pathways involve caspase-mediated apoptosis and inhibition of NF-kappa B signalling.8–10 Both agents enhance the activity of dexamethasone. Findings of several studies have shown the enhanced efficacy of two and three drug combinations including lenalidomide, bortezomib, and dexamethasone.11–13 These studies have also shown a favourable toxicity profile of immunomodulatory drugs and proteasome inhibitor combinations with dexamethasone. A phase 1/2 study14 of lenalidomide, bortezomib, and dexamethasone reported both high efficacy and favourable tolerability in the treatment of newly diagnosed multiple myeloma.
We hypothesised that the addition of bortezomib to lenalidomide and dexamethasone would provide better response rates and improve progression-free survival. This report is the first prospective randomised phase 3 trial of the three drug combination bortezomib, lenalidomide, and dexamethasone versus the two drug combination lenalidomide and dexamethasone in newly diagnosed myeloma without intent for immediate autologous stem-cell transplantation (ASCT).
Methods
Patients and study design
The SWOG S0777 randomised, open-label phase 3 trial was done at Southwest Oncology Group (SWOG) and National Clinical Trials (NCTN) member institutions as listed in the appendix. Patients aged 18 years or older with newly diagnosed myeloma were eligible. Key inclusion criteria were: presence of CRAB criteria (C=calcium elevation; R=renal impairment; A=anaemia; B=bone involvement) with measurable disease (measured by assessment of free light chains).15 No patients with earlier disease were included in this trial. The Eastern Cooperative Oncology Group (ECOG) performance status 0–3 was acceptable.16 Allowable blood count values were: haemoglobin ≥9 g/dL; absolute neutrophil count ≥1× 103 cells per mm3; platelet count ≥80 000/mm3. Major exclusion criteria were: creatinine clearance ≤30 mL/min; cardiac status New York Heart Association class III/IV or recent myocardial infarction; active hepatitis B or C or HIV or uncontrolled other infection; previous cancer prior to study registration or enrolment; or poorly controlled diabetes.
The study protocol was approved by the institutional review boards of all participating institutions. All patients provided written informed consent.
Randomisation
Patients were randomly assigned (1:1) to receive initial treatment of bortezomib with lenalidomide and dexamethasone (VRd) or lenalidomide and dexamethasone (Rd). We used a dynamic allocation algorithm developed by Pocock and Simon to balance treatment assignment by the stratification factors. The randomisation was stratified based on International Staging System stage (I, II, or III) and intent to transplant (yes vs no).17 Patients at participating NCTN institutions were randomly assigned upon registration. Randomisation procedures were developed and maintained by the SWOG statistics and data management centre. There was no masking to treatment interventions.
Procedures
The VRd regimen was given as eight 21-day cycles. Bortezomib was given at 1·3 mg/m2 intravenously on days 1, 4, 8, and 11 combined with 25 mg oral lenalidomide once a day on days 1–14 plus 20 mg oral dexamethasone on days 1, 2, 4, 5, 8, 9, 11, and 12. The Rd regimen was given as six 28-day cycles. The standard Rd regimen was used consisting of 25 mg oral lenalidomide once a day for days 1–21 plus 40 mg oral dexamethasone on days 1, 8, 15, and 22. The total amount of lenalidomide administered for induction was balanced for each group (VRd: 2800 mg lenalidomide total dose; Rd: 3150 mg total dose). Patients in the VRd group received herpes simplex virus prophylaxis. All patients received 325 mg oral aspirin once a day to reduce the risk of thromboembolic complications.
Upon completion of induction, all patients received ongoing maintenance with 25 mg oral lenalidomide once a day for 21 days plus 40 mg oral dexamethasone once a day for days 1, 8, 15, and 22 of each 28-day cycle. Stem-cell collection was allowed for those patients considering future transplant. With dosage adjustments as necessary using slide adjustment scale within the protocol, maintenance was continued until emergence of progressive disease, toxic effects, or patient withdrawal.
Outcomes
The primary endpoint was progression-free survival from the time of randomisation. Secondary endpoints were overall survival, the rate of overall response (partial response or better), safety, and to bank specimens for future translational medicine research. Data were collected and analysed by the SWOG statistical centre team in standard SWOG cooperative group procedural fashion.
Treatment response and disease progression were assessed centrally and followed the international uniform response criteria for multiple myeloma.18 Disease assessments were done at the end of each cycle. After treatment discontinuation because of toxic effects, disease progression, or patient withdrawal, patients were followed up for disease status every 6 months, until death or for a maximum of 6 years after initial randomisation.
We did fluorescence in-situ hybridisation (FISH) analysis of bone marrow cells at trial entry. Preliminary analyses from available data from 316 patients suggested that 33% were deemed high risk by one or more of the high risk features including t(4;14), t(14;16), or chromosome 17 deletion abnormalities. Individual site FISH testing and reports will be further reviewed as part of data assessment in the present study to confirm details including cell numbers and percentages as well as possible coexistence of high, intermediate, and good risk features. We used standard percentage cutoff values for each type of FISH test abnormality (typically 5%, but ranging from 1·5% to 7·5%).
We collected data for adverse events every 3 months while on treatment and again at the end of induction and maintenance treatment. All adverse events were initially graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. From April 6, 2011, serious adverse events were graded according to CTCAE version 4.0. An independent data and safety monitoring committee reviewed unblinded safety data twice a year.
Statistical analysis
The sample size was based on the assumption of an eligible patient accrual rate of 110 patients per year (440 eligible patients over 4 years), a median progression-free survival of about 3 years in the control group, exponential distribution of progression-free survival, and roughly 2·5 years of additional follow up. The study was designed to detect a hazard ratio of 1·5, with approximately 87% power and an overall study alpha of 0·05. Thus, to allow for an interim analysis, a one-sided 0·02 significance level was used to assess the primary progression-free survival endpoint. The primary endpoint was evaluated with the use of a group-sequential design, with two planned interim analyses at 1/3 and 2/3 of the total number of events. A Haybittle–Peto approach was used for alpha spending and a one-sided alpha of 0·0025 was used for each interim analysis.19,20 At the final analysis, a one-sided stratified log-rank test was done at the 0·02 significance level for an overall one-sided alpha of 0·025.21
We compared progression-free survival and overall survival between treatment groups using a log-rank test stratified according to the factors used for randomisation.19,22 Hazard ratios were estimated by means of a stratified Cox proportional-hazards model.23 The multivariate analysis were done with a model that was not stratified by, rather adjusted for stratification factors, to provide some idea as to how the stratification factors were associated with outcome. We used the Kolmogorov-Smirnov test to assess assumptions of proportional hazards. There was no evidence of violation of proportional hazards for any of the covariates. Survival curves were based on the Kaplan-Meier method.22 We compared the overall response rate between groups using a stratified Cochran-Mantel-Haenszel test.24,25 The odds ratio and corresponding 95% confidence interval were estimated with the use of the Mantel-Haenszel method.24,25 Duration of response was summarised by means of the Kaplan-Meier method.22 All primary and secondary endpoint analyses were predefined within the protocol.
Analyses were done on an intention to treat basis that incorporated all eligible patients. Patients with missing parameters of interest were excluded from multivariate analyses. We used SAS (version 4) for all analyses. Baseline variables were compared using Fisher’s exact test. The safety analysis included all eligible patients who received at least one dose of study treatment and who were evaluated for toxic effects.
Role of the funding source
The funder agreed to provide support for the study as designed. The funder had no role in data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between April, 2008, and February, 2012, 525 patients at 139 participating SWOG and NCTN institutions were randomly assigned: 264 to VRd and 261 to Rd. There were no significant changes made to the trial design following initial activation. Baseline characteristics were well balanced between treatment groups, with the exception of sex and age (ie, there were more women and patients were older in the Rd group; table 1).
Table 1.
Baseline characteristics
| Total | Patients given bortezomib with lenalidomide and dexamethasone (VRd group) | Patients given lenalidomide and dexamethasone (Rd group) | |
|---|---|---|---|
| ECOG performance status > 1 | 64/471 (14%) | 28/242 (12%) | 36/229 (16%) |
| Serum beta 2 microglobulin concentration ≥3·5 mg/L | 282/459 (61%) | 141/235 (60%) | 141/224 (63%) |
| C-reactive protein concentration ≥8 mg/L | 104/444 (23%) | 48/225 (21%) | 56/219 (26%) |
| Creatinine concentration ≥2 mg/dL | 22/471 (5%) | 11/242 (5%) | 11/229 (5%) |
| Lactate dehydrogenase concentration ≥190 U/L | 166/462 (36%) | 84/236 (36%) | 82/226 (36%) |
| Albumin concentration <3·5 g/dL | 197/466 (42%) | 98/239 (41%) | 99/227 (44%) |
| Haemoglobin concentration <10 g/dL | 151/471 (32%) | 79/242 (33%) | 72/229 (31%) |
| Platelet count <150 × 109/L | 21/469 (4%) | 11/241 (5%) | 10/228 (4%) |
| International Staging System stage III | 157/471 (33%) | 78/242 (32%) | 79/229 (34%) |
| Age ≥65 years | 202/471 (43%) | 93/242 (38%) | 109/229 (48%) |
| Women | 196/471 (42%) | 89/242 (37%) | 107/229 (47%) |
| Intent to transplant | 324/471 (69%) | 168/242 (69%) | 156/229 (68%) |
Data are n/N (%). ECOG=Eastern Cooperative Oncology Group.
In the randomly assigned patients, 21 patients in the VRd group and 31 in the Rd group were deemed ineligible based mainly on missing, insufficient, or early or late baseline laboratory (figure 1). Two patients, one in each group, were not analysable for efficacy because of consent issues; one patient withdrew consent and one patient who was under guardianship provided consent without guardian approval and so the consent provided was deemed invalid. For VRd, 242 patients were thus eligible and analysable for efficacy, with 241 evaluable for toxic effects and 216 assessable for response. For the Rd group, 229 patients were eligible and analysable for efficacy, with 226 evaluable for toxicity and 214 assessable for response. At the time of prespecified primary efficacy analyses, 66 patients (14% of eligible patients) were still on maintenance therapy. The median overall follow up was 55 months (IQR 48–68), 54 months (IQR 47–66) for VRd and 56 months (50–70) for the Rd group. The median duration of maintenance was 385 days.
Figure 1. Trial profile.
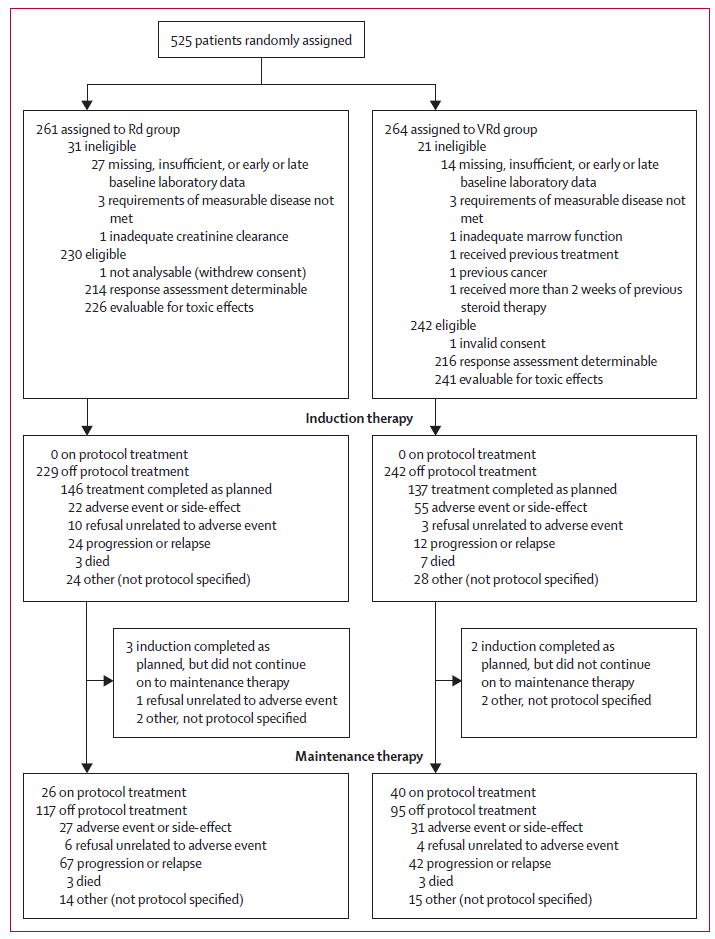
VRd=bortezomib with lenalidomide and dexamethasone. Rd=lenalidomide and dexamethasone.
At the time of analysis, the study met its primary objective of showing that the addition of bortezomib significantly improved progression-free survival. Specifically, the one-sided stratified log-rank p value fell well below the prespecified significance level of 0·02.The stratified hazard ratio and one-sided stratified log-rank p value in favour of VRd versus Rd were 0·712 (96% Wald confidence interval 0·560–0·906; p=0·0018, two-sided p value 0·0037) with an unstratified median progression-free survival of 43 months (95% CI 39–52) for VRd versus 30 months (25–39) for the Rd group (figure 2A). We also assessed response duration with a stratified log-rank test, which suggested improved response duration in patients receiving VRd (HR 0·695, two-sided p value 0·0133). The median response duration was 52 months in the VRd group versus 38 months for the Rd group (figure 2B).
Figure 2. Kaplan-Meier estimates of progression-free survival (A), response duration (B), and overall survival (C) by treatment group.
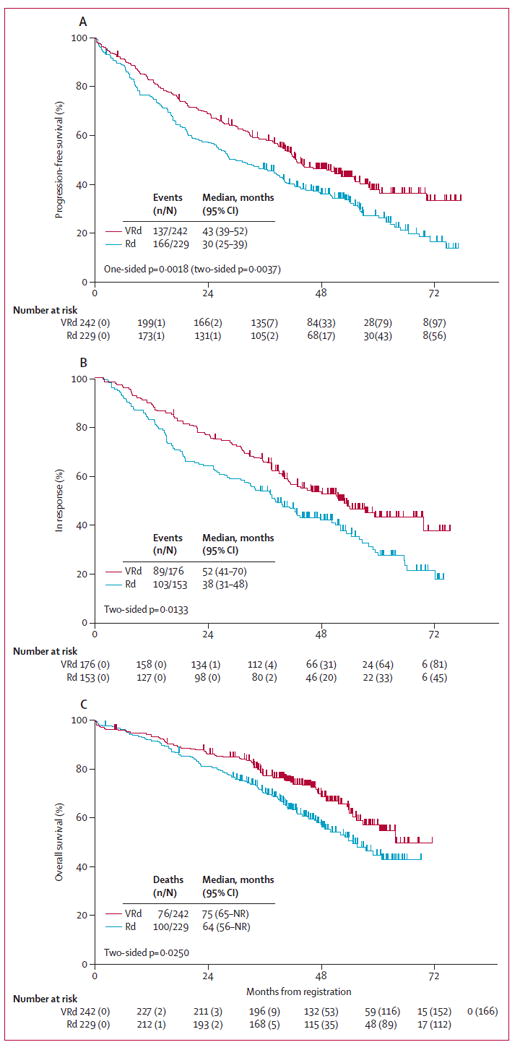
VRd=bortezomib with lenalidomide and dexamethasone. Rd=lenalidomide and dexamethasone. NR=not reached. Response duration was measured from the earliest date of response, partial response, or complete response for patients who had a confirmed partial response or better (this included unconfirmed and confirmed very good partial response, complete response, and stringent complete response), until progression or death, whichever occurred first. Patients who did not die or progress were censored at the date of last contact.
With regard to dose intensity, in the Rd group unplanned dose modifications occurred in 27 (12%) of 223 and 17 (14%) of 121 patients with available dosing data during induction and maintenance, respectively; in the VRd group, unplanned dose modifications occurred in 38 (16%) of 239 and 24 (24%) of 102 patients with available dosing data, respectively. At the time of this report, at least 46 (10%) of 471 patients are estimated to have proceeded to stem-cell harvest and planned transplant after leaving the study. Intent to transplant was a stratification factor and balanced between treatment groups (table 1).
A prespecified secondary endpoint analysis was the assessment of overall survival. The stratified hazard ratio and one-sided stratified log-rank p value in favour of VRd versus Rd were HR 0·709 (95% Wald CI 0·524–0·959; p=0·0125; two-sided p=0·0250) with median overall survival of 75 months for VRd versus 64 months for the Rd group (figure 2C). The median overall survival values were unchanged when patients leaving the study with intent for stem-cell harvest or transplant were censored (medians 75 months and 64 months: p=0·0366).
The median progression-free survival was 16 months for Rd and 38 months with VRd in the 44 patients who were high risk by FISH, and 15 and 34 months in the 17 patients with t(4;14) by FISH, respectively. These differences were not significant (stratified log-rank p=0·19 and 0·96, respectively). In response to the difference in the distribution of patients 65 years and older between treatment groups, and in view of the previously described significance of age as a prognostic factor for both progression-free survival and overall survival, univariately, we did age-adjusted progression-free survival and overall survival multivariate models (table 2). After accounting for the effects of age (≥65 years), the effect of treatment group remained significant for both progression-free survival and overall survival (table 2).
Table 2.
Multivariate age-adjusted progression-free survival and overall survival
| Patients (N=471)* | Progression-free survival
|
Overall survival
|
|||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Patients given bortezomib with lenalidomide and dexamethasone (VRd group) | 242 (51%) | 0·73 (0·58–0·92) | 0·007 | 0·74 (0·55–1·00) | 0·048 |
|
| |||||
| International Staging System stage III | 157 (33%) | 1·58 (1·16–2·13) | 0·003 | 2·16 (1·43–3·25) | 0·0003 |
|
| |||||
| International Staging System stage II | 184 (39%) | 1·16 (0·86–1·57) | 0·322 | 1·18 (0·77–1·81) | 0·447 |
|
| |||||
| Intent to transplant | 324 (69%) | 0·98 (0·74–1·28) | 0·866 | 0·86 (0·61–1·20) | 0·371 |
|
| |||||
| Age ≥65 years | 202 (43%) | 1·32 (1·03–1·71) | 0·031 | 1·88 (1·34–2·62) | 0·0002 |
Data are n (%) unless otherwise stated.
N=471 patients with valid data for factor.
The median age did not differ between treatment groups (63 years [IQR 56–70] for VRd and 63 years [IQR 56–71] for the Rd group). Both progression-free survival and overall survival were improved in each of the three age categories: younger than 65 years; 65–75 years; and older than 75 years (patients older than 75 years had median progression-free survival of 39 months vs 20 months and median overall survival of 63 months vs 31 months). However, these differences were only significant for progression-free survival in patients younger than 65 years and for overall survival in patients older than 75 years. Although the distribution of sex differed between groups, it was not univariately associated with survival outcomes and was thus not included in multivariate modelling.
Table 3 provides confirmed responses. We did sensitivity analyses in which we assessed patients who were assessable for response at the time of analysis and noted improved response in patients in the VRd group over those in the Rd group (81·5% vs 71·5%; p=0·02). Among these assessable patients, 15·7% in the VRd group and 8·4% in the Rd group had a complete response or better (table 3). The overall response rate (confirmed partial response or better, which includes unconfirmed and confirmed very good partial response, complete response, and stringent complete response), with non-assessable patients included as non-responders, was 72·7% and 66·8% in the VRd and Rd groups, respectively (p=0·20).
Table 3.
Confirmed response in assessable patients
| bortezomib with lenalidomide and dexamethasone (VRd group; n=216)* | Lenalidomide and dexamethasone (Rd group; n=214)* | |
|---|---|---|
| Confirmed response | 34 (15·7%) | 18 (8·4%) |
| Very good partial response | 60 (27·8%) | 50 (23·4%) |
| Partial response | 82 (38%) | 85 (39·7%) |
| Overall response rate (partial response or better) | 176 (81·5%) | 153 (71·5%) |
| Stable disease | 34 (15·7%) | 52 (24·3%) |
| Stable disease or better | 210 (97·2%) | 205 (95·8%) |
| Progressive disease or death | 6 (2·8%) | 9 (4·2%) |
The p value for differences in those with confirmed response was 0·02. The results section provides more details (unconfirmed responses are collapsed into the response category one level below).
Outcomes by response category at 6 and 12 months were assessed using landmarked analyses (figure 3). The median progression-free survival for patients with very good partial response or better at 6 months was 49 months versus 34 months for patients with partial response and 18 months for those with stable disease (figure 3A). The median overall survival for patients with partial response at 12 months was 59 months versus 55 months for those with progressive disease and 48 months for patients with stable disease; overall survival was not reached in those with very good partial response or better at 12 months (figure 3B).
Figure 3. Progression-free survival by response status at 6 months (A) and overall survival by response status at 12 months (B).
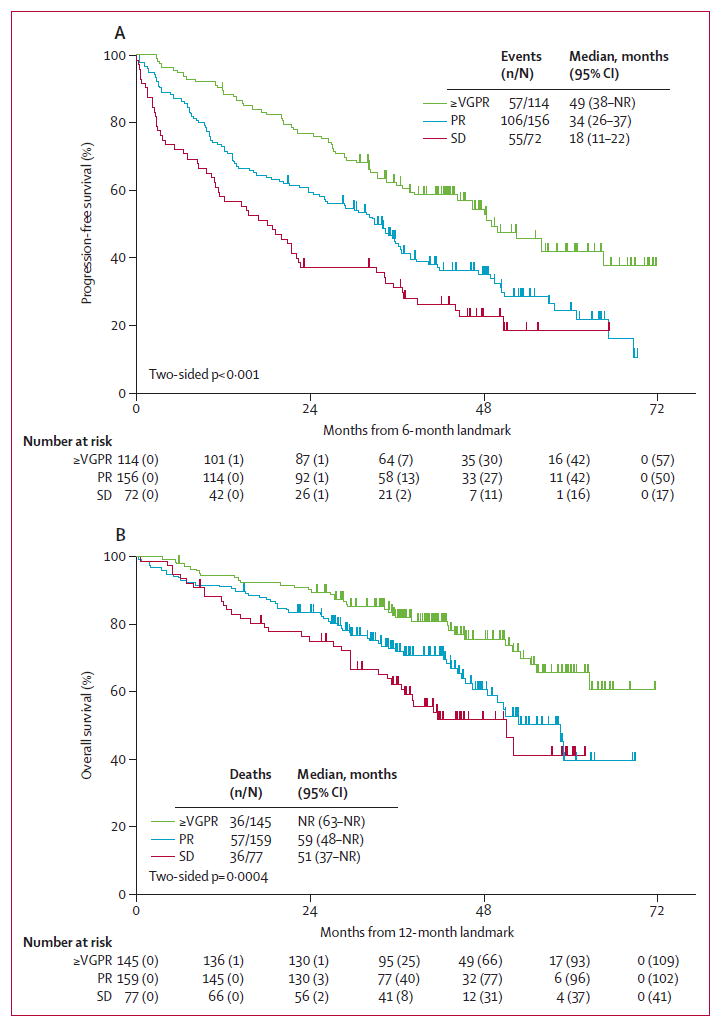
VGPR=very good partial response. PR=partial response. SD=stable disease. NR=not reached
The adverse events defined by Common Toxicity Criteria category and specific toxic effects were fairly well balanced between the two groups (table 4). The commonest haematological adverse events (≥ grade 3 and at least possibly attributable to treatment) were anaemia, lymphopenia, neutropenia, and thrombocytopenia (table 4). The commonest non-haematological adverse events (≥ grade 3 and at least possibly attributable to treatment) were: fatigue, sensory neuropathy, hyperglycaemia, thrombosis or embolism, hypokalaemia, muscle weakness, diarrhoea, and dehydration. As expected, grade 3 or worse neurological toxic effects were more frequent in the VRd group than in the Rd group (33% vs 11%; p<0·0001). 20 patients had a second primary cancer (ten [4%] in the VRd group and ten [4%] in the Rd group; appendix).
Table 4.
Notable toxic effects defined by Common Toxicity Criteria 3.0 category
| Patients given lenalidomide and dexamethasone (Rd group; n=226)
|
Patients given bortezomib with lenalidomide and dexamethasone (VRd group; n=241)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Haematological | ||||||||||
|
| ||||||||||
| Blood or bone marrow | 25 | 50 | 70 | 34 | 0 | 27 | 49 | 73 | 41 | 0 |
| Coagulation | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| Haemorrhage or bleeding | 12 | 2 | 0 | 0 | 0 | 7 | 3 | 7 | 0 | 0 |
| Infection | 1 | 28 | 29 | 2 | 0 | 2 | 31 | 29 | 5 | 1 |
| Lymphatics | 56 | 18 | 2 | 0 | 0 | 66 | 24 | 5 | 0 | 0 |
|
| ||||||||||
| Neurological | ||||||||||
|
| ||||||||||
| Neurological | 78 | 44 | 21 | 3 | 1 | 42 | 72 | 76 | 4 | 0 |
| Pain | 44 | 27 | 9 | 0 | 0 | 55 | 44 | 29 | 0 | 0 |
|
| ||||||||||
| Non-haematological or non-neurological | ||||||||||
|
| ||||||||||
| Cardiac arrhythmia | 5 | 3 | 4 | 0 | 0 | 7 | 2 | 3 | 0 | 0 |
| Cardiac general | 13 | 11 | 8 | 0 | 0 | 15 | 18 | 18 | 0 | 0 |
| Constitutional symptoms | 60 | 83 | 35 | 0 | 0 | 60 | 89 | 46 | 1 | 0 |
| Dermatology or skin | 61 | 21 | 9 | 0 | 0 | 50 | 42 | 6 | 1 | 0 |
| Endocrine | 11 | 8 | 0 | 0 | 0 | 5 | 12 | 0 | 0 | 0 |
| Gastrointestinal | 84 | 65 | 17 | 0 | 0 | 64 | 85 | 49 | 3 | 1 |
| Hepatobiliary or pancreas | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Metabolic or laboratory | 54 | 55 | 51 | 12 | 0 | 50 | 60 | 53 | 9 | 1 |
| Musculoskeletal or soft tissue | 25 | 22 | 13 | 1 | 0 | 20 | 28 | 22 | 1 | 0 |
| Pulmonary or upper respiratory | 42 | 25 | 8 | 1 | 0 | 55 | 16 | 15 | 6 | 0 |
| Ocular or visual | 19 | 8 | 12 | 0 | 0 | 37 | 16 | 6 | 0 | 0 |
| Renal or genitourinary | 2 | 3 | 8 | 1 | 0 | 8 | 3 | 5 | 0 | 0 |
| Secondary cancer | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| Sexual or reproductive function | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| Vascular | 0 | 4 | 16 | 5 | 0 | 1 | 8 | 18 | 4 | 0 |
| Death* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
We excluded events unlikely to be related to treatment. Total number of grade 3 or higher adverse events: 169 (75%) of 226 in the Rd group, 198 (82%) of 241 in the VRd group.
In neither case was the cause of death directly attributable to treatment. One patient had a cardiac arrest and the other patient died in a nursing home, cause undetermined.
Discussion
The addition of bortezomib to lenalidomide and dexamethasone led to significantly improved outcomes for patients with previously untreated multiple myeloma. The progression-free survival was improved by 13 months and overall survival by 11 months. This is the first prospective randomised trial to show the value of the three drug regimen VRd versus the two drug regimen Rd in the absence of front-line transplantation. The value of the three drug regimen is further affirmed by the improved progression-free survival and overall survival achieved with deeper responses (ie, very good partial response or better).
There is precedent for the added benefit of a three drug, proteasome inhibitor, immunomodulatory drugs, steroid combination as a first therapy, using bortezomib plus thalidomide plus dexamethasone for induction.26 A 2015 randomised study27 showed superior response rates in patients given bortezomib plus thalidomide plus dexamethasone versus those given bortezomib plus cyclophosphamide plus dexamethasone, emphasising the particular value of the three drug proteasome inhibitor plus immunomodulatory drugs plus steroid combination, which was first clearly shown in the original phase 1/2 trial of bortezomib plus lenalidomide plus dexamethasone in the previously untreated myeloma setting.14 This synergistic effect with proteasome inhibitor plus immunomodulatory drug plus dexamethasone combination is further shown by results with carfilzomib plus lenalidomide plus dexamethasone combinations in both front-line (high-risk smouldering myeloma and previously untreated myeloma) and relapsed settings.28,29 However, although response rates and progression-free survival were improved with the use of triplet regimens such as bortezomib plus thalidomide plus dexamethasone, no data exist to suggest that overall survival could be improved in trials comparing two modern regimens. Of note, findings of the FIRST trial30 showed the significantly improved outcomes of the two drug regimen Rd (with ongoing maintenance) over the older three drug melphalan-based regimen of melphalan plus prednisone plus thalidomide. Our findings show that overall survival can be further improved by the addition of a proteasome inhibitor to Rd.
The median progression-free survival with Rd noted in our trial (30 months) is longer than the median progression-free survival for the continuous Rd group in the FIRST trial (25·5 months), probably because of differences in age-distribution between the two trials.30 The progression-free survival and overall survival results in the SWOG S0777 trial compare well with the early findings in the IFM portion of the Intergroupe Francophone du Myélome/Dana Farber Cancer Institute (IFM/DFCI) front-line trial using VRd with or without upfront autologous stem-cell transplantation reported at the American Society of Hematology conference in 2015.31
Our trial was conceived in 2007 and accrued patients between 2008 and 2012. It encompassed a time in which bortezomib was given if possible at maximum doses twice a week intravenously. This resulted in almost a quarter of patients assessable for toxic effects stopping VRd induction treatment prematurely and 10% of those in the Rd group. Associated with this was the significantly increased grade 3 or worse neuropathic and gastrointestinal adverse events with the VRd regimen versus the Rd regimen. If bortezomib had been given subcutaneously as it is now, some more serious neuropathic side-effects could have been avoided and additional benefit might have been realised.32
In our study, the number of secondary primary cancers was low at just ten cases in each group.33 The distribution between skin, solid, and bone marrow-derived cancers was as anticipated in view of the median follow up of roughly 4·6 years. The 4% cumulative overall incidence of secondary primary cancers in the SWOG S0777 trial is less than the 6·9% cumulative incidence reported in the recent meta-analysis for lenalidomide-containing groups of treatment at 5 years (3·9% at 3 years).33 The higher 6·9% cumulative incidence is linked to combined use of oral melphalan in the meta-analysis series, which was not a factor in SWOG S0777.
It should be noted that although confirmed responses were superior for the VRd group, the overall responses are lower than in the original front-line VRd study reported by Richardson and colleagues.14 One reason is likely to be failure to confirm a particular level of response with a second reading (ie, second response assessment). For example, 20 patients (9%) of 216 in the VRd group had unconfirmed partial response and were appropriately listed in the confirmed stable disease category. Inclusion of these 20 patients in the overall response rate category would have given an overall response rate of more than 90%. Nevertheless, our findings show that the VRd regimen is very active in newly diagnosed myeloma. Only 2·8% of patients had initial progressive disease or death as a primary assessment.
The SWOG S0777 trial had several limitations. The response assessment was suboptimal as already noted. Because the two published regimens of VRd and Rd were being compared, there were eight 3-week cycles of VRd compared with six 4-week cycles of Rd, rather than perfectly matched 3-week or 4-week cycles. Nonetheless, the administered dose of lenalidomide was well-balanced between groups. Bortezomib was given intravenously twice per week as was standard practice at the time the trial began. This method compromised the outcomes by 2016 standards because resultant neuropathy led to earlier discontinuation of VRd induction therapy. If age had been a stratification factor, it would have simplified the assessment of age with respect to outcomes. The exclusion of patients with impaired renal function or compromised bone marrow function meant that no comments can be made about these groups of patients. Finally, no progression-free survival assessment for the second response and second remission duration was done as part of the study.
In conclusion, the results of the SWOG S0777 trial show that the triplet regimen VRd improved response rates, depth of response, progression-free survival, and overall survival compared with the currently approved front-line regimen Rd. The median overall survival in our study of 75 months with VRd strongly supports the general notion that triplet therapy for induction adds value. Additionally, with the use of weekly subcutaneous bortezomib, fewer toxic effects, and added survival benefit might be anticipated. Therefore, the S0777 study results can inform decision making for front-line therapy using a proteasome inhibitor plus immunomodulatory drugs plus steroid triplet treatment.
Supplementary Material
Research in context.
Evidence before this study
On Oct 15, 2007, we searched PubMed, MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and references from relevant articles with the search terms “multiple myeloma”, “treatment”, “proteasome inhibitor”, “immune modulatory agent”, and “phase III”. We also reviewed all recent reviews on myeloma treatment from the International Myeloma Working Group, and authors were asked to identify any appropriate citation of importance not detected by search strategies. Emphasis was placed on randomised clinical trials, systematic reviews, meta-analyses, and prospective and observational studies through December, 2015. We included only studies of adults, written in English and published between January, 1985, and December, 2015. We identified 3479 studies, and the findings showed that proteasome inhibitors and immune modulatory agents had important activity in the treatment of myeloma but had not been studied in the way proposed in Southwest Oncology Group (SWOG) S0777.
Added value of this study
To our knowledge, the S0777 study is the first to show that an all novel triple drug combination (bortezomib, lenalidomide, and dexamethasone) used in the absence of autologous stem-cell transplant is superior to a double drug novel combination (lenalidomide and dexamethasone) in terms of progression-free survival and overall survival in patients with newly diagnosed myeloma.
Implications of all the available evidence
Data suggest that for patients with newly diagnosed myeloma, the use of triple therapy incorporating an immunomodulatory agent and a proteasome inhibitor improves outcomes, with an acceptable risk-benefi t profi le. The S0777 study results can substantially inform decision making for front-line therapy using the triplet treatment approach in these patients.
Acknowledgments
This study was funded by grants from NIH/NCI/NCTN grants CA180888, CA180819, CA180821, CA180820; NIH/NCI/NCORP grants CA189858, CA189971, CA189808, CA189821, CA189829, CA189804, CA189953, CA189830, CA189957, CA189853, CA189872, CA189856, CA189860, CA139519, CA189854, CA189952, CA189825; NIH/NCI legacy grants CA04919, CA22433, CA58723, CA68183, CA35996, CA73590, CA12644, CA46282, CA13612, CA37981, CA16385, CA45450, CA46113 and partly by Millennium Pharmaceuticals, The Takeda Oncology Company, and Celgene Corporation for provision of study drug under their respective Cooperative Research and Development Agreements with the NCI.
Footnotes
Contributors
AH, MHA, SVR, JE, RS, BB, and AD designed the study. Patient entry was overseen by SPK, MT, FR, and CMR. AH, MHA, and RS collected data, and AH, RS, RZO, BB, and AD analysed data. All authors contributed to the data interpretation, writing of the second draft, and final sign-off of the report. BGMD wrote the first draft and provided overall guidance and support in all responsibilities.
Conflicts of interest
BGMD is a consultant for Johnson & Johnson, Takeda, Onyx, and Celgene. MHA receivesresearch funding from Millennium. JE is employed by University of Arkansas for Medical Sciences. FR and MHA receive research funding from Takeda, Millennium, Novartis, and Celgene. RZO is a member on BioTheryX’s, Janssen Pharmaceuticals’, and Acetylon’s Board of Directors and advisory committees; is a consultant for Celgene, Genentech, and Forma Therapeutics; receives research funding from Spectrum Pharmaceuticals and Onyx Pharmaceuticals; and is a consultant for and receives research funding from Bristol-Myers Squibb, Millennium Pharmaceuticals, and Array BioPharma. BB receives travel stipends from the Dana Farber Cancer Institute, the International Workshop on Waldenström’s Macroglobulinemia, ComtecMed-World Congress on Controversies in Hematology, the European School of Haematology-International Conference on Multiple Myeloma, and Multiple Myeloma Research Foundation; and is a consultant for Celgene; is a consultant and receives research funding from Millennium, Myeloma Health, LLC; and is the co-inventor of patents and patent applications related to use of gene expression profiling in cancer medicine licensed to Myeloma Health, LLC. AH, SVR, SPK, MT, CMR, RS, and AD declare no competing interests.
See Online for appendix
Contributor Information
Prof Brian G M Durie, Cedars-Sinai Samuel Oschin Cancer Center, Los Angeles, CA, USA.
Antje Hoering, Cancer Research and Biostatistics, Seattle, WA, USA.
Muneer H Abidi, Spectrum Health Cancer Center, Michigan State University, Grand Rapids, MI, USA.
Prof S Vincent Rajkumar, Mayo Clinic, Rochester, MN, USA.
Prof Joshua Epstein, Myeloma Institute, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Stephen P Kahanic, Siouxland Regional Cancer Center, Sanford NCORP of the Northern Central Plains, Sioux City, IA, USA.
Mohan Thakuri, Southeast Clinical Oncology Research Consortium NCORP, Cancer Care of Western North Carolina, Asheville, NC, USA.
Frederic Reu, Cleveland Clinic, Taussig Cancer Institute, Cleveland, OH, USA.
Christopher M Reynolds, Michigan Cancer Research Consortium NCORP, St Joseph Mercy Hospital, Ann Arbor, MI, USA.
Rachael Sexton, Cancer Research and Biostatistics, Seattle, WA, USA.
Prof Robert Z Orlowski, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Prof Bart Barlogie, Myeloma Program, Mount Sinai School of Medicine, New York, NY, USA.
Prof Angela Dispenzieri, Division of Hematology, Mayo Clinic, Rochester, MI, USA.
References
- 1.Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23:449–56. doi: 10.1038/leu.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–28. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG) Leukemia. 2009;23:1152–57. doi: 10.1038/leu.2008.402. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Kumar SK. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–19. doi: 10.1016/j.mayocp.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–76. [PubMed] [Google Scholar]
- 9.Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA. 2002;99:14374–79. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–30. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 11.Harousseau JL, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–505. [PubMed] [Google Scholar]
- 12.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82:1179–84. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–86. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4:379–98. [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 17.Griepp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 18.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 19.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomised clinical trials requiring prolonged observation of each patient: I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br JRadiol. 1971;44:793–97. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 23.Cox DR. Regression models and life tables (with discussion) J Roy Stat Soc. 1972;B34:187–220. [Google Scholar]
- 24.Agresti A. Categorical Data Analysis. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of analysis of data from retrospective studies of disease. J Nat Cancer Ins. 1959;22:719–48. [PubMed] [Google Scholar]
- 26.Cavo M, Tacchetti P, Patriarca F, et al. for the GIMEMA Italian Myeloma Network. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075–85. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 27.Moreau P, Hulin C, Macro M, et al. Bortezomib, thalidomide and dexamethasone (VTD) is superior to bortezomib, cyclophosphamide and dexamethasone (VCD) prior to autologous stem cell transplantation for patients with de novo multiple myeloma: results of the prospective IFM 2013–2014. [Nov 10 2015]; https://ash.confex.com/ash/2015/webprogram/Paper81103.html.
- 28.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as frontline treatment for multiple myeloma. Blood. 2010;116:679–86. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1:746–54. doi: 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benbouker L, Dimopoulos M, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906–17. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 31.Attal M, Lauwers-Cances V, Hulin C, et al. Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone Dy Myelome (IFM/DFCI 2009 trial) [Nov 10, 2015]; https://ash.confex.com/ash/2015/webprogram/Paper78452.html.
- 32.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 33.Palumbo A, Bringhen S, Lupparelli G, et al. Second primary maliginancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014;15:333–42. doi: 10.1016/S1470-2045(13)70609-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


