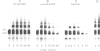Abstract
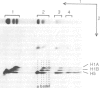
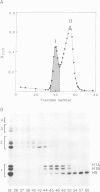
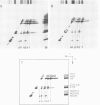
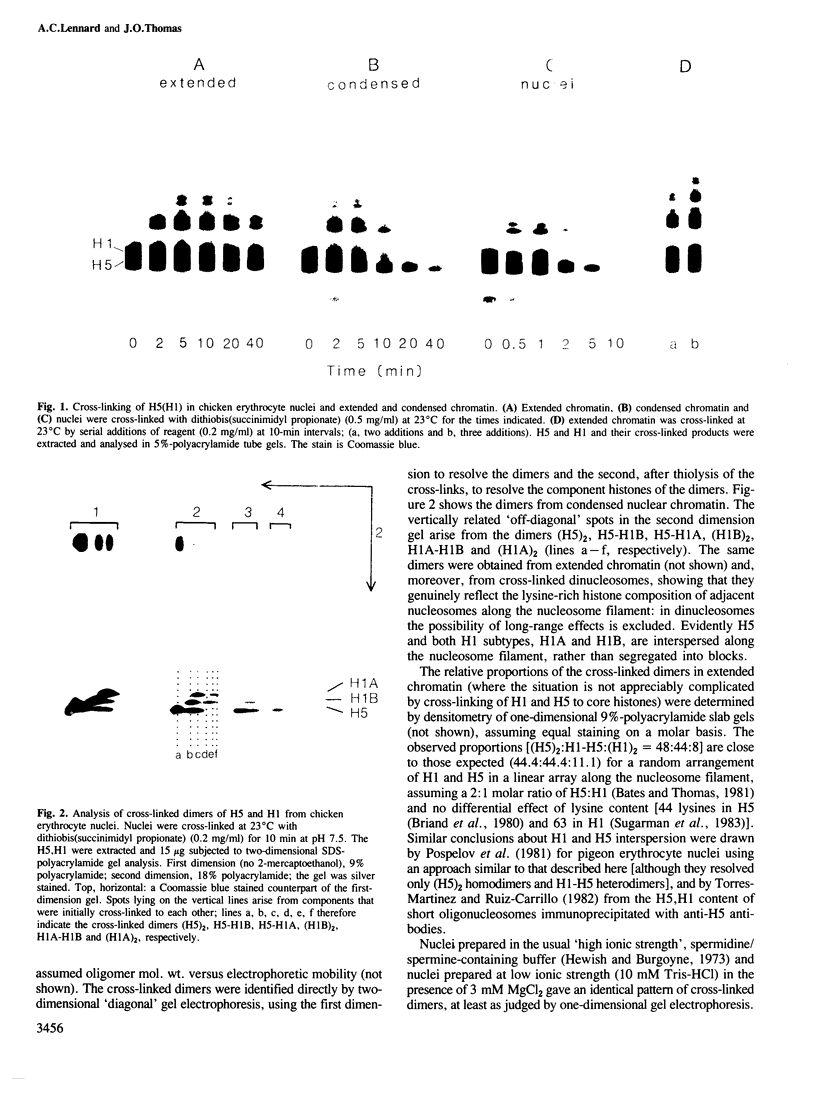
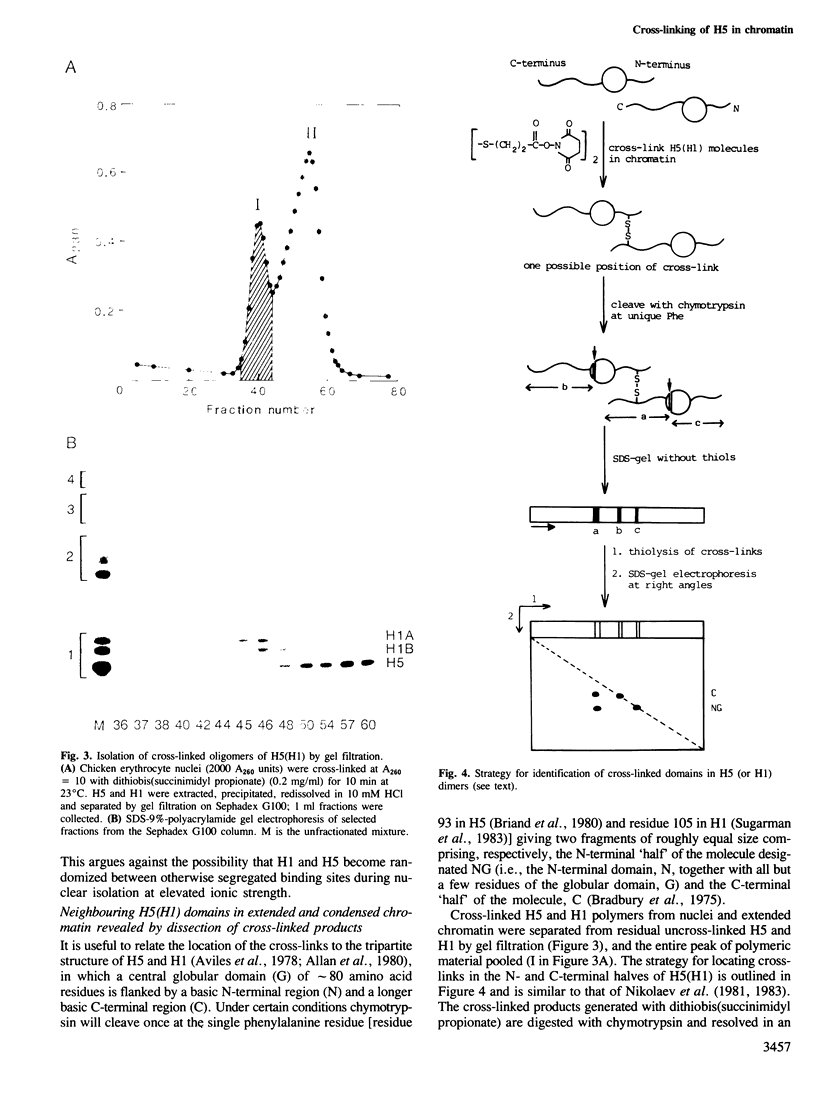
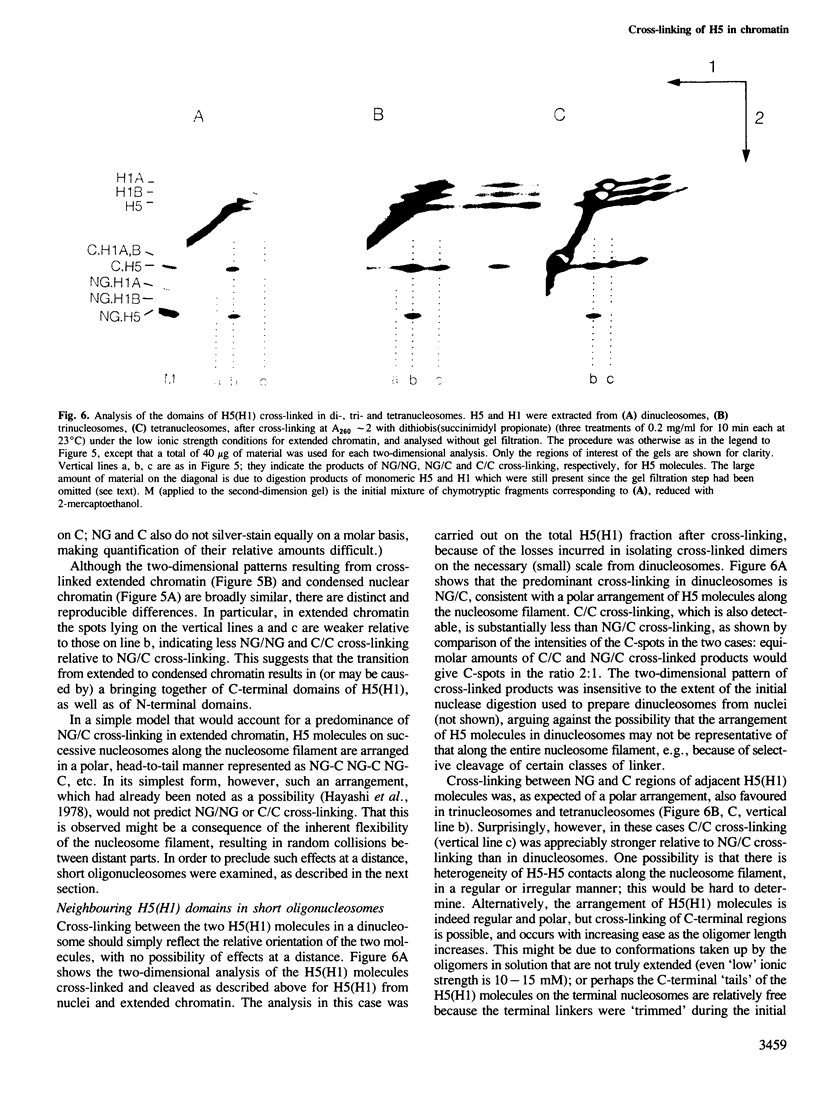
Chemical cross-linking with dithiobis(succinimidyl propionate) has been used to investigate the relative disposition of neighbouring H5 (H1) molecules in chicken erythrocyte chromatin in the extended (nucleosome filament) and condensed (300 A filament) states; in this chromatin H5 and H1 are interspersed along the nucleosome filament, rather than segregated into blocks, as shown by the nature of the cross-linked dimers and their relative amounts. Detailed analysis of the cross-linked H5 homopolymers from extended chromatin and condensed nuclear chromatin indicates which domains of H5 are in contact (or close proximity) in the two states. Two results suggest a polar, head-to-tail arrangement of H5 molecules along the nucleosome filament. This arrangement persists when chromatin adopts higher-order structure but in the folded state neighbouring basic C-terminal domains, in particular, are more closely juxtaposed than they are in extended chromatin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Aviles F. J., Chapman G. E., Kneale G. G., Crane-Robinson C., Bradbury E. M. The conformation of histone H5. Isolation and characterisation of the globular segment. Eur J Biochem. 1978 Aug 1;88(2):363–371. doi: 10.1111/j.1432-1033.1978.tb12457.x. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Butler P. J., Pearson E. C., Thomas J. O. Stability of the higher-order structure of chicken-erythrocyte chromatin in solution. Eur J Biochem. 1981 Oct;119(3):469–476. doi: 10.1111/j.1432-1033.1981.tb05631.x. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Thomas J. O. Histones H1 and H5: one or two molecules per nucleosome? Nucleic Acids Res. 1981 Nov 25;9(22):5883–5894. doi: 10.1093/nar/9.22.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Chapman G. E., Danby S. E., Hartman P. G., Riches P. L. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. The properties of the N-terminal and C-terminal halves of histone H1. Eur J Biochem. 1975 Sep 15;57(2):521–528. doi: 10.1111/j.1432-1033.1975.tb02327.x. [DOI] [PubMed] [Google Scholar]
- Briand G., Kmiecik D., Sautiere P., Wouters D., Borie-Loy O., Biserte G., Mazen A., Champagne M. Chicken erythrocyte histone H5. IV. Sequence of the carboxy-termined half of the molecule (96 residues) and complete sequence. FEBS Lett. 1980 Apr 7;112(2):147–151. doi: 10.1016/0014-5793(80)80167-0. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Thomas J. O. Changes in chromatin folding in solution. J Mol Biol. 1980 Jul 15;140(4):505–529. doi: 10.1016/0022-2836(80)90268-5. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Sollner-Webb B., Felsenfeld G. The organization of histones and DNA in chromatin: evidence for an arginine-rich histone kernel. Cell. 1976 Jul;8(3):333–347. doi: 10.1016/0092-8674(76)90145-8. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Hofstaetter T., Yakuwa N. Asymmetry of chromatin subunits probed with histone H1 in an H1-DNA complex. Biochemistry. 1978 May 16;17(10):1880–1883. doi: 10.1021/bi00603a012. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- NEELIN J. M., CALLAHAN P. X., LAMB D. C., MURRAY K. THE HISTONES OF CHICKEN ERYTHROCYTE NUCLEI. Can J Biochem. 1964 Dec;42:1743–1752. doi: 10.1139/o64-185. [DOI] [PubMed] [Google Scholar]
- Nelson P. P., Albright S. C., Wiseman J. M., Garrard W. T. Reassociation of histone H1 with nucleosomes. J Biol Chem. 1979 Nov 25;254(22):11751–11760. [PubMed] [Google Scholar]
- Nikolaev L. G., Glotov B. O., Dashkevich V. K., Barbashov S. F., Severin E. S. Mutual arrangement of histone H1 molecules in extended chromatin. Chymotryptic digestion of cross-linked H1 histone dimers. FEBS Lett. 1983 Oct 31;163(1):66–68. doi: 10.1016/0014-5793(83)81164-8. [DOI] [PubMed] [Google Scholar]
- Nikolaev L. G., Glotov B. O., Itkes A. V., Severin E. S. Mutual arrangement of histone H1 molecules in chromatin of intact nuclei. FEBS Lett. 1981 Mar 9;125(1):20–24. doi: 10.1016/0014-5793(81)80987-8. [DOI] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Pearson E. C., Butler P. J., Thomas J. O. Higher-order structure of nucleosome oligomers from short-repeat chromatin. EMBO J. 1983;2(8):1367–1372. doi: 10.1002/j.1460-2075.1983.tb01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospelov V. A., Jerkin A. M., Khachatrian A. T. H1 and H5 histone arrangement in chromatin of pigeon erythrocytes. FEBS Lett. 1981 Jun 15;128(2):315–317. doi: 10.1016/0014-5793(81)80106-8. [DOI] [PubMed] [Google Scholar]
- Ring D., Cole R. D. Close contacts between H1 histone molecules in nuclei. J Biol Chem. 1983 Dec 25;258(24):15361–15364. [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Dodgson J. B., Engel J. D. Genomic organization, DNA sequence, and expression of chicken embryonic histone genes. J Biol Chem. 1983 Jul 25;258(14):9005–9016. [PubMed] [Google Scholar]
- Thomas J. O., Khabaza A. J. Cross-linking of histone H1 in chromatin. Eur J Biochem. 1980 Dec;112(3):501–511. doi: 10.1111/j.1432-1033.1980.tb06113.x. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. The study of histone--histone associations by chemical cross-linking. Methods Cell Biol. 1978;18:429–440. [PubMed] [Google Scholar]
- Thomas J. O., Rees C. Exchange of histones H1 and H5 between chromatin fragments. A preference of H5 for higher-order structures. Eur J Biochem. 1983 Jul 15;134(1):109–115. doi: 10.1111/j.1432-1033.1983.tb07538.x. [DOI] [PubMed] [Google Scholar]
- Torres-Martinez S., Ruiz-Carrillo A. Nucleosomes containing histones H1 or H5 are closely interspersed in chromatin. Nucleic Acids Res. 1982 Apr 10;10(7):2323–2335. doi: 10.1093/nar/10.7.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]