Abstract
Context:
Patients with 21-hydroxylase deficiency (21OHD) have long-term complications, resulting from poor disease control and/or glucocorticoid overtreatment. Lack of optimal biomarkers has made it challenging to tailor therapy and predict long-term outcomes.
Objective:
To identify biomarkers of disease control and long-term complications in 21OHD.
Setting and Participants:
Cross-sectional study of 114 patients (70 males), ages 2 to 67 years (median, 15 years), seen in a tertiary referral center.
Methods:
We correlated a mass-spectrometry panel of 23 steroids, obtained before first morning medication, with bone age advancement (children), adrenal volume (adults), testicular adrenal rest tumors (TART), hirsutism, menstrual disorders, and pituitary hormones.
Results:
Total adrenal volume correlated positively with 18 steroids, most prominently 21-deoxycortisol and four 11-oxygenated-C19 (11oxC19) steroids: 11β-hydroxyandrostenedione (11OHA4), 11-ketoandrostenedione (11ketoA4), 11β-hydroxytestosterone (11OHT), and 11-ketotestosterone (11ketoT) (r ≈ 0.7, P < 0.0001). Nine steroids were significantly higher (P ≤ 0.01) in males with TART compared with those without TART, including 11OHA4 (6.8-fold), 11OHT (4.9-fold), 11ketoT (3.6-fold), 11ketoA4 (3.3-fold), and pregnenolone sulfate (PregS; 4.8-fold). PregS (28.5-fold) and 17-hydroxypregnenolone sulfate (19-fold) levels were higher (P < 0.01) in postpubertal females with menstrual disorders. In males, testosterone levels correlated positively with all 11oxC19 steroids in Tanner stages 1 and 2 (r ≈ 0.7; P < 0.001) but negatively in Tanner stage 5 (r = −0.3 and P < 0.05 for 11ketoA4 and 11ketoT). In females, testosterone level correlated positively with all four 11oxC19 steroids across all Tanner stages (r ≈ 0.8; P < 0.0001).
Conclusion:
11oxC19 steroids and PregS might serve as clinically useful biomarkers of disease control and long-term complications in 21OHD.
Using LC-MS/MS, we show 11-oxygenated 19-carbon steroids and pregnenolone sulfate are biomarkers of disease control and long-term complications in adults and children with 21-hydroxylase deficiency.
Congenital adrenal hyperplasia (CAH) is a group of inherited defects in cortisol biosynthesis, and 21-hydroxylase deficiency (21OHD) accounts for the overwhelming majority of cases (1, 2). The most severe forms of 21OHD are defined by clinically apparent glucocorticoid (GC) insufficiency and are termed “classic.” Classic 21OHD is further subclassified as “salt-wasting” (SW) or “simple virilizing” (SV), depending if the mineralocorticoid deficiency leads to neonatal adrenal crisis. In all 21OHD forms, impaired cortisol synthesis leads to chronic compensatory adrenocorticotropic hormone (ACTH) elevation, and the enzymatic blockage redirects the steroidogenic flux toward adrenal androgens.
GCs have been the mainstay of 21OHD treatment, not only for replacement, as in other forms of adrenal insufficiency, but also to suppress ACTH and the ensuing excessive androgen synthesis. For the latter goal, supraphysiologic GC therapy is often needed. Many of the long-term clinical complications characteristic of 21OHD result from the intimate relationship between the intrinsic hormonal excess and exogenous GC therapy. The sustained ACTH elevation in CAH is thought to promote the expansion of orthotopic and ectopic adrenal tissue. Patients with 21OHD have enlarged adrenal glands (3) and a higher prevalence of adrenal masses (4, 5). Adrenal rest tumors have been commonly reported in males (6–10) and occasionally in females (11–14) with classic 21OHD. Both endogenous sex steroids and exogenous GC influence growth velocity and final height; consequently, precise titration of GC treatment is critical during childhood.
The management of 21OHD has been limited by inadequate biomarkers of disease control (15). The advent of liquid chromatography-tandem mass spectrometry (LC-MS/MS) panels has expanded the repertoire of steroid biomarkers for 21OHD (16–18). Steroids synthesized with the participation of 11β-hydroxylase (CYP11B1), such as 11β-hydroxyandrostenedione (11OHA4) and 11-ketotestosterone (11ketoT), are abundant in patients with 21OHD, and these 11-oxygenated-C19 (11oxC19) steroids have been proposed to be clinically relevant androgens (16, 18). Herein, we examined the relationship between the serum steroid metabolome of children and adults with classic 21OHD and clinical findings reflecting long-standing poor disease control, such as increased adrenal volume, advanced bone age, presence of testicular adrenal rest tumors (TARTs) in males, and presence of hirsutism and menstrual disorders in females.
Subjects and Methods
Patients
Patients with classic 21OHD seen at the National Institutes of Health (NIH; Bethesda, Maryland) from 2010 to 2016 were included. The diagnosis was confirmed by hormonal and genetic analyses, and all patients were enrolled in a Natural History Study at the NIH (NCT00250159) (19). Patients who had undergone adrenalectomy were excluded. All serum samples were obtained at approximately 0800 hours, before receiving their first morning medications. Studies were conducted under a Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Board Review-approved protocol, with written informed consent obtained from all adults and parents of participating minors. All minors at least 8 years old gave written assent.
For children, Tanner stage was assessed by physical examination by a pediatric endocrinologist (D.P.M. or A.M.), according to the criteria of Tanner for breast development in females and genital staging based on the average volume of both testes in males (20). Patients were classified as adults if the bone age was >16 years for females or 17 years for males. For postpubertal females, hirsutism was defined as a Ferriman-Gallwey total score ≥8 and menstrual disorder was defined as amenorrhea (lack of menses for >6 months) or oligomenorrhea (cycle >35 days). A standardized GC equivalent (GCE) was calculated for each patient by multiplying the current GC dose by 1 for hydrocortisone, by 5 for prednisone, and by 80 for dexamethasone (19). For the one patient receiving continuous hydrocortisone infusion, the GCE was the total daily hydrocortisone dose (21). Current GCE was adjusted for body surface area.
Hormonal assays
We quantified 23 steroids by LC-MS/MS, including three unconjugated ∆5-steroids, four steroid sulfates, 14 ∆4-steroids, and three 3α,5α-reduced steroids. Steroid extraction and quantitation was carried out as previously described (16, 17). The 3α,5α-reduced steroids (Supplemental Table 1 (25.5KB, docx) ) were derivatized with 50 µL of 1 M ammonium hydroxide and 100 µL of 1 M hydroxylamine hydrochloride, as described for Δ5 steroids (16, 22).
Plasma ACTH was analyzed at the NIH Clinical Center (Bethesda, MD) by chemiluminescent immunoassay on a Siemens Immulite 200 XPi analyzer or on a Siemens Immulite 2500 analyzer (before 2012; Munich, Germany). The assay had a sensitivity of 5 pg/mL, reference range of 0 to 46 pg/mL, and a within-run coefficient of variation of 1.61% to 3.36% at a mean concentration of 33.97 and 466 pg/mL, respectively, and a day-to-day variability of 3.09% to 4.16% at a mean concentration of 34 and 469 pg/mL, respectively. Plasma renin activity was analyzed by LC-MS/MS and inhibin B by enzyme-linked immunosorbent assay at the Mayo Medical Laboratories (Rochester, MN).
Radiological studies
All growing children had a bone-age assessment by performing a radiograph of the left hand and wrist. Bone age was determined by a pediatric endocrinologist using the Greulich and Pyle method (23). Testicular ultrasound was performed in all males by a trained sonographer and radiologist using a linear, high-frequency, 7.5-MHz transducer in the transverse and longitudinal planes. The testicles were evaluated for size, echogenicity, and intratesticular lesions (TART). Color-flow Doppler ultrasound was performed if a mass was detected on grayscale sonography. Testicular and TART dimensions were retrospectively reviewed and determined by one radiologist (J.M.). Total testicular and TART volumes were calculated by applying the formula for a prolate ellipse (length × width × thickness × 0.52). Because pediatric patients were included, functional testicular volumes were calculated as a percent and defined as (1 – TART volume/testicular volume) × 100.
Adult patients (46 of 52) had a computed tomography (CT) scan of the adrenal glands. Adrenal volumes were measured with GE ADW4 (General Electric, Milwaukee, WI) and Vital Solutions Vitrea V6.74 (Vital Images, Minnetonka, MN) workstations using a 19-inch monitor (Tyco Electronics, Concord, CA) with a 1280 × 1024 matrix. All images were viewed with the default abdominal window and level settings (window, 400 HU; level, 40 HU). The scans, with slice thickness ranging from 1 to 2.5 mm, were used to create three-dimensional models of each adrenal gland. This was done by an experienced technologist trained to identify and outline the adrenal contour in each slice, and all measurements were reviewed by a single radiologist (N.A.A.). This tracing included any bulges of the contour caused by nodules or masses in the adrenal periphery. The adrenal volume was calculated from the sum of each axial slice area (24). Adrenal hypertrophy was defined as volumes greater than published norms: right adrenal gland, 5.7 mL for males and 4.9 mL for females; left adrenal gland, 5.7 mL for males, 4.4 mL for females (25).
Statistical analyses
The nonparametric Mann-Whitney U test was used to compare targeted hormones between prespecified groups. The nonparametric Spearman correlation test assessed the relation between pairs of continuous variables. Logistic (for binary variables) or linear (for continuous variables) regression was used to find the best multivariate correlation models. Statistical significance was accepted for P < 0.05.
Results
Clinical characteristics of participants
We enrolled 114 patients (70 males) with classic 21OHD (76 SW, 38 SV; Table 1). The median age of the cohort was 15 years (range, 2 to 67 years) and 52 (45.6%) were classified as adults. The median current GCE was 13.3 mg/m2/d (range, 5.8 to 26.3 mg/m2/d) for children and 17.5 mg/m2/d (range, 0 to 52 mg/m2/d) for adults. Of note, three adults with SV CAH had voluntarily discontinued GC therapy for varying durations. Of the pediatric patients, 32 (51.6%), 10 (16.1%), five (8%), seven (11.3%), and eight (13%) were in Tanner stages 1, 2, 3, 4, and 5, respectively.
Table 1.
Clinical Characteristics of Pediatric and Adult Patients With CAH Due to 21OHD According to Sex
| Male | Female | |
|---|---|---|
| Pediatric patients | ||
| No. | 39 | 23 |
| Phenotype, n (%) | ||
| SW | 25 (64.1) | 20 (87.0) |
| SV | 14 (35.9) | 3 (13.0) |
| Age, y | 10.8 ± 3.8 | 8.0 ± 3.7 |
| BA – CA, y | 1.2 ± 1.6 | 0.1 ± 1.6a |
| Tanner stage, n (%) | ||
| 1 | 15 (38.5) | 17 (73.9) |
| 2 | 9 (23.1) | 1 (4.3) |
| 3 | 4 (10.3) | 1 (4.3) |
| 4 | 4 (10.3) | 3 (13.0) |
| 5 | 7 (17.9) | 1 (4.3) |
| With TART, n (%) | 7 (17.9) | N/A |
| GCE, mg/m2/db | 14.0 ± 3.5 | 13.5 ± 4.9 |
| Adult patients, n | ||
| No. | 31 | 21 |
| Phenotype | ||
| SW, n (%) | 16 (51.6) | 15 (71.4) |
| SV, n (%) | 15 (48.4) | 6 (28.6) |
| Age, y | 29 ± 13.3 | 26 ± 8.9 |
| Adrenal volume, mL | ||
| R adrenal | 10.7 ± 6.8 | 9.7 ± 6.3 |
| L adrenal | 17.9 ± 32.0 | 22.3 ± 46.1 |
| With TART, n (%) | 14 (45.2) | N/A |
| Hirsutism, n (%)c | N/A | 9 (42.9) |
| Menstrual disorder, n (%)d | N/A | 7 (33.3) |
| GCE, mg/m2/db | 18.7 ± 8.5 | 16.9 ± 10.0 |
Values are expressed as means ± SD unless otherwise specified.
Abbreviations: BA, bone age; CA, chronological age; N/A, not applicable.
Bone-age assessment performed for children >4 years.
Total GCE dose divided by body surface area (mg/m2/d) × 1 for hydrocortisone, × 5 for prednisone, and × 80 dexamethasone (19).
Females with a Ferriman-Gallwey Score ≥8.
Amenorrhea or menstrual cycle length >35 days.
TARTs were found in 13 of 41(32%) of males with SW CAH and eight of 29 (28%) males with SV CAH. The maximum diameter ranged from 0.1 to 4.7 cm (mean, 1.8 cm). TARTs were present bilaterally in 17 of 21 (81%) of patients and the asymmetry between the two sides varied from 0.001 to 2.8 cm3. Of 46 patients who had an abdominal CT scan, 29 (63%) had adrenal hypertrophy, either with (n = 25 patients) or without nodularity (n = 4 patients), and 6 patients (13%) had adrenal nodules but normal gland size. One patient had adrenal atrophy. The median total adrenal volume was 20 mL (range, 4 to 229 mL).
Of 21 postpubertal females, seven (33%) had menstrual disorders (five with SW CAH) and nine (43%) had hirsutism (six with SW CAH). One patient had only oligomenorrhea, three patients had only hirsutism, and six patients had both hirsutism and irregular menses.
Hormonal correlations with clinical parameters of poor long-term disease control
Total adrenal volume correlated positively and significantly with 18 of 23 steroids. Of these, the strongest correlations were with the four 11oxC19 steroids and 21-deoxycortisol (21dF; r = 0.67 to 0.70; P < 0.0001 for all), followed by androstenedione (A4), 17α-hydroxyprogesterone (17OHP), and 16α-hydroxyprogesterone (16OHP; r = 0.57 to 0.62; P < 0.0001 for all; Table 2). Multivariate linear regression analyses, which are limited by the codependence of these variables, identified 21dF and progesterone as the best predictors of adrenal volume. Total adrenal volume also correlated directly with ACTH (r = 0.4; P = 0.005), but inversely with luteinizing hormone (LH) (r = −0.5; P < 0.001). There was no significant correlation between adrenal volume and follicle-stimulating hormone (FSH) (r = −0.25; P = 0.1) or inhibin B (r = −0.4; P = 0.13).
Table 2.
Hormonal Correlations With Total Adrenal Volume
| r | 95% CI | P | |
|---|---|---|---|
| Pregnenolone | 0.47 | 0.2 to 0.68 | 0.0009 |
| 17OH-pregnenolone | 0.31 | 0.02 to 0.56 | 0.03 |
| Dehydroepiandrosterone | 0.23 | −0.071 to 0.50 | 0.12 |
| Androsterone | 0.56 | 0.31 to 0.73 | < 0.0001 |
| Allopregnanolone | 0.48 | 0.21 to 0.68 | 0.0007 |
| 5α-Pregnane-3α,17α-diol-20-one | 0.43 | 0.15 to 0.65 | 0.003 |
| PregS | 0.52 | 0.26 to 0.70 | 0.0002 |
| 17OHPregS | 0.39 | 0.11 to 0.62 | 0.007 |
| Dehydroepiandrosterone sulfate | 0.37 | 0.08 to 0.6 | 0.01 |
| Androstenediol-3-sulfate | 0.16 | −0.15 to 0.44 | 0.29 |
| Cortisol | 0.41 | 0.13 to 0.63 | 0.005 |
| 21dF | 0.67 | 0.46 to 0.81 | < 0.0001 |
| 11-Deoxycortisol | 0.24 | −0.06 to 0.50 | 0.11 |
| 11-Deoxycorticosterone | 0.26 | −0.05 to 0.51 | 0.09 |
| 16OHP | 0.57 | 0.33 to 0.74 | < 0.0001 |
| 17OHP | 0.58 | 0.35 to 0.75 | < 0.0001 |
| A4 | 0.62 | 0.39 to 0.77 | < 0.0001 |
| Testosterone | −0.02 | −0.31 to 0.28 | 0.91 |
| Progesterone | 0.50 | 0.23 to 0.69 | 0.0004 |
| 11OHA4 | 0.70 | 0.50 to 0.82 | < 0.0001 |
| 11ketoA4 | 0.68 | 0.48 to 0.81 | < 0.0001 |
| 11OHT | 0.68 | 0.48 to 0.81 | < 0.0001 |
| 11ketoT | 0.67 | 0.46 to 0.80 | < 0.0001 |
| Inhibin | −0.37 | −0.72;0.13 | 0.13 |
| ACTH | 0.42 | 0.12 to 0.64 | 0.005 |
| PRA | 0.42 | 0.14 to 0.64 | 0.004 |
| LH | −0.51 | −0.71 to -0.23 | 0.0006 |
| FSH | −0.25 | −0.52 to 0.06 | 0.11 |
Abbreviations: CI, confidence interval; PRA, plasma renin activity.
The youngest patient with TART in our cohort was 14 years old. To eliminate hormonal differences due to age, we compared males with and without TART who were age 14 years and older. The phenotype and age distribution were similar in males with and without TART (P > 0.99). ACTH, LH, FSH, inhibin B and current GCE were no different (P > 0.1) between patients with or without TART. We found that nine of the 23 steroids quantified were significantly higher in males with as compared with those without TART, including 11OHA4 (6.8-fold; P = 0.004), 11β-hydroxytestosterone (11OHT; 4.9-fold; P = 0.002); 11ketoT (3.6-fold; P = 0.004); 11-ketoandrostenedione (11ketoA4; 3.3-fold; P = 0.01); A4 (3.3-fold; P = 0.03); pregnenolone sulfate (PregS; 4.8-fold; P = 0.007); 17-hydroxypregnenolone sulfate (17OHPregS; 2.3-fold; P = 0.01); androsterone (2.7-fold; P = 0.02) and allopregnanolone (2.6-fold; P = 0.01; Table 3; Supplemental Table 2 (25.5KB, docx) ). In the subset of patients who had both testicular ultrasound and abdominal CT imaging, the median adrenal volume was higher in patients with than in those without TART (25 vs 17 mL; P = 0.02). None of the steroid biomarkers demonstrated a meaningful correlation with TART size or percent functional testicular volume.
Table 3.
Summary of Relationships Between Key Biomarkers and Clinical Findings
| Steroid | r Value: Adrenal Volume | TART/No TART | ∆ Bone Age | Menstrual Disorders/Regular Menses | r Value: ACTH | Testosterone | ||
|---|---|---|---|---|---|---|---|---|
| Prepubertal Males | Postpubertal Males | Females | ||||||
| 17OHP | 0.7a | NS | NS | 5.5b | 0.57a | |||
| 16OHP | 0.7a | NS | NS | 6.8b | 0.58a | |||
| 21dF | 0.64a | NS | NS | 3.6b | 0.67a | |||
| A4 | 0.62a | 3.3b | NS | 3.9b | 0.53a | |||
| 11OHA4 | 0.6a | 6.8c | NS | 4.5b | 0.7a | 0.6c | NS | 0.84a |
| 11ketoA4 | 0.6a | 3.3b | NS | 4.7b | 0.68a | 0.8a | −0.3b | 0.75a |
| 11OHT | 0.44a | 4.9c | NS | 7.2b | 0.68a | 0.79a | NS | 0.8a |
| 11ketoT | 0.64a | 3.6c | NS | 7.3b | 0.67a | 0.66d | −0.32b | 0.83a |
| PregS | 0.57a | 4.8c | NS | 28.5c | 0.5d | |||
Abbreviations: ∆, change in; NS, no statistical significance.
P < 0.0001.
P < 0.05.
P < 0.01.
P < 0.001.
Most steroid hormones were significantly higher in women with menstrual disorders than in those with regular menses, most notably PregS (28.5-fold; P < 0.01) and 17OHPregS (19-fold; P < 0.01; Table 3). ACTH was also higher in patients with menstrual disturbances as compared with women with regular menses (4.7-fold; P = 0.014), whereas LH, FSH, and current GCE values were no different between the two groups. Similar results were found for hirsutism (data not shown).
We did not find any predictors of bone-age advancement. Subgroup analysis of patients with delayed, normal, and advanced bone age did not reveal any significant differences in steroid biomarkers (data not shown).
Interhormonal correlations
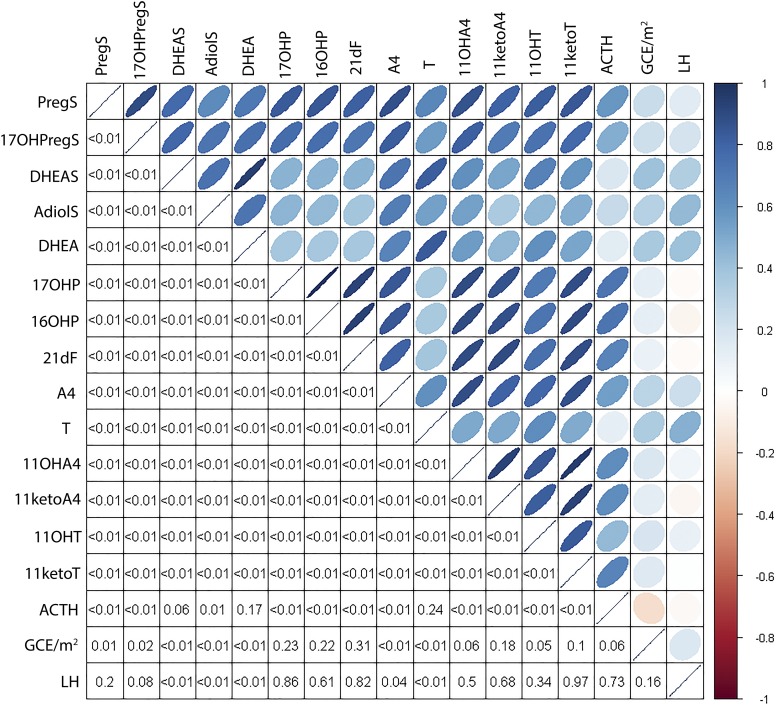
Of the 23 steroids measured, 20 correlated directly with ACTH. The tightest ACTH correlations were observed for 17OHP and 16OHP (r = 0.72; P < 0.0001 for both), followed by 21dF, 11ketoT, progesterone, 11OHA4, and 11ketoA4 (r = 0.61 to 0.66; P < 0.0001 for all; Fig. 1). Conversely, ACTH did not correlate with dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), and testosterone (T).
Figure 1.
Interhormonal correlation matrix (lower panel shows P values). AdiolS, androst-5-ene-3β,17β-diol-3-sulfate;16/17OHP, DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate.
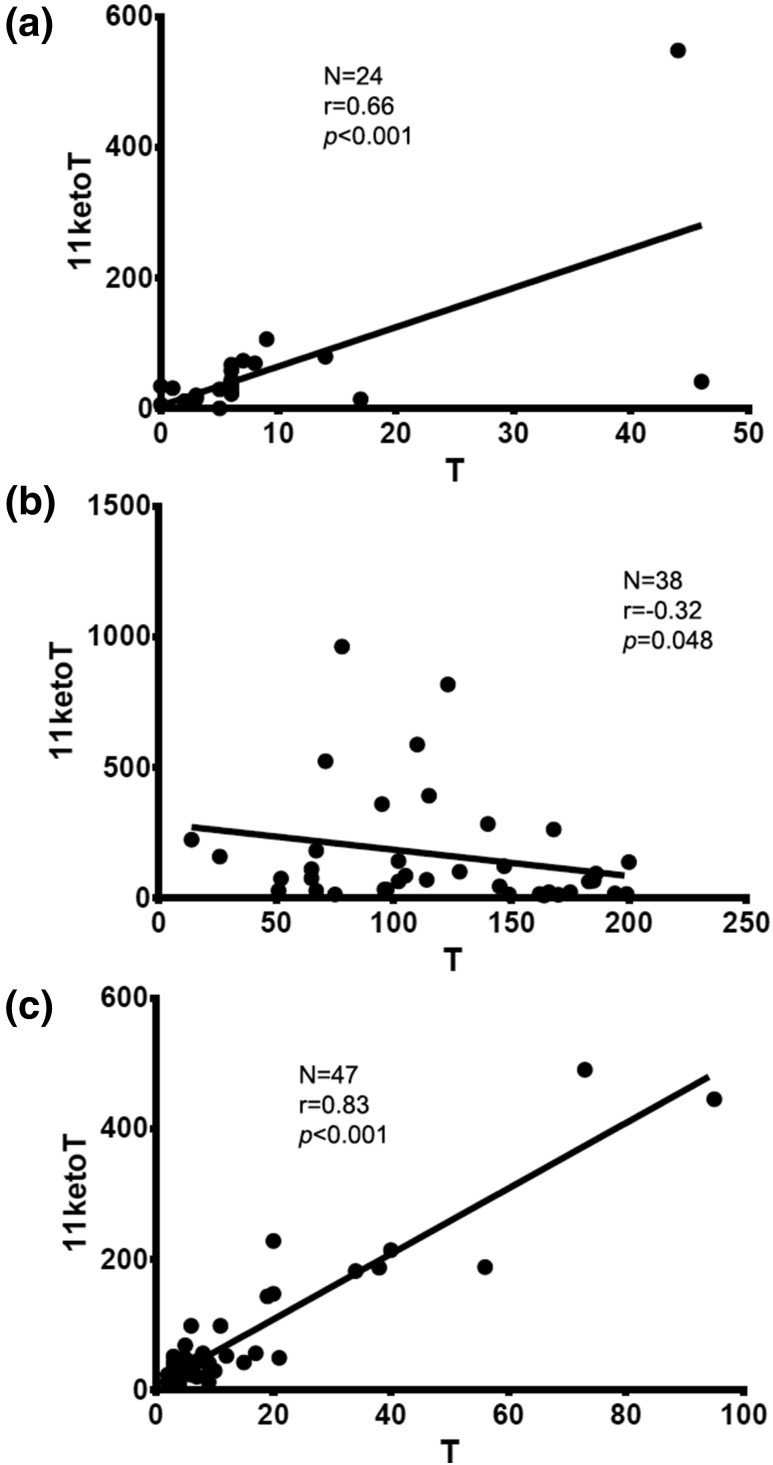
T correlated positively with LH in males (r = 0.7; P < 0.0001) but not females (r = 0.4; P = 0.052). LH did not correlate with either that of 11OHT or 11ketoT in females of all ages and prepubertal boys. In males in Tanner stage 5, a negative correlation with LH was observed for both 11OHT (r = −0.43; P = 0.01) and 11ketoT (r = −0.41; P = 0.02). T demonstrated a positive correlation with all four 11oxC19 steroids in boys in Tanner stages 1 and 2 [r = 0.6 to 0.8; P < 0.001; Fig. 2(a)]. In contrast, 11ketoT and 11ketoA4 correlated negatively with T in boys in Tanner stage 5 [r = −0.3; P = 0.04 for 11ketoA4 and 0.048 for 11ketoT; Fig. 2(b)]. In females, there was a direct correlation between T and all four 11oxC19 steroids for all Tanner stages [r = 0.75 to 0.84; P < 0.0001 for all; Fig 2(c)]. Overall, four 11oxC19 steroids and PregS were found to have the most significant correlations with key clinical characteristics (Table 3).
Figure 2.
Correlations between 11ketoT and T in boys in Tanner stages 1 and 2 (upper graph), males in Tanner stage 5 (middle graph), and females of all ages (lower graph).
Discussion
To our knowledge, this is the first study to implement LC-MS/MS to comprehensively analyze the relationship between a wide spectrum of traditional and emerging serum steroid biomarkers and clinical outcomes reflecting poor long-term disease control of classic 21OHD. Adverse effects solely deriving from iatrogenic Cushing syndrome were not within the scope of our study. We identified associations of several steroid biomarkers with adrenal volume, presence of TART, menstrual disturbances, and hirsutism, but no predictors of advanced bone age or TART size.
In total, 18 of the 23 measured steroids correlated directly with adrenal volume. The tightest correlation of adrenal volume was with 21dF and the 11oxC19 steroids 11OHA4, 11ketoA4, 11OHT, and 11ketoT, followed closely by A4, 17OHP, and 16OHP. Along with 17OHP, 21dF (26, 27) and 16OHP (17) have been found to be elevated in patients with 21OHD. The 21dF and all 11oxC19 steroids result from the further metabolism of precursors via CYP11B1. CYP11B1 catalyzes the final step in cortisol synthesis and is predominantly expressed in the adrenal gland, with similar expression in zona fasciculata and zona reticularis (28). As we have recently shown, the 11oxC19 steroids 11OHA4, 11ketoA4, 11OHT, and 11ketoT are three- to fourfold higher in treated patients with classic 21OHD compared with sex- and age-matched control subjects (16). Our results further demonstrate that the production of all four 11oxC19 steroids is proportional with adrenal volume. In addition, adrenal volume correlated inversely with LH, suggesting that the abundant production of adrenal androgens leads to hypothalamic-pituitary-gonadal axis suppression. In a cross-sectional study of 26 men with classic 21OHD, Reisch and colleagues (3) found a similar positive correlation of the total adrenal volume with the established biomarkers, 17OHP and A4, as measured by immunoassays, and a negative correlation with LH.
We identified nine steroid biomarkers to be significantly higher in males with TART as compared with their counterparts of similar ages without TART. Of these, 11OHA4 was the most significant. In contrast, T was similar in both groups, a finding in agreement with similar reports (7). Interestingly, two 3α,5α-reduced metabolites, androsterone and allopregnanolone, were higher in the TART group. Kamrath and colleagues (29) demonstrated a sevenfold increase in urinary androsterone concentration in 142 children and young adults with 21OHD compared with that of 138 similarly aged control subjects. In a recent study of 150 children with classic 21OHD treated with thrice-daily hydrocortisone, however, 24-hour urinary androsterone excretion was similar to that of etiocholanolone, a T metabolite (18). Our findings suggest that the 3α,5α-reduction of progesterone and 17OHProg to allopregnanolone and androsterone, respectively, might become more significant when disease control is poor.
Interestingly, two conjugated steroids, PregS and 17OHPregS, were significantly higher in males with than in those of the same age without TART, whereas DHEAS and androst-5-ene-3β,17β-diol-3-sulfate were similar. We have previously shown that PregS is higher in patients with classic 21OHD than in age- and sex-matched control subjects, whereas DHEAS and androst-5-ene-3β,17β-diol-3-sulfate were both dramatically lower in 21OHD (16). Taken together, these findings suggest that the upstream conjugated steroids PregS and 17OHPregS could be useful biomarkers of poor disease control, whereas the ordinarily major adrenal C19 steroid DHEAS cannot serve this purpose.
We found no meaningful hormonal correlations with TART volume, in concordance with previous reports (3). Because none of the steroids correlated positively with tumor size, it is unlikely that the additional adrenal-like tissue is sufficient to account for the higher amounts of these novel biomarkers in patients with TART. Although adrenal volume and TART size were not correlated, the median adrenal volume was higher in the TART group compared with males without TART, suggesting poorer disease control in the preceding months to years.
The origins of TARTs and factors that influence their growth have been controversial. These tumors are thought to originate from adrenocortical cells from the common gonadal-adrenal primordium, which migrate along with the gonads to their final location. TARTs share several common features with adrenal-cortex tissue, such as expression of ACTH and angiotensin II receptors (30–33). Poor disease control has been proposed to promote tumor growth, whereas intensive GC treatment can decrease TART size and restore fertility (34–36). Conversely, some TARTs do not respond to GC treatment (37, 38), possibly due to an advanced fibrotic stage (39). More recently, it has been proposed that TART might arise from totipotent embryonic cells, expressing markers of both adrenal cortex and Leydig cells (40, 41). Regardless of this aspect, the lack of TART size correlation with specific steroid biomarkers or long-term 21OHD treatment in this and other studies (3, 6, 42) is not surprising, because factors predisposing to TART are not fully understood and only a subset of males with 21OHD develop TART. An important contribution of intrauterine exposure to elevated ACTH for adrenal rest development has been proposed (39). Autopsy studies in infants showed that adrenal rests are more prevalent in 21OHD (43%) than in unaffected neonates (3.5%) (43, 44). Furthermore, the prevalence of TART correlates with the 21OHD phenotype severity, being most common in patients with SW 21OHD and very rarely described in nonclassic 21OHD (3, 6–8). Collectively, these lines of evidence suggest that, although the presence and ultimate dimensions of TART depend on a suite of prenatal events, the chronicity and amplitude of ACTH elevation contributes to TART enlargement.
Female patients with menstrual disorders and/or hirsutism tended to have higher ACTH and higher concentrations of several steroid biomarkers than females without clinical evidence of androgen excess, despite being treated with similar doses of GCs. Remarkably, in females with menstrual disturbances, T was only double, whereas PregS was >28-fold higher than in those with normal menses. In congruence with our findings in males with TART, PregS once again distinguished itself as a biomarker of poor disease control.
It is not surprising that we did not identify any steroid predictors of bone-age advancement. Children with 21OHD may have accelerated prepubertal growth with advanced bone maturation and shorter final height than unaffected individuals or than predicted by parental height (45–48). In general, advanced bone age is an indicator of poor disease control but may also reflect late diagnosis or an antecedent period of poor control.
To understand the dynamic regulation of the studied biomarkers, we examined their correlations with pituitary hormones, as well as with the biomarkers currently used in clinical practice. Of the steroids measured, only DHEA, DHEAS, and T did not show a significant correlation with ACTH. Expectedly, 17OHP and 16OHP demonstrated the tightest correlation with ACTH, followed closely by 21dF, progesterone, 11ketoT, 11OHA4, and 11ketoA4. T correlated positively with that of LH in males. In contrast, a negative correlation of 11OHT and 11ketoT with LH was observed in sexually mature males. Moreover, T correlated positively with 11ketoT in males in Tanner stages 1 to 3 and all female patients, whereas the same correlation was negative in males in Tanner stage 5. Collectively, these findings suggest that 11ketoT synthesis relies predominantly on adrenal precursors. It has been suggested that 11ketoT might be produced by Leydig cells and theca cells (49); however, CYP11B1, a key enzyme in the synthesis of all 11oxC19 steroids, is expressed in only trivial amounts in the gonads as compared with the adrenal cortex. Moreover, although T concentrations are dramatically higher in adult men than in women, 11ketoT concentration is comparable in both sexes, not only in adrenal vein samples but also in peripheral serum (16, 49). These findings once again demonstrate that the gonadal capacity to synthesize 11ketoT is negligible. Because the androgenic activity of 11ketoT parallels that of T (50–52), we have previously proposed that 11ketoT might act as the major circulating androgen in many patients with 21OHD. Several findings from our present studies, including the positive correlation with adrenal volume, the higher concentration in males with TART, and the positive correlation with ACTH and lack of correlation with LH, further promote 11ketoT as a promising clinical biomarker in patients with 21OHD.
Our study has several limitations inherent in its cross-sectional design. Thus, the associations between the steroids and the outcomes do not indicate if the associations are related to the development of the outcomes or if the steroids are consequences of the outcomes. The predictive value of these potential markers needs to be investigated in prospective studies.
In summary, this is, to our knowledge, the first study to implement LC-MS/MS for an extensive analysis of the serum steroid metabolome across a wide range of ages and in relation with long-term complications in patients with 21OHD. We found a link between the morning serum steroid metabolome and long-term complications in patients with classic 21OHD. We identified a set of steroid biomarkers that are associated with increased adrenal volume and the presence of TART, but not TART size, in males and menstrual disorders in females. In particular, four 11oxC19 steroids and PregS emerged as biomarkers with promising clinical value for monitoring treatment of 21OHD. LC-MS/MS offers the benefit of sensitive and specific quantitation of multiple analytes simultaneously. This approach is likely to expand into clinical laboratories, thereby allowing the development of valuable biomarker tools for optimizing patient care. Our study constitutes only an early step in the discovery of greatly needed biomarkers in 21OHD management. Longitudinal prospective studies that adopt a similar steroid metabolome approach are necessary for further selection of relevant biomarkers to guide treatment of patients with 21OHD.
Acknowledgments
We thank Mr. Robert Chomic for technical assistance with LC-MS/MS.
Acknowledgments
This work was supported, in part, by Grants 1K08DK109116 (to A.F.T.) and R01GM086596 (to R.J.A.) from the National Institutes of Health, Michigan Institute for Clinical and Health Research Translational Science Award/U046500 (to A.F.T.), and the Intramural Research Program of the National Institutes of Health. Mass spectrometry used core services supported by Grant DK089503 from the National Institutes of Health to the University of Michigan under the Michigan Nutrition Obesity Center.
Clinical trial registry: ClinicalTrials.gov no. NCT00250159 (registered 5 November 2005)
Disclosure Summary: D.P.M. received unrelated research funds from Diurnal Limited and Millendo Therapeutics through the National Institutes of Health Cooperative Research and Development Agreement and is a commissioned officer in the US Public Health Service. R.J.A. received unrelated research funds from Millendo Therapeutics. The remaining authors have nothing to disclose.
Footnotes
- 11ketoA4
- 11-ketoandrostenedione
- 11ketoT
- 11-ketotestosterone
- 11OHA4
- 11β-hydroxyandrostenedione
- 11OHT
- 11β-hydroxytestosterone
- 11oxC19
- 11-oxygenated-C19
- 16OHP
- 16α−hydroxyprogesterone
- 17OHP
- 17β-hydroxyprogesterone
- 17OHPregS
- 17-hydroxypregnenolone sulfate
- 21dF
- 21-deoxycortisol
- 21OHD
- 21-hydroxylase deficiency
- A4
- androstenedione
- ACTH
- adrenocorticotropic hormone
- CAH
- congenital adrenal hyperplasia
- CYP11B1
- 11β-hydroxylase
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- GC
- glucocorticoid
- GCE
- glucocorticoid equivalent
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LH
- luteinizing hormone
- NIH
- National Institutes of Health
- PregS
- pregnenolone sulfate
- SV
- simple virilizing
- SW
- salt-wasting
- TART
- testicular adrenal rest tumor.
References
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349(8):776–788. [DOI] [PubMed] [Google Scholar]
- 2.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125–2136. [DOI] [PubMed] [Google Scholar]
- 3.Reisch N, Scherr M, Flade L, Bidlingmaier M, Schwarz HP, Müller-Lisse U, Reincke M, Quinkler M, Beuschlein F. Total adrenal volume but not testicular adrenal rest tumor volume is associated with hormonal control in patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2010;95(5):2065–2072. [DOI] [PubMed] [Google Scholar]
- 4.Jaresch S, Kornely E, Kley HK, Schlaghecke R. Adrenal incidentaloma and patients with homozygous or heterozygous congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1992;74(3):685–689. [DOI] [PubMed] [Google Scholar]
- 5.Nermoen I, Rørvik J, Holmedal SH, Hykkerud DL, Fougner KJ, Svartberg J, Husebye ES, Løvås K. High frequency of adrenal myelolipomas and testicular adrenal rest tumours in adult Norwegian patients with classical congenital adrenal hyperplasia because of 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2011;75(6):753–759. [DOI] [PubMed] [Google Scholar]
- 6.Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, Hermus AR. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(12):5721–5728. [DOI] [PubMed] [Google Scholar]
- 7.Claahsen-van der Grinten HL, Sweep FC, Blickman JG, Hermus AR, Otten BJ. Prevalence of testicular adrenal rest tumours in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2007;157(3):339–344. [DOI] [PubMed] [Google Scholar]
- 8.Falhammar H, Nyström HF, Ekström U, Granberg S, Wedell A, Thorén M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2012;166(3):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvattier C, Esterle L, Renoult-Pierre P, de la Perrière AB, Illouz F, Kerlan V, Pascal-Vigneron V, Drui D, Christin-Maitre S, Galland F, Brue T, Reznik Y, Schillo F, Pinsard D, Piguel X, Chabrier G, Decoudier B, Emy P, Tauveron I, Raffin-Sanson ML, Bertherat J, Kuhn JM, Caron P, Cartigny M, Chabre O, Dewailly D, Morel Y, Touraine P, Tardy-Guidollet V, Young J. Clinical outcome, hormonal status, gonadotrope axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French national survey. J Clin Endocrinol Metab. 2015;100(6):2303–2313. [DOI] [PubMed] [Google Scholar]
- 10.Delfino M, Elia J, Imbrogno N, Argese N, Mazzilli R, Toscano V, Mazzilli F. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: prevalence and sonographic, hormonal, and seminal characteristics. J Ultrasound Med. 2012;31(3):383–388. [DOI] [PubMed] [Google Scholar]
- 11.Zaarour MG, Atallah DM, Trak-Smayra VE, Halaby GH. Bilateral ovary adrenal rest tumor in a congenital adrenal hyperplasia following adrenalectomy. Endocr Pract. 2014;20(4):e69–e74. [DOI] [PubMed] [Google Scholar]
- 12.Tiosano D, Vlodavsky E, Filmar S, Weiner Z, Goldsher D, Bar-Shalom R. Ovarian adrenal rest tumor in a congenital adrenal hyperplasia patient with adrenocorticotropin hypersecretion following adrenalectomy. Horm Res Paediatr. 2010;74(3):223–228. [DOI] [PubMed] [Google Scholar]
- 13.Stikkelbroeck NM, Hermus AR, Schouten D, Suliman HM, Jager GJ, Braat DD, Otten BJ. Prevalence of ovarian adrenal rest tumours and polycystic ovaries in females with congenital adrenal hyperplasia: results of ultrasonography and MR imaging. Eur Radiol. 2004;14(10):1802–1806. [DOI] [PubMed] [Google Scholar]
- 14.Crocker MK, Barak S, Millo CM, Beall SA, Niyyati M, Chang R, Avila NA, Van Ryzin C, Segars J, Quezado M, Merke DP. Use of PET/CT with cosyntropin stimulation to identify and localize adrenal rest tissue following adrenalectomy in a woman with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(11):E2084–E2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC; Endocrine Society . Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ. Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency. J Clin Endocrinol Metab. 2015;100(6):2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF, Wudy SA. The urinary steroidome of treated children with classic 21-hydroxylase deficiency. J Steroid Biochem Mol Biol. 2017;165(Pt B):396–406. [DOI] [PubMed] [Google Scholar]
- 19.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weise M, Eisenhofer G, Merke DP. Pubertal and gender-related changes in the sympathoadrenal system in healthy children. J Clin Endocrinol Metab. 2002;87(11):5038–5043. [DOI] [PubMed] [Google Scholar]
- 21.Nella AA, Mallappa A, Perritt AF, Gounden V, Kumar P, Sinaii N, Daley LA, Ling A, Liu CY, Soldin SJ, Merke DP. A phase 2 study of continuous subcutaneous hydrocortisone infusion in adults with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2016;101(12):4690–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng HM, Im SC, Pearl NM, Turcu AF, Rege J, Waskell L, Auchus RJ. Cytochrome b5 activates the 17,20-lyase activity of human cytochrome P450 17A1 by increasing the coupling of NADPH consumption to androgen production. Biochemistry. 2016;55(31):4356–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greulich WWPS. Radiographic Skeletal Development of the Hand and Wrist. Stanford: Stanford University Press; 1985. [Google Scholar]
- 24.Chrysostomou PP, Lodish MB, Turkbey EB, Papadakis GZ, Stratakis CA. Use of 3-dimensional volumetric modeling of adrenal gland size in patients with primary pigmented nodular adrenocortical disease. Horm Metab Res. 2016;48(4):242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geraghty EM, Boone JM, McGahan JP, Jain K. Normal organ volume assessment from abdominal CT. Abdom Imaging. 2004;29(4):482–490. [DOI] [PubMed] [Google Scholar]
- 26.Costa-Barbosa FA, Tonetto-Fernandes VF, Carvalho VM, Nakamura OH, Moura V, Bachega TA, Vieira JG, Kater CE. Superior discriminating value of ACTH-stimulated serum 21-deoxycortisol in identifying heterozygote carriers for 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2010;73(6):700–706. [DOI] [PubMed] [Google Scholar]
- 27.Milewicz A, Vecsei P, Gruszka S, Szymczak J, Bednarek-Tupikowska G, Grabiński M. Diagnosis of congenital adrenal hyperplasia based on plasma 21-deoxycortisol level determined with a specific radioimmunoassay. Mater Med Pol. 1984;16(2-4):95–98. [PubMed] [Google Scholar]
- 28.Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J Clin Endocrinol Metab. 2014;99(3):E518–E527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97(3):E367–E375. [DOI] [PubMed] [Google Scholar]
- 30.Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, Hermus AR. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab. 2007;92(9):3674–3680. [DOI] [PubMed] [Google Scholar]
- 31.Bercovici JP, Fiet J, Gibault L, Volant A, Abalain JH, Floch HH, Sonnet E, Fournier G. Testicular adrenal rest tumours in salt wasting congenital adrenal hyperplasia (in vivo and in vitro studies). J Steroid Biochem Mol Biol. 2005;93(1):67–72. [DOI] [PubMed] [Google Scholar]
- 32.Franco-Saenz R, Antonipillai I, Tan SY, McCorquodale M, Kropp K, Mulrow PJ. Cortisol production by testicular tumors in a patient with congenital adrenal hyperplasia (21-hydroxylase deficiency). J Clin Endocrinol Metab. 1981;53(1):85–90. [DOI] [PubMed] [Google Scholar]
- 33.Clark RV, Albertson BD, Munabi A, Cassorla F, Aguilera G, Warren DW, Sherins RJ, Loriaux DL. Steroidogenic enzyme activities, morphology, and receptor studies of a testicular adrenal rest in a patient with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1990;70(5):1408–1413. [DOI] [PubMed] [Google Scholar]
- 34.Stikkelbroeck NM, Hermus AR, Suliman HM, Jager GJ, Otten BJ. Asymptomatic testicular adrenal rest tumours in adolescent and adult males with congenital adrenal hyperplasia: basal and follow-up investigation after 2.6 years. J Pediatr Endocrinol Metab. 2004;17(4):645–653. [DOI] [PubMed] [Google Scholar]
- 35.Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Hermus AR. Repeated successful induction of fertility after replacing hydrocortisone with dexamethasone in a patient with congenital adrenal hyperplasia and testicular adrenal rest tumors. Fertil Steril. 2007;88(3):705e5–8. [DOI] [PubMed] [Google Scholar]
- 36.Hamwi GJ, Gwinup G, Mostow JH, Besch PK. Activation of testicular adrenal rest tissue by prolonged excessive ACTH production. J Clin Endocrinol Metab. 1963;23:861–869. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera MS, Vogiatzi MG, New MI. Long term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(7):3070–3078. [DOI] [PubMed] [Google Scholar]
- 38.Walker BR, Skoog SJ, Winslow BH, Canning DA, Tank ES. Testis sparing surgery for steroid unresponsive testicular tumors of the adrenogenital syndrome. J Urol. 1997;157(4):1460–1463. [PubMed] [Google Scholar]
- 39.Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MM, Sweep FC, Hermus AR. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):209–220. [DOI] [PubMed] [Google Scholar]
- 40.Smeets EE, Span PN, van Herwaarden AE, Wevers RA, Hermus AR, Sweep FC, Claahsen-van der Grinten HL. Molecular characterization of testicular adrenal rest tumors in congenital adrenal hyperplasia: lesions with both adrenocortical and Leydig cell features. J Clin Endocrinol Metab. 2015;100(3):E524–E530. [DOI] [PubMed] [Google Scholar]
- 41.Knudsen JL, Savage A, Mobb GE. The testicular ‘tumour’ of adrenogenital syndrome--a persistent diagnostic pitfall. Histopathology. 1991;19(5):468–470. [DOI] [PubMed] [Google Scholar]
- 42.Reisch N, Rottenkolber M, Greifenstein A, Krone N, Schmidt H, Reincke M, Schwarz HP, Beuschlein F. Testicular adrenal rest tumors develop independently of long-term disease control: a longitudinal analysis of 50 adult men with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2013;98(11):E1820–E1826. [DOI] [PubMed] [Google Scholar]
- 43.Shanklin DR, Richardson AP Jr, Rothstein G. Testicular hilar nodules in adrenogenital syndrome. The nature of the nodules. Am J Dis Child. 1963;106:243–250. [DOI] [PubMed] [Google Scholar]
- 44.Bouman A, Hulsbergen-van de Kaa C, Claahsen-van der Grinten HL. Prevalence of testicular adrenal rest tissue in neonates. Horm Res Paediatr. 2011;75(2):90–93. [DOI] [PubMed] [Google Scholar]
- 45.Van der Kamp HJ, Otten BJ, Buitenweg N, De Muinck Keizer-Schrama SM, Oostdijk W, Jansen M, Delemarre-de Waal HA, Vulsma T, Wit JM. Longitudinal analysis of growth and puberty in 21-hydroxylase deficiency patients. Arch Dis Child. 2002;87(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hargitai G, Sólyom J, Battelino T, Lebl J, Pribilincová Z, Hauspie R, Kovács J, Waldhauser F, Frisch H; MEWPE-CAH Study Group . Growth patterns and final height in congenital adrenal hyperplasia due to classical 21-hydroxylase deficiency. Results of a multicenter study. Horm Res. 2001;55(4):161–171. [DOI] [PubMed] [Google Scholar]
- 47.Cordeiro GV, Silva IN, Goulart EM, Chagas AJ, Kater CE. Final height in congenital adrenal hyperplasia: the dilemma of hypercortisolism versus hyperandrogenism. Arq Bras Endocrinol Metabol. 2013;57(2):126–131. [DOI] [PubMed] [Google Scholar]
- 48.Muthusamy K, Elamin MB, Smushkin G, Murad MH, Lampropulos JF, Elamin KB, Abu Elnour NO, Gallegos-Orozco JF, Fatourechi MM, Agrwal N, Lane MA, Albuquerque FN, Erwin PJ, Montori VM. Clinical review: Adult height in patients with congenital adrenal hyperplasia: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2010;95(9):4161–4172. [DOI] [PubMed] [Google Scholar]
- 49.Imamichi Y, Yuhki KI, Orisaka M, Kitano T, Mukai K, Ushikubi F, Taniguchi T, Umezawa A, Miyamoto K, Yazawa T. 11-ketotestosterone is a major androgen produced in human gonads. J Clin Endocrinol Metab. 2016;101(10):3582–3591. [DOI] [PubMed] [Google Scholar]
- 50.Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campana C, Rege J, Turcu A, Pezzi V, Gomez-Sanchez CE, Robins DM, et al. . Development of a novel cell based androgen screening model. J Steroid Biochem Mol Biol. 2016;156:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377(1-2):135–146. [DOI] [PubMed] [Google Scholar]