Abstract
Context:
Patients with nonalcoholic fatty liver disease have a high cardiovascular risk, but statins are rarely prescribed because of fear of hepatotoxicity.
Objective:
To prospectively assess the long-term safety of statins in patients with prediabetes/type 2 diabetes mellitus (T2DM) and nonalcoholic steatohepatitis (NASH).
Design:
Post hoc analysis of statin use during a randomized, controlled trial assessing pioglitazone vs placebo for NASH.
Patients:
A total of 101 patients (86 receiving statins) with biopsy-proven NASH and prediabetes/T2DM were followed for up to 36 months.
Interventions:
Oral glucose tolerance test and percutaneous liver biopsy (baseline, month 18, and month 36); liver magnetic resonance spectroscopy and euglycemic insulin clamp (baseline and month 18).
Main Outcome Measures:
Histologic and biochemical safety of statin use among patients with NASH.
Results:
Only 37% of patients were receiving statins at enrollment despite their high cardiovascular risk. Statin nonusers had higher plasma alanine aminotransferase levels but similar histologic severity of liver disease at baseline. In both statin users and nonusers, the same number of patients (n = 4) had a twofold or greater increase in plasma aminotransferases during follow-up. One statin nonuser was discontinued from the study because of this elevation. Values returned to normal without any active measure in all other cases. No changes on liver histology or hepatic insulin resistance were observed in patients with NASH newly started on a statin and receiving placebo during the main study.
Conclusions:
Statin therapy is safe in patients with prediabetes/T2DM and NASH. Given their high cardiovascular risk, statin therapy should be encouraged in this population.
This study prospectively assessed the histologic safety of statins in patients with prediabetes or T2DM and NASH and found that statins can safely be used in this high-risk population.
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver condition that ranges from isolated liver fat accumulation to severe inflammation and necrosis [known as nonalcoholic steatohepatitis (NASH)], in the absence of alcohol abuse and other causes of liver disease, such as viruses, autoimmunity, or drug-induced hepatitis (1, 2). It is characterized by insulin resistance and the coexistence of other components of the metabolic syndrome (MetS), such as obesity, prediabetes or type 2 diabetes (T2DM), hypertriglyceridemia with increased apolipoprotein B secretion, low high-density lipoprotein cholesterol (HDL-C), and small, dense low-density lipoproteins (LDLs) (3, 4).
Patients with NAFLD are at increased risk of cardiovascular disease (5). Many mechanisms have been postulated to explain this association (i.e., more severe insulin resistance, subclinical inflammation, ectopic fat accumulation with potential cardiolipotoxicity, and worse dyslipidemia) (6, 7). Because of their highly atherogenic profile, patients with NAFLD are excellent candidates for the use of statins (8).
Unfortunately, concerns remain regarding their safety in patients with NASH, especially in those with elevated plasma aminotransferases. There is a belief among clinicians that patients with NASH are at a higher risk for hepatotoxicity when prescribed a statin (9), and it has even been postulated that statins could increase hepatic fat accumulation in patients with NAFLD (10). This perception has had serious clinical implications because many patients with NAFLD are denied statins and other lipid-lowering therapies on a daily basis (11). For instance, a previous report found that only 9% of 638 patients with NAFLD were treated with a statin, despite most of them having dyslipidemia (12). The use of statins was also significantly low (45%) in a cohort of 346 patients with T2DM and NAFLD (13).
Some studies have suggested that statins may be safe in patients with NAFLD, but these studies have had serious limitations imposed by their cross-sectional (12, 14) or retrospective (15–17) nature. More important, most lacked a histologic diagnosis of NASH (12, 15, 16, 18–22), as recently reviewed elsewhere (23). The few prospective studies in patients with NASH have been short (≤12 months) and included only 5 to 43 patients (24–28). Overall, results for plasma aminotransferases, hepatic steatosis, and histology were rather inconsistent, with some of these studies even showing mild beneficial effects of statins in NASH (29).
The aim of the current study was to perform a long-term prospective evaluation of the safety of statins in patients with prediabetes or T2DM and biopsy-proven NASH.
Methods
Patients
A total of 101 patients were recruited from the general population of San Antonio, Texas, between December 2008 and 2014, as part of a randomized controlled trial (RCT) assessing the long-term efficacy of pioglitazone in patients with NASH. Complete inclusion/exclusion criteria and results from the main study have been previously reported (30). In summary, the study included patients aged 18 to 70 years with prediabetes or T2DM and biopsy-proven NASH. They were identified from responses to local newspaper advertisements and were diagnosed with NAFLD during a screening with proton magnetic resonance spectroscopy (1H-MRS) or from referrals from endocrinology and hepatology clinics. Participants were in good general health without evidence of any significant chronic disease, as determined by history, physical examination, routine blood and urine chemistries, and electrocardiography. Patients with established cardiovascular disease were not excluded from the trial as long as they were stable for at least 6 months before screening. Volunteers were excluded if they had a history of alcohol abuse (≥30 g/d in men or ≥20 g/d in women); liver disease other than NASH (i.e., hepatitis B or C, autoimmune hepatitis, hemochromatosis, Wilson disease, drug-induced hepatitis); type 1 diabetes; or a history of clinically significant renal disease, pulmonary disease, or congestive heart failure (New York Heart Association classification >II). The study was approved by the University of Texas Health Science Center at San Antonio institutional review board, and each patient provided written informed consent before participation.
Study design
After enrollment, there was a run-in phase of ∼4 weeks, in which baseline metabolic studies were performed (see below). During this phase, patients not taking a statin were prescribed therapy if indicated (31, 32). In patients already taking a statin, the dosage was titrated as needed to achieve LDL-cholesterol (LDL-C) targets, in accordance with prevailing guidelines at the time (31).
Baseline metabolic measurements included (1) fasting plasma glucose, hemoglobin A1c, lipid profile, plasma aminotransferase levels, insulin, and free fatty acids; (2) total body fat by dual-energy x-ray absorptiometry; (3) liver fat content by 1H-MRS; (4) euglycemic hyperinsulinemic clamp with 3-[3H] glucose for the measurement of glucose turnover; (5) 75-g oral glucose tolerance test to diagnose normal glucose tolerance or T2DM according to current criteria (32); and (6) liver biopsy to diagnose NASH and determine the grade and stage of the disease.
After the preceding baseline metabolic measurements were performed, patients were prescribed a hypocaloric diet (500-kcal/d deficit from the calculated weight-maintaining diet) and followed every 1 to 2 months at the clinical research center by research staff. During follow-up visits, vital signs, physical examination, home glucose monitoring results (if individuals had diabetes), adverse events, medication adherence, and blood chemistries were assessed. On the basis of guidelines criteria, patients who were not started on statins during the run-in phase were prescribed a statin when required (or, on occasion, the dose titrated) during follow-up as needed to reach lipid treatment goals. In patients who completed 18 and 36 months of follow-up, we repeated the metabolic studies and liver biopsy as part of the trial evaluating the use of pioglitazone vs placebo in patients with NASH (30). Liver 1H-MRS and euglycemic hyperinsulinemic clamp were performed only at baseline and month 18. To assess the safety of statins regarding liver function, we measured plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) at enrollment, at baseline, and at every follow-up visit.
Measurements of total body and liver fat content
Total body fat content was measured by dual-energy x-ray absorptiometry (Hologic Inc., Waltham, MA). For the measurement of hepatic fat content, localized 1H-MRS of the liver was performed on a Siemens TIM-Trio 3.0-T magnetic resonance imaging whole-body scanner (Siemens Healthcare, Erlanger, Germany), using methods previously described (33). A liver fat content >5.5% was considered diagnostic of NAFLD (34).
Euglycemic hyperinsulinemic clamp
After an overnight fast, patients underwent a two-step euglycemic hyperinsulinemic clamp with the infusion of 3-[3H] glucose as previously described (35). A primed [25 µCi × (fasting glucose/100)] continuous (0.25 µCi/min) infusion of 3-[3H] glucose (DuPont-NEN, Boston, MA) was initiated and continued until the end of the study. After the basal equilibration period, insulin was administered as a primed continuous infusion at 10 mIU/(m2 × minute) for 120 minutes to assess suppression of endogenous glucose production, followed by another 2 hours at an infusion rate of 80 mIU/(m2 × minute) to assess skeletal muscle insulin-stimulated glucose disposal. A variable infusion of 20% glucose was adjusted according to the negative feedback principle to maintain the plasma glucose concentration at approximately 90 to 100 mg/dL with a coefficient of variation <5%.
Liver biopsy
An ultrasonography-guided liver biopsy was performed in patients with elevated liver aminotransferase levels when all other causes of liver disease were ruled out, or in patients with normal liver aminotransferase levels if they were diagnosed with NAFLD by 1H-MRS and had significant risk factors for the development of NASH, such as T2DM, MetS, and/or insulin resistance. Biopsy specimens were evaluated by a pathologist who was unaware of the patients' identity or clinical information. Histologic characteristics for the diagnosis of NASH were determined using standard criteria (36).
Statistical analysis
Data were summarized in percentages for categorical variables and as mean ± standard error of the mean for numeric variables, except for plasma triglyceride concentrations, which were expressed as median (interquartile range). Comparisons between two groups were performed with χ2 or Fisher exact test for categorical variables and Kruskal-Wallis or Student t test for numeric variables depending on their distribution. Changes in continuous variables within groups (before vs after) were tested by means of Wilcoxon signed-rank test or paired t test depending on the variables’ distribution. A two-tailed P value <0.05 was considered to indicate statistical significance. Analyses were performed with Stata software, version 11.1 (Stata Corp. LP, College Station, TX).
Results
Patient characteristics at enrollment
We enrolled and followed 101 patients with biopsy-proven NASH. Table 1 summarizes patient characteristics according to whether they were receiving or not receiving a statin at enrollment. Body mass index (BMI), total body fat, and presence of the MetS did not significantly differ between statin users and nonusers. As expected, patients receiving statins were slightly older (55 ± 1 years vs 48 ± 1 years; P < 0.001) and had a trend toward higher prevalence of T2DM (63% vs 44%; P = 0.07). We also observed a strong trend toward a lower percentage of women among patients already receiving statin therapy at enrollment (18% vs 37%; P = 0.05). Although patients receiving statins had lower plasma levels of total cholesterol (166 ± 6 vs 196 ± 6 mg/dL; P < 0.001) and LDL-C (93 ± 5 mg/dL vs 119 ± 5 mg/dL; P < 0.001), we found no differences regarding plasma triglyceride or HDL-C concentration between the groups.
Table 1.
Patients’ Clinical Characteristics at Enrollment
| Characteristic | Not Receiving Statins at Enrollment (n = 63) | Receiving Statins at Enrollment (n = 38) | P Value |
|---|---|---|---|
| Age, y | 48 ± 1 | 55 ± 1 | <0.001 |
| Men, % | 63 | 82 | 0.05 |
| Body mass index, kg/m2 | 34.5 ± 0.5 | 34.3 ± 0.9 | 0.82 |
| Total body fat, % | 34 ± 1 | 33 ± 1 | 0.66 |
| Liver fat, % | 19 ± 1 | 14 ± 1 | 0.02 |
| MetS, % | 89 | 89 | 0.93 |
| T2DM, % | 44 | 63 | 0.07 |
| Total cholesterol, mg/dL | 196 ± 6 | 166 ± 6 | <0.001 |
| LDL-C, mg/dL | 119 ± 5 | 93 ± 5 | <0.001 |
| Triglycerides, mg/dL | 166 (133–224) | 152 (82–227) | 0.23 |
| HDL-C, mg/dL | 36 ± 1 | 38 ± 2 | 0.23 |
| Receiving other lipid-lowering drug, % | 8 | 18 | 0.13 |
| ALT, IU/mL | 75 ± 5 | 57 ± 5 | 0.03 |
| AST, IU/mL | 53 ± 4 | 43 ± 3 | 0.09 |
| Fasting plasma glucose, mg/dL | 122 ± 4 | 127 ± 5 | 0.44 |
| Hemoglobin A1c, % | 6.2 ± 0.1 | 6.5 ± 0.2 | 0.11 |
| Systolic blood pressure, mmHg | 133 ± 2 | 131 ± 2 | 0.45 |
| Diastolic blood pressure, mmHg | 77 ± 1 | 75 ± 2 | 0.19 |
| Hypertension, % | 71 | 87 | 0.07 |
| NAFLD activity score | 4.4 ± 0.2 | 4.6 ± 0.2 | 0.49 |
| Steatosis grade | 2.0 ± 0.1 | 2.0 ± 0.1 | 0.85 |
| Inflammation grade | 1.6 ± 0.1 | 1.8 ± 0.1 | 0.12 |
| Ballooning grade | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.86 |
| Fibrosis stage | 0.9 ± 0.1 | 1.3 ± 0.2 | 0.05 |
Numeric variables are expressed as mean ± standard error of the mean, except for triglycerides, which are expressed as median (interquantile range). Qualitative data are expressed as percentages. P values were calculated with χ2 or Fisher exact test for categorical variables and t test or Kruskal-Wallis test for numeric variables depending on their distribution.
Patients receiving a statin had lower levels of plasma ALT (57 ± 5 IU/L vs 75 ± 5 IU/L; P = 0.03), and this was associated with lower liver fat content by 1H-MRS (14±1% vs 19±1%; P = 0.02). However, histologic severity of NASH did not significantly differ between statin users and nonusers in steatosis, inflammation, ballooning, or fibrosis (Table 1).
Only 37% of patients with NASH at enrollment were receiving statins and 12% were receiving other lipid-lowering drugs [fenofibrate (n = 2), gemfibrozil (n = 7), niacin (n = 2), and fish oil (n = 1)] despite the overall high cardiovascular risk of this population. Most patients not prescribed a statin had a clear indication based on the guidelines at the time (31, 32), given the high prevalence of obesity (81%), T2DM (51%), MetS (89%), and dyslipidemia (59% with plasma triglyceride levels > 150 mg/dL, 55% with LDL-C levels > 100 mg/dL, and 74% with low HDL-C levels). The statin most frequently used at enrollment was simvastatin (84% of statin users), followed by rosuvastatin (11%), influenced largely by the local health care plan formulary guidelines at the time of the study. Statins doses were classified as low (≤10 mg of simvastatin, 20 mg of lovastatin, or 10 to 20 mg of pravastatin), medium (20 to 40 mg of simvastatin, 40 to 80 mg of pravastatin, 10 to 20 mg of atorvastatin, or 5 to 10 mg of rosuvastatin), and high (40 to 80 mg of atorvastatin or 20 to 40 mg of rosuvastatin) according to recent guidelines (37). Only 8% of the patients were receiving high-intensity statin therapy at enrollment, whereas the majority (79%) of patients were receiving moderate-intensity therapy. Overall, patients receiving statins at enrollment were undertreated, with high percentages of patients out of lipid targets according to current guidelines (51% with triglyceride levels > 150 mg/dL, 35% with LDL-C levels >100 mg/dL, and 65% with low HDL-C levels).
Short-term safety of statin therapy (before and after run-in phase)
At the beginning of the run-in phase (average of ∼4 weeks), patients had upward titration of statin dose or were started on a statin according to prevailing guidelines at the time (31, 32). Patients not prescribed a statin at this time had a contraindication for these drugs or were unwilling to start them.
Among patients who were newly prescribed a statin during the run-in phase, most were started cautiously on a low or medium dose (45% and 48%, respectively). As expected, statin initiation led to a significant reduction in plasma total cholesterol (P < 0.002) and LDL-C (P = 0.003). No deleterious effects on plasma aminotransferase concentration or the proportion of patients with elevated plasma aminotransferases were observed after statin initiation. None of the patients newly started on a statin experienced any significant increase in plasma ALT or AST levels (defined as a twofold elevation) during the run-in phase. Indeed, patients newly prescribed a statin and those already taking a statin before enrollment showed a slight reduction of plasma ALT during this phase (from 64 ± 4 IU/L to 56 ± 3 IU/L; P = 0.007). This was associated with a small reduction in BMI (from 34.4 ± 0.5 kg/m2 to 34.1 ± 0.5 kg/m2; P = 0.007). During this phase, 13 patients with plasma ALT or AST levels >80 IU/L were prescribed a statin despite their significant elevation of plasma aminotransferase concentration. Plasma aminotransferase levels did not increase further among this group of patients. In fact, mean plasma ALT and AST significantly decreased during the run-in phase after statin initiation in this group of patients [from 109 ± 6 IU/L to 83 ± 5 IU/L (P = 0.001) for plasma ALT and from 66 ± 5 IU/L to 53 ± 5 IU/L (P = 0.01) for plasma AST].
Among patients who were already receiving a statin at enrollment, 28% had an upward titration of their dose during the run-in period, resulting in an increase in the number of patients in the medium- and high-dose group (from 28% to 33% and from 28% to 36%, respectively). Upward titration of statin dose during this phase led to a further decrease in plasma total cholesterol (189 ± 12 mg/dL vs 145 ± 9 mg/dL; P = 0.01) and LDL-C (114 ± 15 mg/dL vs 68 ± 7 mg/dL; P = 0.004) levels and a trend toward lower median plasma triglyceride [190 (126 to 299) mg/dL vs 126 (111 to 154) mg/dL, P = 0.06] concentrations. Overall, mean plasma ALT and AST levels did not change in this group during this period or later. Regarding the hepatic safety of statin titration, only one patient in whom the statin was increased from low to medium dose had a twofold increase in plasma ALT concentration that spontaneously returned to baseline at the next follow-up visit without any active measure. Two patients not receiving statins during the run-in phase also showed an elevation in plasma aminotransferase levels that returned to normal spontaneously.
Follow-up
A total of 86 patients receiving statins and 15 not receiving statins with biopsy-proven NASH were followed as part of a pioglitazone vs placebo randomized clinical trial (30) for a total of 194 patient-years of statin therapy (range, 0.1 to 3 years). Eighteen of the 101 patients were prematurely discontinued from the study after a mean follow-up of 7 months (range, 1 to 16 months) because of loss to follow-up or other causes unrelated to statin use. Most withdrawals were due to safety concerns with pioglitazone and bladder cancer at the time of a report suggesting such an association, as detailed elsewhere (30). We found no significant differences in clinical and laboratory measures between patients discontinued and the rest of the participants. Of these patients, 12 were receiving statins at the time of disenrollment and the remaining 6 were never prescribed a statin. Only 1 patient discontinued statins during follow-up against medical advice. No drug-related adverse events were identified in this patient.
After all patients were started on statins, 67% of them were taking simvastatin, 21% were taking rosuvastatin, and the remaining 12% were equally divided between atorvastatin and pravastatin. At the end of the study, only 30% of patients were receiving a low-dose statin, whereas the rest were receiving medium- or high-dose therapy (46% and 24%, respectively). Approximately 21% of patients receiving a statin were taking combination therapy at the end of the study [fenofibrate (n = 4), gemfibrozil (n = 11), niacin (n = 2), and fish oil (n = 1)].
Long-term safety
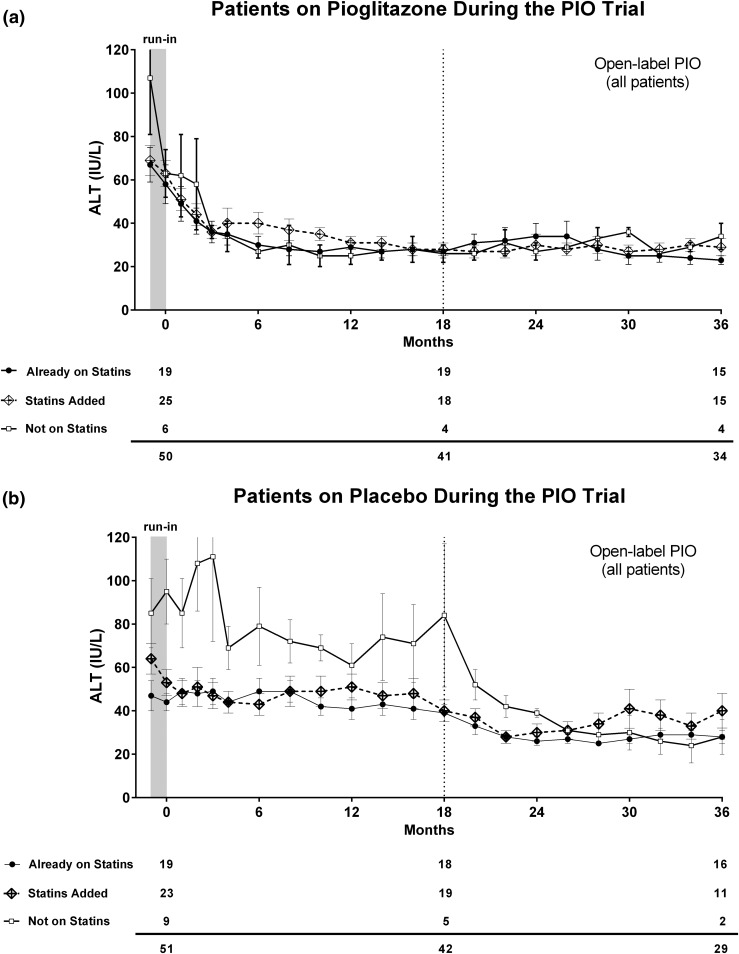
As can be observed in Fig 1(a), plasma ALT levels tended to decrease over time in all patients receiving pioglitazone, whether they were taking statin therapy or not, likely because of the effects of the thiazolidinedione on liver histology (30). Before pioglitazone initiation, plasma ALT reductions were already observed but were similar for all groups, as plotted in Fig. 1(a). Mean plasma aminotransferase levels did not significantly increase during the 3-year follow-up, either in patients who were started on or were already receiving a statin at study enrollment.
Figure 1.
Plasma ALT levels over time in patients not taking a statin (white squares), those receiving statins from enrollment (black dots), and those newly started on a statin during the study (dotted line). Patients were divided according to their original randomization to (a) pioglitazone or (b) placebo. PIO, pioglitazone.
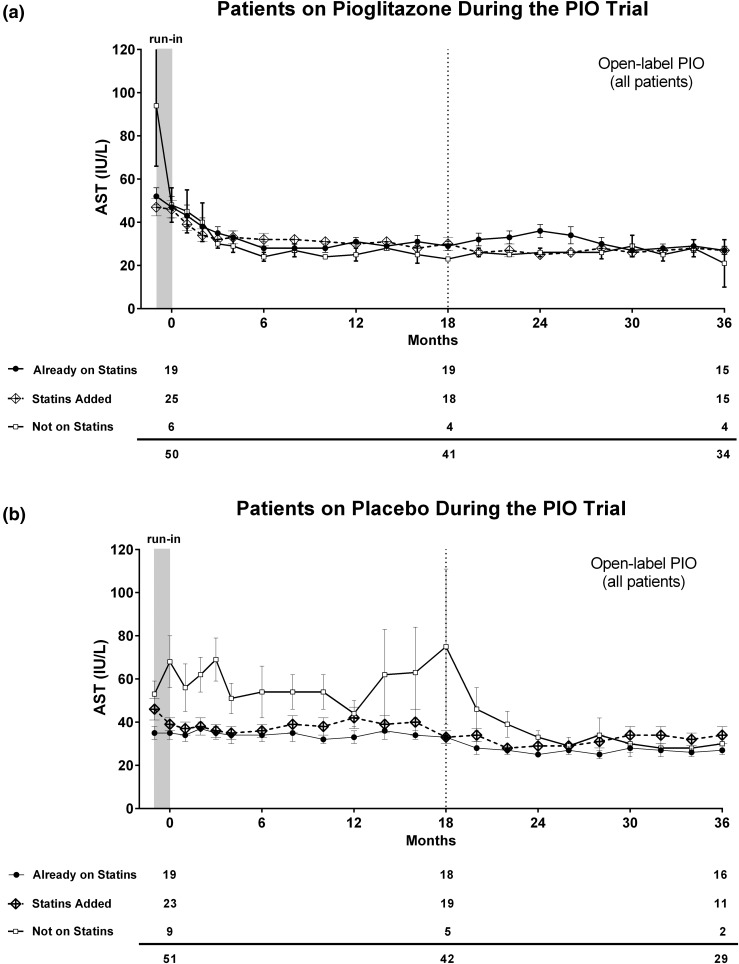
In patients randomly assigned to placebo [Fig. 1(b)], mean plasma ALT levels remained abnormally elevated (>40 IU/L) throughout the first 18 months. Of note, patients not receiving a statin showed persistently higher levels of plasma ALT compared with patients taking a statin. The difference in plasma ALT levels between patients not receiving statins and those who started statin treatment occurred early (it reached statistical significance by the end of the run-in phase; P = 0.002) and persisted for the first 18 months of follow-up. This reduction in plasma ALT during the run-in in patients starting statins was associated with weight loss as mentioned above [Fig. 1(b)]. All groups normalized their mean plasma ALT levels after pioglitazone initiation in the open-label phase. Those patients who had started a statin during the study showed a nonsignificant increase in plasma ALT at month 24 that decreased spontaneously at months 32 and 34. An overall similar pattern was observed when plasma AST levels were plotted over time (Fig. 2).
Figure 2.
Plasma AST levels over time in patients not taking a statin (white squares), those receiving statins from enrollment (black dots), and those newly started on a statin during the study (dotted line). Patients were divided according to their original randomization to (a) pioglitazone or (b) placebo. PIO, pioglitazone.
For further analyses, we took into account patients who showed at least a doubling of ALT/AST levels during follow-up. Overall, five patients showed at least a twofold increase in liver enzymes levels after the run-in phase (including the above-mentioned patient who was discontinued for this reason). Two of these five patients were not receiving a statin when their plasma aminotransferase levels increased. Of the other three, two were already taking a statin at enrollment, and only one was newly started on the medication. In all cases, liver aminotransferases returned to normal without a change in the statin dosing. Regarding muscular adverse events, 11 patients reported muscle cramps or muscle aches. None of these patients had an elevation in plasma creatine phosphokinase, and symptoms spontaneously resolved without any treatment.
Long-term efficacy
To assess possible long-term beneficial effects of statins in patients with NASH, we compared clinical and laboratory measures of patients who started a statin during the study and had not been randomly assigned to active medication (pioglitazone) during the trial (Table 2).
Table 2.
Clinical, Metabolic, and Liver Histologic Profile Before and After 18 Months in Patients for Whom Statin Therapy Was Added During Follow-Up but Who Were Receiving Only Placebo During the Trial
| Variable |
Patients Newly Started on a Statin (n = 19) |
P Value | |
|---|---|---|---|
| At Enrollment | After 18 Months | ||
| BMI, kg/m2 | 35.1 ± 0.9 | 34.6 ± 0.9 | 0.06 |
| Liver fat by 1H-MRS, % | 13 ± 2 | 8 ± 2 | <0.001 |
| ALT, IU/L | 66 ± 8 | 38 ± 5 | <0.001 |
| AST, IU/L | 48 ± 6 | 31 ± 3 | 0.006 |
| Patients with ALT > 40 IU/L, % | 67 | 39 | 0.10 |
| Hemoglobin A1c, % | 6.2 ± 0.2 | 6.2 ± 0.2 | 0.86 |
| Total cholesterol, mg/dL | 201 ± 11 | 148 ± 10 | <0.001 |
| Triglycerides, mg/dL | 166 (132–210) | 141 (94–189) | 0.04 |
| HDL-C, mg/dL | 37 ± 2 | 41 ± 2 | 0.01 |
| LDL-C, mg/dL | 127 ± 8 | 77 ± 6 | <0.001 |
| Muscle insulin sensitivity (Rd, mg/kg LBM per min) | 5.8 ± 0.7 | 5.5 ± 0.6 | 0.45 |
| Suppression of EGP, % | 43 ± 5 | 37 ± 5 | 0.34 |
| Suppression of FFA, % | 41 ± 5 | 49 ± 4 | 0.02 |
| NAFLD activity score | 3.9 ± 0.3 | 3.7 ± 0.5 | 0.40 |
| Steatosis grade | 1.7 ± 0.2 | 1.4 ± 0.2 | 0.10 |
| Inflammation grade | 1.5 ± 0.1 | 1.6 ± 0.2 | 0.50 |
| Ballooning grade | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.43 |
| Fibrosis stage | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.50 |
Numeric variables are expressed as mean ± standard error of the mean, except for triglycerides, which are expressed as median (interquantile range). Qualitative data are expressed as percentages. P values were calculated with χ2 or Fisher exact test for categorical variables and paired t test or Wilcoxon matched-pairs signed-rank test depending on the variable’s distribution for continuous variables. EGP, endogenous glucose production; FFA, free fatty acid; LBM, lean body mass; Rd, insulin-stimulated glucose disposal.
As expected, after 18 months of statin use, all lipid measures improved. Both plasma ALT (66 ± 8 IU/L vs 38 ± 5 IU/L; P < 0.001) and AST (48 ± 6 IU/L vs 31 ± 3 IU/L; P = 0.006) levels improved significantly after 18 months of statin therapy. This remained true when we analyzed the proportion of patients with abnormal plasma ALT concentration (ALT > 40 IU/L) before and after 18 months of follow-up. In agreement with this biochemical improvement, liver fat by 1H-MRS also decreased after 18 months (13% ± 2% vs 8% ± 2%; P < 0.001). These improvements were strongly associated with changes in BMI [35.1 ± 0.9 kg/m2 vs 34.6 ± 0.9 kg/m2 (P = 0.06), corresponding to a reduction of 1.5 kg] and a small but significant improvement in the suppression of free fatty acids by low-dose insulin during the euglycemic insulin clamp (41% ± 5% vs 49% ± 4%; P = 0.02). Liver and skeletal muscle insulin resistance did not change after 18 months of statin therapy. None of the above changes in plasma ALT or liver fat by 1H-MRS translated into any histologic improvement in the NAFLD activity score (3.9 ± 0.3 vs 3.7 ± 0.5; P = 0.40) or any of the individual components of the score: steatosis (1.7 ± 0.2 vs 1.4 ± 0.2; P = 0.10), inflammation (1.5 ± 0.1 vs 1.6 ± 0.2; P = 0.50), and ballooning (0.8 ± 0.1 vs 0.7 ± 0.1; P = 0.43). There were also no differences in fibrosis stage (0.6 ± 0.2 vs 0.5 ± 0.2; P = 0.50). Of note, we observed no significant metabolic or histologic changes among patients already receiving a statin at enrollment who continued statin therapy for 18 months (Table 3).
Table 3.
Clinical, Metabolic, and Liver Histologic Profile Before and After 18 Months in Patients Already Receiving Statin Therapy at Enrollment and Only Receiving Placebo During the Trial
| Variable |
Patients Already Receiving a Statin at Enrollment (n = 18) |
P Value | |
|---|---|---|---|
| At Enrollment | After 18 Months | ||
| BMI, kg/m2 | 35.1 ± 1.5 | 34.9 ± 1.4 | 0.77 |
| Liver fat by 1H-MRS, % | 14 ± 3 | 12 ± 2 | 0.40 |
| ALT, IU/L | 46 ± 7 | 39 ± 4 | 0.22 |
| AST, IU/L | 33 ± 2 | 32 ± 3 | 0.91 |
| Patients with ALT > 40 IU/L, % | 50 | 39 | 0.50 |
| Hemoglobin A1c, % | 6.7 ± 0.3 | 6.4 ± 0.2 | 0.05 |
| Total cholesterol, mg/dL | 155 ± 7 | 148 ± 9 | 0.55 |
| Triglycerides, mg/dL | 137 (79–196) | 137 (88–198) | 0.96 |
| HDL-C, mg/dL | 39 ± 2 | 40 ± 2 | 0.47 |
| LDL-C, mg/dL | 84 ± 5 | 79 ± 8 | 0.59 |
| Muscle insulin sensitivity (Rd, mg/kg LBM per min) | 4.4 ± 0.3 | 5.1 ± 0.5 | 0.15 |
| Suppression of EGP, % | 42 ± 5 | 39 ± 6 | 0.49 |
| Suppression of FFA, % | 39 ± 6 | 42 ± 7 | 0.61 |
| NAFLD activity score | 4.8 ± 0.2 | 4.3 ± 0.3 | 0.16 |
| Steatosis grade | 2.0 ± 0.2 | 2.0 ± 0.2 | 0.99 |
| Inflammation grade | 1.9 ± 0.1 | 1.6 ± 0.1 | 0.06 |
| Ballooning grade | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.27 |
| Fibrosis stage | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.85 |
Numeric variables are expressed as mean ± standard error of the mean, except for triglycerides, which are expressed as median (interquantile range). Qualitative data are expressed as percentages. P values were calculated with χ2 or Fisher exact test for categorical variables and paired t test or Wilcoxon matched-pairs signed-rank test depending on the variable’s distribution for continuous variables. EGP, endogenous glucose production; FFA, free fatty acid; LBM, lean body mass; Rd, insulin-stimulated glucose disposal.
Discussion
Health care providers commonly face the clinical dilemma of elevated plasma aminotransferases in an obese patient with the MetS and/or T2DM. Moreover, this situation is likely to increase in parallel with the worsening of the epidemics of obesity and T2DM. Clinicians are confronted with prescribing a statin to a patient with high cardiovascular risk vs withholding such treatment in the face of a perceived risk for harm in the setting of NASH. Few studies have served as guidance, and most of these suffered from many limitations: retrospective nature, small sample size (5 to 43 patients), short follow-up (mostly 6 to 12 months), and/or use of surrogate markers of liver disease (AST/ALT or liver imaging) rather than the gold standard, liver biopsy (12, 15–22, 24–28).
The current report is the largest prospective study assessing the safety of statins in patients with biopsy-proven NASH, with a cohort of 101 patients closely followed for a total follow-up of 194 patient-years. Our results offer compelling evidence that statins can be safely used in patients with NASH and should not be denied to these patients, who already have a very high risk for cardiovascular disease.
At enrollment, we found that only 37% of patients were receiving a statin, with an additional 12% receiving other lipid-lowering therapies. This very low use of statins among patients with NASH is in accordance with prior studies (12). Of note, this low statin use occurred despite a high prevalence of obesity (81%), T2DM (51%), and MetS (89%). For instance, most patients had dyslipidemia; 55% had a plasma LDL-C level > 100 mg/dL, 59% had a plasma triglyceride level > 150 mg/dL, and 74% had an HDL-C level <40 mg/dL (men) or <50 mg/dL (women). There are several potential explanations for this. On the one hand, patient adherence to lipid-lowering therapy remains a challenge (38). It is also well established that primary care providers may not be aggressive enough in starting or upwardly titrating statin therapy to meet established treatment goals (39). Female patients appear to be at particular risk for statin therapy undertreatment, despite compelling evidence suggesting a similar benefit with statin therapy in both sexes (40, 41).
However, the most likely cause (expressed by many referring primary care physicians) was the concern over safety of prescribing lipid-lowering agents in the face of elevated liver enzymes or knowledge that the patient had NASH. Supporting this view, there was an inverse correlation between the magnitude of the liver aminotransferase elevation in patients with NASH and the use of statins: Whereas 36% of patients were receiving statins at enrollment, this percentage declined to 33% and 21% when patients with plasma ALT > 40 U/L or > 80 U/L were respectively considered. Despite higher plasma aminotransferases and liver fat content by 1H-MRS in statin nonusers, both statin users and nonusers had similar severity of liver disease at baseline based on histology. This indicates that elevated plasma aminotransferases and imaging (even 1H-MRS) poorly reflect liver disease in NASH and offer inadequate information with which to make clinical decisions. This discordance between aminotransferases and histology has been reported before in clinical trials (42–44). The National Lipid Association Statin Safety Assessment Task Force clearly stated that patients with NAFLD or NASH may safely receive statin therapy, recommending their use, but there is still strong resistance to do so in clinical practice (45). In the current study, 75% of patients not taking a statin at enrollment were safely prescribed one. Because of this, our results are important in creating a paradigm shift for the use of statins in patients with NASH.
Most patients receiving a statin at study entry were not at goal (Table 1). About a third had a plasma LDL-C level >100 mg/dL, >50% had an elevated plasma triglyceride concentration, and as many as two thirds a low HDL-C level. Indeed, in 75% the plasma non–HDL-C level was >100 mg/dl. Again, lack of upward statin titration was related to the fear of liver toxicity in patients with elevated plasma aminotransferases. However, after a 3-year follow-up period, statin therapy proved to be safe. The fact that more patients were able to reach plasma LDL-C rather than the non–HDL-C target highlights the difficulties of improving plasma triglyceride and HDL-C concentration in such patients and the potential for combination therapy with fibrates in this population. Fibrates, with or without statins, have been tested for the treatment of NAFLD, with rather neutral effects on liver fat content on imaging or histology; however, in some reports plasma aminotransferases were reported to decrease (46). In this regard, no patient receiving combination therapy experienced a significant increase in creatine phosphokinase or liver enzymes. Taken together, fibrates also appear to be safe when added to statins in this population.
Finally, there has been some interest in the potential role of statins to improve liver histology in NASH and biochemical measures. In cell culture and animal models, statins may improve NASH by several potential mechanisms (10). For instance, in macrophages and monocytes, statins activate the peroxisome proliferator-activated receptor γ (47). They also activate peroxisome proliferator-activated receptor α and promote fatty acid oxidation in vivo (48). Other possible mechanisms include improvement of plasma levels of tumor necrosis factor-α, interleukin-6, and C-reactive protein, markers frequently associated with advanced histology in NASH (49). However, it is unlikely that statins have a major effect on liver histology in humans with NASH. Although several uncontrolled studies have reported reductions in plasma aminotransferases and steatosis on imaging, these reports were characterized by their small numbers of patients, concomitant use of lifestyle intervention, lack of appropriate controls, and short follow-up (8, 29). Confirming that statins do not improve liver histology in NASH, patients in the placebo group receiving statins had no significant change in hepatic steatosis, necroinflammation, or fibrosis after 18 months of follow-up.
The modest reduction in plasma aminotransferases and hepatic triglyceride content by 1H-MRS that we observed in these patients (Table 2) was likely related to weight reduction in this subset of patients [BMI, 35.1 ± 0.9 kg/m2 vs 34.6 ± 0.9 kg/m2 (P = 0.06), corresponding to a reduction of 1.5 kg in body weight]. Moreover, the ALT/AST changes we report are similar to those in the placebo groups of other RCTs in patients with NASH. For instance, in our prior RCT of pioglitazone vs placebo in patients with NASH (43), plasma ALT and AST also improved in the placebo group from 61 to 40 IU/L (P = 0.03) and AST from 42 to 33 IU/L (P = 0.08). Reductions of plasma ALT/AST of similar magnitude have been reported for the placebo groups of the PIVENS (42) and FLINT (50) trials in patients with NASH. These findings suggest that a small decrease in plasma aminotransferases is to be expected in placebo-treated patients and highlight the need for adequate controls in intervention studies in patients with NASH. Although we recognize that this study is a post hoc analysis of a randomized, single-center study with a relatively small sample size, the difficulty of performing paired percutaneous liver biopsies makes this current work the largest prospective study assessing the histologic safety of statins in patients with NASH. Statin adherence was not formally assessed during follow-up, but study drug adherence was overall good (95% by pill counting), suggesting a similarly high rate of adherence for statin use.
In summary, in the largest prospective study to date in patients with biopsy-proven NASH, we report that statins can be safely prescribed in this population. These are timely findings that call for a change in current practice as patients with NASH have the highest cardiovascular risk but dyslipidemia remains often undertreated. We hope that this work will increase awareness about the need for and safety of statin use in patients with NASH and that in the future they will not be deprived from this much-needed therapy. However, more work is needed to fully understand the role of statins in this setting.
Acknowledgments
This work was supported by the Burroughs Wellcome Fund (K.C.), by the American Diabetes Association (1-08-CR-08, K.C.), and by US Department of Veterans Affairs Merit Review Award 1 I01 CX000167-01. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Disclosure Summary: The authors have nothing to disclose.
Author contributions: F.B. contributed to patient recruitment and follow-up, data collection, statistical analysis, and manuscript writing and takes responsibility for the integrity of the data and the accuracy of the data analysis; P.P.S., R.L., B.O., and J.H. contributed to patient recruitment and follow-up and data collection; F.T. assessed the liver biopsy findings; K.C. provided funding, contributed to patient recruitment and follow-up, data collection, and manuscript writing, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
- 1H MRS
- proton magnetic resonance spectroscopy
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- BMI
- body mass index
- HDL-C
- high-density lipoprotein cholesterol
- LDL-C
- low-density lipoprotein cholesterol
- MetS
- metabolic syndrome
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- RCT
- randomized controlled trial
- T2DM
- type 2 diabetes mellitus.
References
- 1.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49(1):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Targher G, Bellis A, Fornengo P, Ciaravella F, Pichiri I, Cavallo Perin P, Trimarco B, Marchesini G. Prevention and treatment of nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(5):331–340. [DOI] [PubMed] [Google Scholar]
- 3.Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490–3497. [DOI] [PubMed] [Google Scholar]
- 4.Bril F, Sninsky JJ, Baca AM, Superko HR, Portillo Sanchez P, Biernacki D, Maximos M, Lomonaco R, Orsak B, Suman A, Weber MH, McPhaul MJ, Cusi K. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab. 2016;101(2):644–652. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30(8):2119–2121. [DOI] [PubMed] [Google Scholar]
- 6.Perseghin G. The role of non-alcoholic fatty liver disease in cardiovascular disease. Dig Dis. 2010;28(1):210–213. [DOI] [PubMed] [Google Scholar]
- 7.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725 e716. [DOI] [PubMed] [Google Scholar]
- 8.Bril F, Lomonaco R, Cusi K. The challenge of managing dyslipidemia in patients with nonalcoholic fatty liver disease. Clin Lipidol. 2012;7:471–481. [Google Scholar]
- 9.Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4):690–695. [DOI] [PubMed] [Google Scholar]
- 10.Argo CK, Loria P, Caldwell SH, Lonardo A. Statins in liver disease: a molehill, an iceberg, or neither? Hepatology. 2008;48(2):662–669. [DOI] [PubMed] [Google Scholar]
- 11.Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F. Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Dig Dis Sci. 2016;61(6):1714–1720. [DOI] [PubMed] [Google Scholar]
- 12.Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44(2):466–471. [DOI] [PubMed] [Google Scholar]
- 13.Nascimbeni F, Aron-Wisnewsky J, Pais R, Tordjman J, Poitou C, Charlotte F, Bedossa P, Poynard T, Clément K, Ratziu V; LIDO Study Group . Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, Maggioni M, Kakela P, Wiklund O, Mozzi E, Grimaudo S, Kaminska D, Rametta R, Craxi A, Fargion S, Nobili V, Romeo S, Pihlajamaki J, Valenti L. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705–712. [DOI] [PubMed] [Google Scholar]
- 15.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287–1292. [DOI] [PubMed] [Google Scholar]
- 16.Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329(2):62–65. [DOI] [PubMed] [Google Scholar]
- 17.Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47(1):135–141. [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Domínguez E, Gisbert JP, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23(11):1643–1647. [DOI] [PubMed] [Google Scholar]
- 19.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106(1):71–77. [DOI] [PubMed] [Google Scholar]
- 20.Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, Kakafika AI, Tziomalos K, Burroughs AK, Elisaf MS. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22(5):873–883. [DOI] [PubMed] [Google Scholar]
- 21.Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis. 2006;184(1):233–234. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators . Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453–1463. [DOI] [PubMed] [Google Scholar]
- 23.Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12(12):CD008623. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y, Hyogo H, Yamagishi S, Takeuchi M, Ishitobi T, Nabeshima Y, Arihiro K, Chayama K. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J Gastroenterol. 2010;45(7):750–757. [DOI] [PubMed] [Google Scholar]
- 25.Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):990–994. [DOI] [PubMed] [Google Scholar]
- 26.Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis. 2004;174(1):193–196. [DOI] [PubMed] [Google Scholar]
- 27.Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis. 2007;16(1):39–46. [PubMed] [Google Scholar]
- 28.Kiyici M, Gulten M, Gurel S, Nak SG, Dolar E, Savci G, Adim SB, Yerci O, Memik F. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol. 2003;17(12):713–718. [DOI] [PubMed] [Google Scholar]
- 29.Barb D, Cusi K. Reply to “statins and non-alcoholic steatohepatitis”. Metabolism. 2017;66:e3–e5. [DOI] [PubMed] [Google Scholar]
- 30.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–315. [DOI] [PubMed] [Google Scholar]
- 31.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. [DOI] [PubMed] [Google Scholar]
- 32.Standards of medical care in diabetes-2015: summary of revisions. Diabetes Care. 2015;38(Suppl):S4. [DOI] [PubMed] [Google Scholar]
- 33.Bril F, Ortiz-Lopez C, Lomonaco R, Orsak B, Freckleton M, Chintapalli K, Hardies J, Lai S, Solano F, Tio F, Cusi K. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35(9):2139–2146. [DOI] [PubMed] [Google Scholar]
- 34.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. [DOI] [PubMed] [Google Scholar]
- 35.Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, Weber MH, Budd JT, Lupi ME, Cusi K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–1144. [DOI] [PubMed] [Google Scholar]
- 36.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 38.Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev. 2010; (3):CD004371. [DOI] [PubMed] [Google Scholar]
- 39.Koren MJ. Statin use in a “real-world” clinical setting: aggressive lipid lowering compared with usual care in the Aggressive Lipid-Lowering Initiation Abates New Cardiac Events (ALLIANCE) trial. Am J Med. 2005;118(Suppl 12A):16–21. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Plutzky J, Shubina M, Turchin A. Drivers of the sex disparity in statin therapy in patients with coronary artery disease: a cohort study. PLoS One. 2016;11(5):e0155228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–1405. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN . Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307. [DOI] [PubMed] [Google Scholar]
- 44.Maximos M, Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Biernacki D, Suman A, Weber M, Cusi K. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology. 2015;61(1):153–160. [DOI] [PubMed] [Google Scholar]
- 45.Bays H, Cohen DE, Chalasani N, Harrison SA; The National Lipid Association’s Statin Safety Task Force . An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S47–S57. [DOI] [PubMed] [Google Scholar]
- 46.Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, Klein S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95(6):2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yano M, Matsumura T, Senokuchi T, Ishii N, Murata Y, Taketa K, Motoshima H, Taguchi T, Sonoda K, Kukidome D, Takuwa Y, Kawada T, Brownlee M, Nishikawa T, Araki E. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res. 2007;100(10):1442–1451. [DOI] [PubMed] [Google Scholar]
- 48.Sanguino E, Roglans N, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC. Atorvastatin reverses age-related reduction in rat hepatic PPARalpha and HNF-4. Br J Pharmacol. 2005;145(7):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40(1):46–54. [DOI] [PubMed] [Google Scholar]
- 50.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]