Abstract
Context:
The cellular basis of persistent β-cell function in type 1 diabetes (T1D) remains enigmatic. No extensive quantitative β-cell studies of T1D pancreata have been performed to test for ongoing β-cell regeneration or neogenesis.
Objective:
We sought to determine the mechanism of β-cell persistence in T1D pancreata.
Design:
We studied T1D (n = 47) and nondiabetic control (n = 59) pancreata over a wide range of ages from the Juvenile Diabetes Research Foundation Network of Pancreatic Organ Donors with Diabetes via high-throughput microscopy.
Intervention and Main Outcome Measures:
We quantified β-cell mass, β-cell turnover [via Ki-67 and terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)], islet ductal association, and insulin/glucagon coexpression in T1D and control pancreata.
Results:
Residual insulin-producing β cells were detected in some (but not all) T1D cases of varying disease duration. Several T1D pancreata had substantial numbers of β cells. Although β-cell proliferation was prominent early in life, it dramatically declined after infancy in both nondiabetic controls and T1D individuals. However, β-cell proliferation was equivalent in control and T1D pancreata. β-cell death (assessed by TUNEL) was extremely rare in control and T1D pancreata. Thus, β-cell turnover was not increased in T1D. Furthermore, we found no evidence of small islet/ductal neogenesis or α-cell to β-cell transdifferentiation in T1D pancreata, regardless of disease duration.
Conclusion:
Longstanding β-cell function in patients with T1D appears to be largely a result of β cells that persist, without any evidence of attempted β-cell regeneration, small islet/ductal neogenesis, or transdifferentiation from other islet endocrine cell types.
We imaged and analyzed control and T1D pancreata and determined that β cells persist in T1D without any evidence for compensatory proliferation, regeneration, or α-cell transdifferentiation.
Type 1 diabetes (T1D) is an autoimmune disease that leads to dramatic β-cell loss and insulin deficiency (1–6). Reports of patients with T1D with persistent β-cell function have led to various theories to explain β-cell persistence, including ongoing β-cell regeneration, surviving hyperfunctioning β cells, β-cell neogenesis, and β cells that avoid immune system detection. However, these theories have not been thoroughly tested due to a variety of challenges, including scarcity of human T1D pancreata, low basal rates of endogenous β-cell turnover, and absence of high-throughput imaging methodologies. Consequently, the cellular basis of variable β-cell function in T1D pancreata remains poorly understood. Conclusive studies to determine the histopathology of β-cell persistence and turnover are essential to understand the pathogenesis of T1D and to direct the development of therapies.
Although exogenous insulin can transiently improve β-cell function in patients with T1D, insulin production [measured by connecting peptide (C-peptide)] progressively declines thereafter (7–9). Thus, major efforts are currently underway to preserve β-cell function in newly diagnosed patients with T1D by using various immunosuppressive agents (10). Several investigators have reported that a few β cells continue to function in longstanding T1D, as measured by C-peptide (11, 12). These observations imply that some β-cell function can persist in longstanding T1D. However, the anatomical correlates to this persistent β-cell function remain poorly understood.
Pioneering histological studies by Maclean and Ogilvie (13), Gepts et al. (2), Evans (14), Löhr and Klöppel (15), Foulis et al. (16, 17), Butler et al. (4, 18), and others (6, 19, 20) established that β cells persist in T1D pancreata. β cells are readily detected in pancreata of patients with new-onset T1D during autoimmune attack and gradually disappear (1, 13). However, β cells often persist in T1D pancreata several years after diagnosis, even in rare individuals with longstanding T1D (2, 4, 6, 13–20).
The cellular basis for persistent β cells in longstanding T1D remains enigmatic. In 1985, Gepts et al. (21) noted that “a major problem in the pathology of diabetes is whether the β-cells regenerate.” More than 30 years later, the debate continues regarding the mechanism of β-cell persistence in T1D. Studies revealed that substantial β-cell proliferation could occur during autoimmune attack in rodents (22–25). Similar phenomena have been asserted in human T1D pancreata (18, 26). The various theories of β-cell persistence in T1D parallel the major theories proposed in postnatal β-cell mass expansion, including self-renewal of β cells, contribution of tissue stem cells, and transdifferentiation from other islet endocrine sources such as α cells (27, 28). However, the lack of consensus regarding the mechanism of β-cell persistence is not surprising, given the technical challenges involved in studying β cells in T1D.
We performed the first comprehensive study of human β-cell mass and turnover between nondiabetic and T1D pancreata across ages. We analyzed T1D and control pancreatic samples for β-cell mass, β-cell proliferation, β-cell death, ductal neogenesis, and α- to β-cell transdifferentiation. We found that T1D pancreata do not show any evidence of increased compensatory β-cell turnover, neogenesis, or α- to β-cell transdifferentiation. We concluded that β cells simply persist in longstanding T1D, without ongoing generation of new β cells.
Research Design and Methods
Human pancreatic samples
Anonymized, formalin-fixed, paraffin-embedded pancreas tissue sections were obtained from the Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) after acquiring a waiver from the Baylor College of Medicine Institutional Review Board. All consecutive T1D cases were selected on the basis of availability at the time of the onset of the study. Other cases were added later to fill out age cohorts. Over time, the nPOD pool grew to include 128 T1D samples. All tissues were processed by nPOD according to standardized operating procedures (http://www.jdrfnpod.org/for-investigators/standard-operating-procedures/). Paraffin-embedded tissues were fixed in 10% neutral buffered formalin for 24 hours, and for up to 40 hours for pancreata with high fat content. Hematoxylin and eosin stainings were obtained through nPOD.
Sample population
A total of 59 nondiabetic control individuals (37 male, 22 female) and 47 individuals with T1D (26 male, 21 female) were selected across age groups: infants (0 to 1.4 years), children (1.5 to 13.9 years), adolescents (13 to 20.9 years), young adults (21 to 39 years), and older adults (≥40 years). Recent-onset T1D is defined as disease duration ≤10 years. Additional cohort information can be found in Tables 1 and 2 and Supplemental Tables 1 and 2 (206.5KB, xlsx) . An additional 32 nondiabetic control and 60 T1D pancreas weights were acquired from the nPOD Datashare.
Table 1.
Nondiabetic Control Sample Population
| nPOD Case No. | Age (y) | Sex | Ethnicity | Cause of Death | C-Peptide (ng/mL) | HbA1c (%) | Autoantibody Count | |
|---|---|---|---|---|---|---|---|---|
| Infants (0–1.4 y) | 6200 | 0.005 | F | H/L | PH | 0.2 | n/a | n/a |
| 6214 | 0.014 | M | W | AX | 0.2 | n/a | n/a | |
| 6164 | 0.030 | M | W | AX | n/a | 5.7 | n/a | |
| 6218 | 0.080 | F | AA | AX | 1.5 | n/a | n/a | |
| 6222 | 0.170 | M | W | AX | n/a | n/a | n/a | |
| 6117 | 0.330 | M | W | HT | 3.27 | n/a | n/a | |
| 6115 | 0.420 | M | W | AX | 4.59 | n/a | n/a | |
| 6092 | 0.500 | F | AA | AX | 0.35 | n/a | n/a | |
| Children (1.5–13.9 y) | 6103 | 1.5 | M | W | AX | 0.98 | 6.1 | n/a |
| 6107 | 2.2 | M | AA | AX | 5.9 | 5.2 | n/a | |
| 6094 | 2.9 | M | AA | AX | 3.55 | n/a | n/a | |
| 6106 | 2.9 | M | W | AX | 7.36 | n/a | n/a | |
| 6005 | 5.0 | F | W | C/S | n/a | n/a | n/a | |
| 6112 | 6.3 | F | H/L | HT | 5.11 | 5.6 | n/a | |
| 6007 | 9.0 | M | AA | AX | n/a | n/a | n/a | |
| 6278 | 10.0 | F | AA | AX | 4.54 | 6.3 | n/a | |
| Adolescents (14–20.9 y) | 6233 | 14.0 | M | W | AX | 7.26 | n/a | n/a |
| 6232 | 14.0 | F | W | HT | 19.5 | n/a | n/a | |
| 6099 | 14.2 | M | W | HT | 5.37 | n/a | n/a | |
| 6153 | 15.2 | M | H/L | HT | 8.38 | 5.5 | n/a | |
| 6075 | 16.0 | M | AA | AX | 2.94 | n/a | n/a | |
| 6096 | 16.0 | F | AA | HT | 2.97 | n/a | n/a | |
| 6230 | 16.0 | M | W | HT | 5.22 | 5.3 | n/a | |
| 6271 | 17.0 | M | W | HT | 11.47 | n/a | n/a | |
| 6227 | 17.0 | F | W | C/S | 2.75 | n/a | n/a | |
| 6098 | 17.8 | M | W | HT | 1.41 | 4.9 | n/a | |
| 6279 | 19.0 | M | W | HT | 8.01 | n/a | n/a | |
| 6253 | 19.0 | F | AA | HT | 7.22 | n/a | n/a | |
| 6238 | 20.0 | M | AA | HT | 1.17 | n/a | n/a | |
| 6234 | 20.0 | F | W | HT | 6.89 | 5.8 | n/a | |
| 6174 | 20.8 | M | W | C/S | 3 | n/a | n/a | |
| Young adults (21–39 y) | 6024 | 21.0 | M | W | HT | 3.52 | n/a | n/a |
| 6179 | 21.8 | F | W | HT | 2.74 | n/a | n/a | |
| 6001 | 22.0 | M | W | HT | 1.58 | n/a | n/a | |
| 6057 | 22.0 | M | W | HT | 16.23 | n/a | n/a | |
| 6162 | 22.7 | M | AA | HT | 7.61 | n/a | n/a | |
| 6003 | 23.0 | F | W | HT | n/a | n/a | n/a | |
| 6029 | 24.0 | F | H/L | C/S | n/a | n/a | n/a | |
| 6131 | 24.2 | M | W | AX | 1.01 | n/a | n/a | |
| 6053 | 25.0 | M | W | HT | 1.77 | n/a | n/a | |
| 6126 | 25.2 | M | W | HT | 0.88 | n/a | n/a | |
| 6058 | 27.0 | M | H/L | HT | 9.09 | n/a | n/a | |
| 6235 | 30.0 | M | W | HT | 8.1 | n/a | n/a | |
| 6048 | 30.0 | M | W | C/S | 17.91 | n/a | n/a | |
| 6030 | 30.1 | M | W | HT | 2.54 | n/a | n/a | |
| 6229 | 31.0 | F | W | HT | 6.23 | 5.5 | n/a | |
| 6034 | 32.0 | F | W | HT | 3.15 | n/a | n/a | |
| 6004 | 33.0 | M | W | HT | n/a | n/a | n/a | |
| 6002 | 39.0 | M | W | HT | n/a | n/a | n/a | |
| 6015 | 39.0 | F | W | AX | 1.99 | n/a | n/a | |
| Older adults (≥40 y) | 6009 | 45.0 | M | W | AX | 11.32 | n/a | n/a |
| 6011 | 46.0 | F | AA | C/S | n/a | n/a | n/a | |
| 6010 | 47.0 | F | W | C/S | n/a | n/a | n/a | |
| 6008 | 50.0 | F | W | HT | n/a | n/a | n/a | |
| 6168 | 51.0 | M | H/L | C/S | n/a | 6.2 | n/a | |
| 6017 | 59.0 | F | W | C/S | 9.89 | n/a | n/a | |
| 6020 | 60.0 | M | W | C/S | 2.82 | n/a | n/a | |
| 6016 | 64.0 | F | W | C/S | n/a | n/a | n/a | |
| 6013 | 65.0 | M | W | C/S | 2.8 | n/a | n/a |
Nondiabetic sample population: nPOD case number, age (years), sex, ethnicity, cause of death, C-peptide (ng/mL), HbA1c (%).
Abbreviations: A, Asian; AA, African American; AX, anoxia; CE, cerebral edema; C/S, cerebrovascular/stroke; DKA/CE, diabetic keto-acidosis/cerebral edema; H/L, Hispanic/Latino; HT, head trauma; n/a, not applicable; PH, pulmonary hypoplasia; W, white.
Table 2.
T1D Sample Population
| nPOD Case No. | Age (y) | Duration of Diabetes | Sex | Ethnicity | Cause of Death | C-Peptide (ng/mL) | HbA1c (%) | Autoantibody Count | |
|---|---|---|---|---|---|---|---|---|---|
| Children (1.5–13.9 y) | 6063 | 4.4 | 3.0 | M | W | AX | <0.05 | n/a | 1 |
| 6209 | 5.0 | 0.3 | F | W | DKA/CE | 0.1 | n/a | 3 | |
| 6062 | 10.7 | 6.0 | M | AA | DKA/CE | n/a | 12.4 | n/a | |
| 6265 | 11.0 | 8 | M | W | C/S | 0.06 | n/a | 2 | |
| 6052 | 12.0 | 1.0 | M | AA | CE | 0.18 | n/a | 2 | |
| 6264 | 12.0 | 9 | F | W | DKA | 0.001 | 8.9 | 0 | |
| 6268 | 12.0 | 3 | F | W | AX | 0.05 | 9.8 | 1 | |
| 6228 | 13.0 | 0 | M | W | AX | 0.1 | 13.3 | 3 | |
| 6243 | 13.0 | 5 | M | W | C/S | 0.42 | 13.1 | 1 | |
| 6113 | 13.1 | 1.6 | F | W | HT | <0.05 | n/a | 1 | |
| Adolescents (14–20.9 y) | 6084 | 14.2 | 4.0 | M | W | AX | <0.05 | n/a | 1 |
| 6089 | 14.3 | 8.0 | M | W | AX | <0.05 | 10.4 | 1 | |
| 6049 | 15.0 | 10.0 | F | AA | AX | <0.05 | n/a | 2 | |
| 6083 | 15.2 | 11.0 | F | W | DKA/CE | <0.05 | n/a | 1 | |
| 6207 | 16.0 | 10 | F | AA | C/S | 0.001 | n/a | 3 | |
| 6261 | 16.0 | 14.2 | M | W | AX | 0.001 | 7.2 | 2 | |
| 6148 | 17.1 | 7 | M | W | AX | 0.001 | n/a | 2 | |
| 6087 | 17.5 | 4.0 | M | W | HT | <0.05 | n/a | 2 | |
| 6145 | 18.0 | 11.0 | M | W | HT | 0.06 | n/a | 3 | |
| 6237 | 18.0 | 12 | F | W | HT | 0.001 | n/a | 2 | |
| 6195 | 19.2 | 5.0 | M | W | HT | <0.05 | n/a | 2 | |
| 6161 | 19.2 | 7 | F | W | C/S | 0.001 | 11.1 | 3 | |
| 6064 | 19.6 | 9.0 | F | W | AX | <0.05 | n/a | 4 | |
| 6212 | 20.0 | 5 | M | W | AX | 0.001 | 6.4 | 1 | |
| Young adults (21–39 y) | 6224 | 21.0 | 1.5 | F | W | AX | 0.001 | n/a | 0 |
| 6198 | 22.0 | 3 | F | H/L | C/S | 0.001 | n/a | 2 | |
| 6245 | 22.0 | 7 | M | W | HT | 0.001 | n/a | n/a | |
| 6026 | 22.4 | 14.0 | M | W | HT | <0.05 | n/a | 2 | |
| 6070 | 22.6 | 7.0 | F | W | AX | <0.05 | n/a | 4 | |
| 6069 | 22.9 | 7.0 | M | AA | HT | n/a | n/a | 1 | |
| 6025 | 23.8 | 19.0 | M | W | AX | <0.05 | n/a | 1 | |
| 6211 | 24.0 | 4 | F | AA | AX | 0.001 | 10.5 | 4 | |
| 6247 | 24.0 | 0.6 | M | W | HT | 0.47 | n/a | 1 | |
| 6196 | 26.0 | 15 | F | AA | AX | 0.48 | n/a | 0 | |
| 6041 | 26.3 | 23.0 | M | W | C/S | <0.05 | n/a | 2 | |
| 6039 | 28.7 | 12.0 | F | W | HT | <0.05 | n/a | 4 | |
| 6088 | 31.2 | 5.0 | M | W | HT | <0.05 | n/a | 4 | |
| 6081 | 31.4 | 15.0 | M | H/L | C/S | 0.24 | n/a | 0 | |
| 6035 | 32.1 | 28.0 | M | W | C/S | <0.05 | n/a | 1 | |
| 6054 | 35.1 | 30.0 | F | W | C/S | <0.05 | n/a | 1 | |
| 6038 | 37.2 | 20.0 | F | W | AX | 0.2 | n/a | 0 | |
| 6031 | 39.0 | 35.0 | M | W | C/S | <0.05 | n/a | 1 | |
| Older adults (≥40 y) | 6150 | 41.2 | 35.0 | M | W | AX | <0.05 | n/a | 1 |
| 6135 | 43.5 | 21.0 | M | W | AX | <0.05 | n/a | 2 | |
| 6036 | 49.2 | 34.0 | F | AA | AX | <0.05 | n/a | 1 | |
| 6138 | 49.2 | 41.0 | F | W | AX | <0.05 | n/a | 1 | |
| 6040 | 50.0 | 20.0 | F | W | C/S | <0.05 | 7.3 | 1 |
T1D sample population: nPOD case number, age (years), duration of diabetes (years), sex, ethnicity, cause of death, C-peptide (ng/mL), HbA1c (%), positive autoantibodies and total autoantibody count (out of the four autoantibodies tested). T1D diagnosis for nPOD cases was based on review of terminal charts, clinical and biochemical testing, and histopathology. Consideration from medical records includes the donor’s admission course, age, BMI, laboratory profiles (chemistry, urinalysis, toxicology), diagnoses, and medications. nPOD expert clinicians and pathologists assessed medical records in conjunction with the results of biochemical tests and histopathological analysis. These include autoantibody and C-peptide testing in addition to high-resolution HLA typing. Tissue sections were screened for histological features such as presence of amyloid, islet hormones, inflammation, and fibrosis.
Abbreviations: A, Asian; AA, African American; AX, anoxia; CE, cerebral edema; C/S, cerebrovascular/stroke; DKA/CE, diabetic keto-acidosis/cerebral edema; H/L, Hispanic/Latino; HT, head trauma; n/a, not applicable; PH, pulmonary hypoplasia; W, white.
Immunohistochemistry
Paraffin sections were incubated with primary antisera, guinea pig anti-insulin (A0564; Dako, Carpinteria, CA), mouse anti-Ki-67 (550609; BD Biosciences, San Jose, CA), mouse anti-Nkx6.1 (DSHB, Iowa City, IA), sheep anti-ARX (AF7069; R&D Systems, Minneapolis, MN), rabbit antiglucagon (ab8055; Abcam, Cambridge, United Kingdom), mouse antiglucagon (ab10988; Abcam), rabbit antisynaptophysin (18-0130; Thermo Fisher Scientific, Waltham, MA), followed by secondary antisera conjugated to aminomethylcoumarin, Cy2, Cy3, or Cy5 (Jackson ImmunoResearch, West Grove, PA) and 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR).
Morphometry
Slides were imaged to quantify β-cell morphometry using Volocity 6.1.1 (PerkinElmer, Waltham, MA), as previously described (29). Images were acquired with Zeiss Axio Imager M1 (Carl Zeiss Microscopy, Thornwood, NY) with an automated X-Y stage and captured with Orca ER camera (Hamamatsu, Bridgewater, NJ), resulting in images of tens of thousands of individual nuclei per sample (summarized in Supplemental Table 3 (206.5KB, xlsx) ).
Proliferation analysis
Ki-67+ cells were measured within insulin-stained slides or ×20 magnification colorimetric images from optimal cutting temperature compound (OCT)–embedded pancreas sections prepared by the nPOD core laboratory, available through the nPOD Datashare. Colorimetric insulin and DAPI were identified using a sample-specific red-green-blue color range, established through sampling of multiple data points. Ki-67+ β-cell and Ki-67+ acinar proliferation was calculated as percent total cell population. Acinar cells were identified as nonislet cells containing DAPI.
Total terminal deoxynucleotide transferase–mediated dUTP nick end labeling
Apoptosis analysis was performed in a random sampling of T1D cases, as previously described (30), with modification; sections were predigested in 0.004% trypsin and identified with Cy5-labeled reagents. Total terminal deoxynucleotide transferase–mediated dUTP nick end labeling (TUNEL)–positive β cells were assessed in >95,000 islet cells per condition. In every sample, TUNEL-positive pancreatic ducts were imaged to ensure adequate TUNEL staining.
Islet ductal neogenesis
Individuals with T1D who had residual β cells and controls were evaluated for evidence of ductal neogenesis. Islet images were classified into three possible categories: (1) solitary insulin-positive β cell in duct; (2) insulin-positive islet in duct; and (3) insulin-positive islets not associated with ducts. Results were quantified per individual and expressed as percent total islets.
α-cell to β-cell transdifferentiation
Control and T1D pancreata with persistent β cells were stained for insulin, Nkx6.1, glucagon, and ARX. Subjects with T1D with persistent β cells were defined as individuals with ≥1000 β cells in one pancreatic section.
Statistics
Results are reported as mean ± standard error of the mean and compared with independent Student t tests (unpaired, two tailed). P < 0.05 was considered significant. Linear regression analysis was performed for correlations studies.
Results
Disordered islet histology in T1D
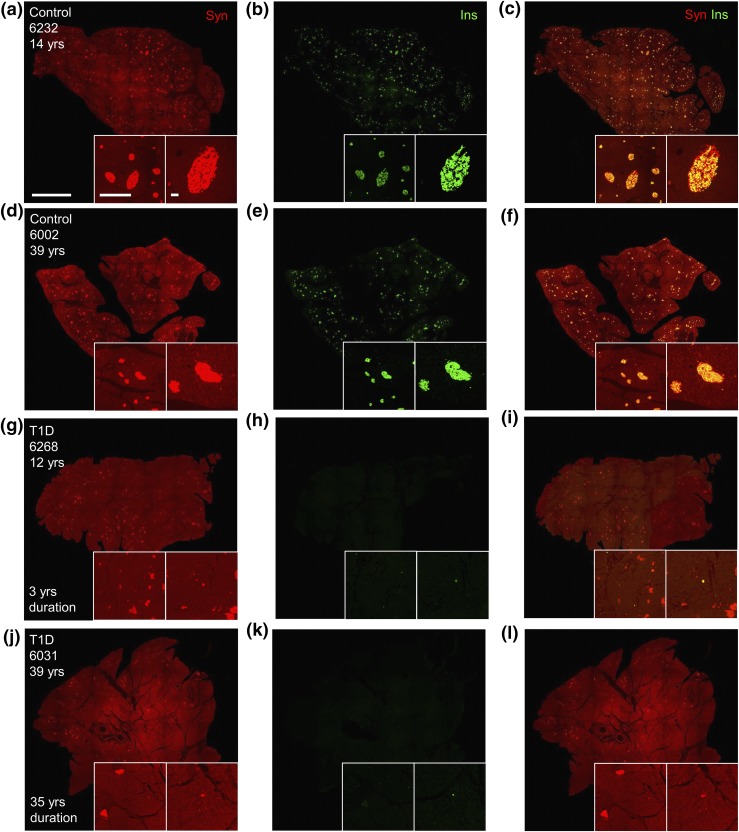
We carried out studies to determine islet and β-cell histopathology in T1D from a large cohort (Table 1; Supplemental Tables 1 and 2 (206.5KB, xlsx) ). Slides were stained with antisera against insulin and synaptophysin (panendocrine islet cell marker) to characterize β cells and islet endocrine cells, respectively. Islet histology was dramatically disturbed in T1D pancreata. β cells were drastically reduced in individuals with T1D compared with controls (Fig. 1). β cells were widely present in islets of a few individuals with both recent-onset and longstanding T1D (Supplemental Fig. 1 (207.2MB, pdf) ). However, most T1D samples contained few β cells, which were randomly scattered among pancreatic parenchyma (Fig. 1(g–l); Supplemental Fig. 2 (207.2MB, pdf) ). No T1D samples contained β cells equivalent to age-matched controls (Supplemental Tables 1 and 2 (206.5KB, xlsx) ). In contrast, islet endocrine cells were readily detected in all pancreatic samples, including those from individuals with T1D of long duration [Fig. 1(a), 1(d), 1(g), and 1(j); Supplemental Fig. 1(a), 1(d), 1(g), and 1(j) (207.2MB, pdf) ]. Pancreatic histology was grossly unaltered in many T1D samples and markedly abnormal in others, with interstitial fibrosis and acinar atrophy present in multiple pancreata (Supplemental Fig. 3 (207.2MB, pdf) ; Supplemental Table 2 (206.5KB, xlsx) ).
Figure 1.
β-cell deficiency in T1D pancreata of longstanding diabetes duration. Pancreatic islet cell and β-cell area images of (a–f) control and (g–l) T1D pancreata stained for (a, d, g, j) synaptophysin-positive (syn; red) and (b, e, h, k) insulin-positive (ins; green) islets with corresponding merged images (c, f, i, l). Insets provide ×4 (left) and ×10 (right) magnification of image. Image scale bar: 5 mm; left inset scale bar: 1 mm; right inset scale bar: 100 μm.
Regional preservation of β cells within some T1D pancreata
As expected from previous studies (2, 19), a few T1D samples exhibited regional preservation of β cells within distinct pancreatic lobes. These residual β cells were contained within islet clusters in specific areas of the pancreas [Supplemental Fig. 1(b), 1(e), 1(h), and 1(k) (207.2MB, pdf) ]. Islet clusters containing residual β cells were not specifically located around ducts [Supplemental Fig. 4(a–l) (207.2MB, pdf) ]. In contrast, islet endocrine cells were otherwise unaltered within pancreata containing residual β cells [Supplemental Fig. 1(a), 1(d), 1(g), and 1(j) (207.2MB, pdf) ].
β cells are greatly reduced in T1D but persist in some cases
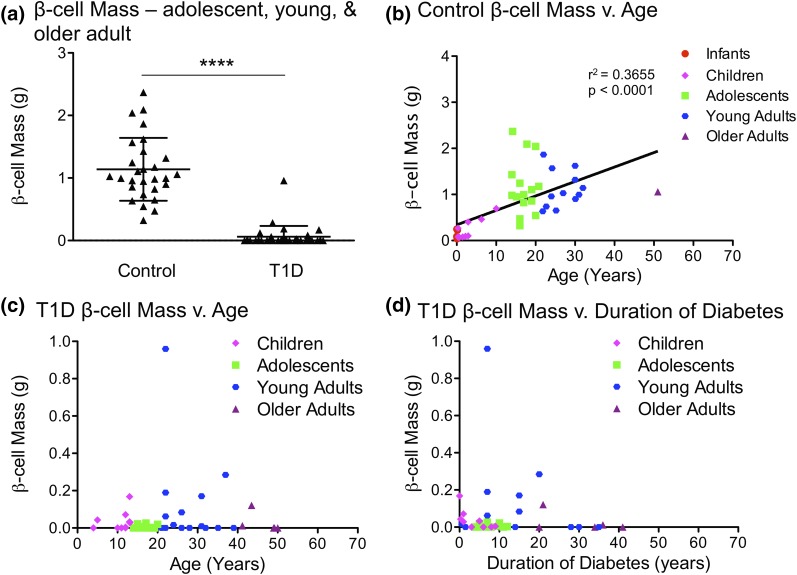
Although 36% of T1D pancreata samples (17 of 47) had complete β-cell deficiency, 64% of T1D pancreata samples (30 of 47) retained some β cells (Supplemental Tables 4 and 5 (206.5KB, xlsx) ). We quantified β-cell persistence in T1D and control samples of various ages. As expected, β-cell area and mass were severely reduced (∼88%) in pancreata from children with T1D compared with controls. β-cell area and mass were also reduced (91% and 95%, respectively), collectively, in pancreata from adolescents, young adults, and older adults with T1D [Fig. 2(a–c); Supplemental Fig. 5(a–c) (207.2MB, pdf) ]. β-cell mass increased with age in controls, but did not increase with age in T1D samples [Fig. 2(b) and 2(c)]. As expected, β-cell area and mass decreased with duration of diabetes [Fig. 2(d); Supplemental Fig. 5(d) (207.2MB, pdf) ]. Several samples exhibited substantial β cells after prolonged T1D, most of which were from individuals with recent onset of disease (Supplemental Fig. 1 (207.2MB, pdf) ) (31). Surprisingly, some samples with persistent β cells were from individuals with disease duration of >15 years.
Figure 2.
β-cell mass is greatly reduced in T1D; substantial β-cells persist in some cases of T1D. (a) β-cell mass (in grams) of adolescent, young, and older adults in control and T1D pancreata. (b–c) β-cell mass (in grams) vs. age (years) in (b) control and (c) T1D pancreata across all ages. β-cell mass increases with age in controls, as seen in (b). β-cell mass does not increase with age in T1Ds, as seen in (c). (d) β-cell mass (in grams) vs. duration of diabetes (years) in T1D pancreata. β-cell mass declines with duration of diabetes. (a) Results are expressed as mean ± standard error of the mean for 28 controls and 33 T1D pancreata. ****P < 0.0001. (b–d) Data points represent the mean value for each of 59 control and 47 T1D pancreata.
Pancreatic mass is severely reduced in T1D
Although a recent report indicates that pancreatic mass is reduced in T1D (32), the impact of T1D upon age-related pancreatic mass expansion remains unclear. Consequently, we examined the relationship between pancreas mass and age in control and T1D pancreata. Pancreas mass increased with age, weight, and body mass index (BMI) in controls [Supplemental Fig. 6(a–g) (207.2MB, pdf) ; Supplemental Table 6 (206.5KB, xlsx) ], a finding consistent with results of prior studies (33, 34). In contrast, pancreas mass did not increase with age in T1D samples. T1D pancreas mass was similar in children compared with controls (24.3 g vs. 26.9 g; P = 0.56). But pancreas mass was reduced by 49% in adolescent, young, and older adult T1D pancreata [Supplemental Fig. 6(h–k) (207.2MB, pdf) ]. Pancreas mass was reduced more severely in young (40.4 g vs. 84.8 g; P < 0.0001) and older adults (40.8 g vs. 81.7 g; P < 0.0001) with T1D. Collectively, these findings indicate that T1D impairs pancreatic mass expansion with age.
Relationship between residual β cells and serum C-peptide
Residual β-cell function in patients with longstanding T1D could be associated with anatomical preservation or regeneration of β cells. We compared serum C-peptide measurements with β-cell area and mass in T1D samples. C-peptide was undetectable if <0.05 in many longstanding T1D donors with the assay used in the current study, but low levels of C-peptide might have been detectable with the current generation of C-peptide assays (12). C-peptide was associated with both β-cell area and mass in T1Ds but not controls (Supplemental Fig. 7 (207.2MB, pdf) ; Supplemental Tables 4 and 5 (206.5KB, xlsx) ). Thus, residual β-cell function is positively associated with β-cell persistence in T1D.
β-cell replication declines with age; no compensatory β-cell proliferation in T1D
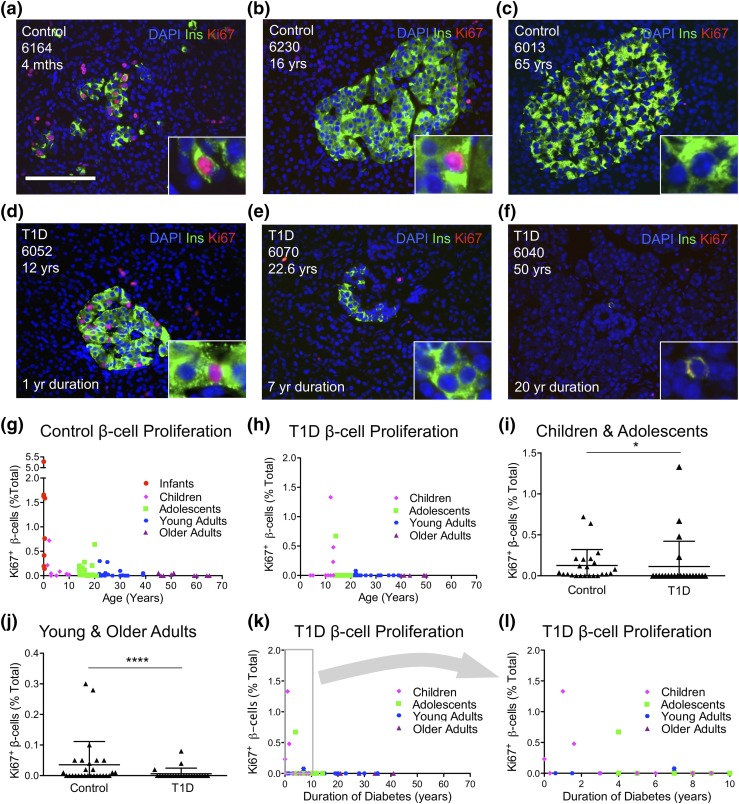
To test if persistent β-cell function in T1D is due to ongoing generation of new β cells, we measured β-cell proliferation to detect possible compensatory β-cell turnover. As expected from previous studies (33, 35), β-cell proliferation was high in infants but decreased dramatically thereafter in control pancreata [Fig. 3(a–c) and 3(g); Supplemental Table 7 (206.5KB, xlsx) ]. Control infants exhibited the highest amount of β-cell proliferation (up to 5.28%). In contrast, young and older control adults displayed little β-cell turnover (0% to 0.30%). Children with TID exhibited the highest β-cell replication rates (up to 1.33%), whereas young and older adults exhibited the lowest (0% to 0.08%) [Fig. 3(d–f) and 3(h); Supplemental Table 8 (206.5KB, xlsx) ]. There was no relationship between β-cell proliferation and body weight or BMI in control or T1D pancreata (Supplemental Fig. 8 (207.2MB, pdf) ).
Figure 3.
No compensatory β-cell proliferation in T1D. (a–h) β-cell proliferation declines with age in control and T1D pancreata. (a–f) Representative pancreatic islet images for (a–c) control and (d–f) T1D stained for DAPI (blue), insulin (ins; green), and Ki-67 (red). Scale bar: 100μm. (g–h) Ki-67+ β cells (% total β cells) in (g) control and (h) T1D vs. age (years) demonstrate that β-cell proliferation is reduced in T1D pancreata. (i–j) Ki-67+ β cells (% total β cells) were reduced in T1D pancreata from (i) children and adolescents and also in pancreata from (j) young and older adults. (k–l) β-cell proliferation vs. T1D duration (years) reveal that most pancreata from individuals with T1D exhibited no evidence of β-cell proliferation, regardless of duration of diabetes. (l) Panel inset from (k) represents individuals with duration of diabetes <10 years. (i–j) Results are expressed as mean ± standard error of the mean for 28 controls and 33 T1D pancreata. *P < 0.05; ****P < 0.0001. (g–h, k–l) Data points represent the mean value for each of 59 control and 47 T1D pancreata.
Surprisingly, β-cell proliferation rates for individuals with T1D were similar to those of controls in both children and adolescents and young and older adults [Fig. 3(i) and 3(j)]. Notably, β-cell proliferation was higher in individuals with T1D who had a shorter duration of diabetes compared with those with longstanding disease [Fig. 3(k) and 3(l)]. Although this result could be interpreted as attempted β-cell regeneration in T1D, the samples with higher β-cell proliferation represented the youngest age groups with T1D samples. Indeed, β-cell proliferation was unaltered in these T1D pancreata when compared with age-matched controls (Supplemental Tables 7 and 8 (206.5KB, xlsx) ). Our results therefore confirm age-dependent reduction in β-cell proliferation and refute a β-cell proliferative response to TID.
We considered the possibility that pancreatic tissue degradation could reduce Ki-67 immunoreactivity within β cells of some samples. Consequently, we quantified acinar cell proliferation in every control and T1D sample. Reassuringly, Ki-67 was readily detectable within acinar cells within every case in abundance (Supplemental Fig. 9 (207.2MB, pdf) ; Supplemental Tables 7 and 8 (206.5KB, xlsx) ). Although this result does not rule out the possibility of pancreatic sample degradation, minimal β-cell turnover does not seem to be associated with a lack of Ki-67 immunoreactivity throughout the pancreas.
We studied whether tissue fixation could reduce Ki-67 in β cells. We examined images from a random sampling of control and T1D cases from the nPOD online database to ensure that Ki-67 could be reliably detected within T1D samples with low Ki-67+ insulin-positive cells (Supplemental Fig. 10 (207.2MB, pdf) ). We quantified images from the nPOD image database of OCT-embedded frozen pancreas, paraffin-embedded pancreas, and paraffin-embedded spleen tissues. Reassuringly, no obvious differences were discernible between paraffin and OCT-embedded tissues (Supplemental Fig. 10 (207.2MB, pdf) ; Supplemental Table 9 (206.5KB, xlsx) ).
T1D individuals with residual β cells do not have increased β-cell death
To test for ongoing β-cell destruction in T1D, we quantified β-cell TUNEL in a representative cohort of control and T1D pancreata. TUNEL was readily detectable within occasional pancreatic ducts [Supplemental Fig. 11(a) (207.2MB, pdf) ]. Controls exhibited minimal β-cell death, as assessed by TUNEL (Supplemental Fig. 11 (207.2MB, pdf) ; Supplemental Table 10 (206.5KB, xlsx) ). Controls exhibited only 0.01% TUNEL-positive β cells. In comparison, subjects with T1D, including some with residual β cells, exhibited 0.00% TUNEL-positive β cells. Results suggest that there is no apparent or measurable increase in β-cell death as assessed by TUNEL. More importantly, individuals with T1D and residual β cells are not experiencing substantial ongoing β-cell destruction, as measured by TUNEL.
β-cell turnover was not associated with pancreas harvest conditions
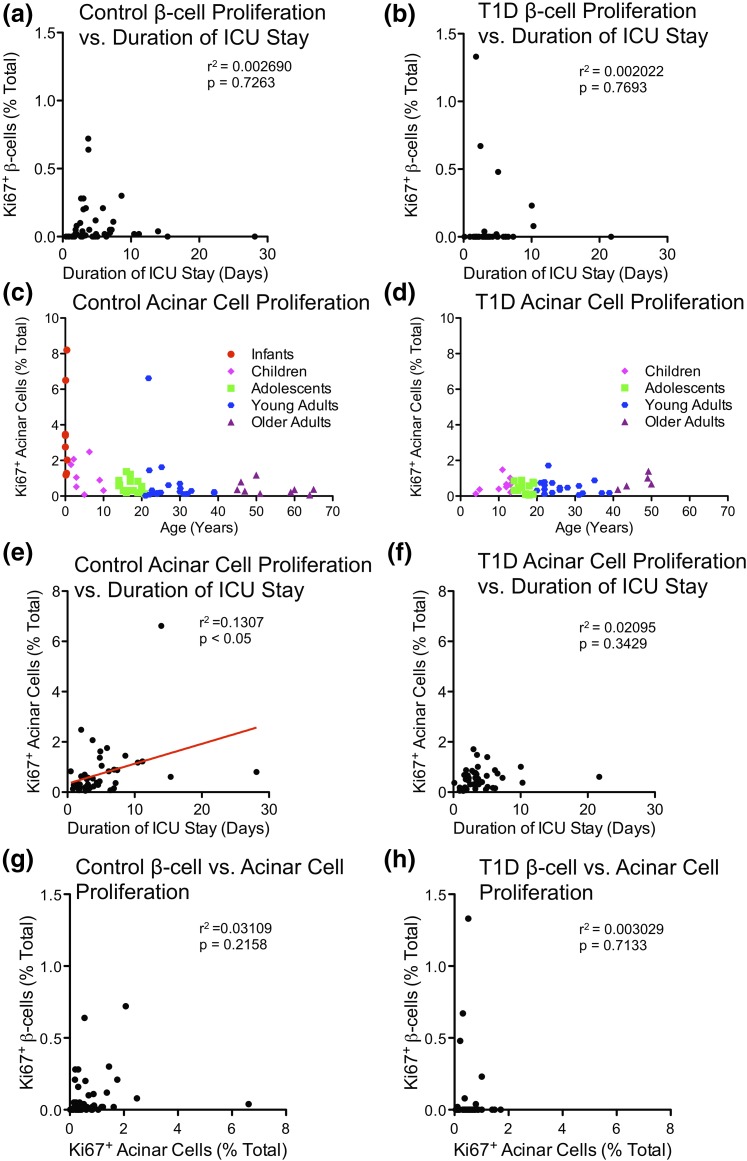
Pipeleers and colleagues (36) reported that β-cell proliferation was increased in samples of donor organs from young patients after prolonged life support or intensive care unit (ICU) stay (≥3 days). We considered whether β-cell turnover might be confounded by postmortem state or organ harvest conditions. However, there was no association between duration of ICU stay and β-cell proliferation within control or T1D populations [Fig. 4(a) and 4(b); Supplemental Table 11 (206.5KB, xlsx) ]. Acinar cell proliferation was strongly associated with prolonged ICU stay in controls, but was not associated with β-cell proliferation, T1D status, or duration of diabetes [Fig. 4(c–h); Supplemental Fig. 9(a–e) (207.2MB, pdf) ]. Thus, proliferation within pancreatic parenchyma is strongly correlated with the length of ICU stay. Although length of ICU stay appears to have opposing effects on acinar cell and β-cell proliferation, we cannot formally exclude possible contributions of the length of ICU stay on β-cell proliferation without a large cohort that matches the strict criteria used by Pipeleers et al. (36).
Figure 4.
Duration of ICU stay is unassociated with β-cell proliferation in infants to older adults. (a–b) Ki-67+ β cells (% total β cells) vs. duration of ICU stay (days) in (a) control and (b) T1D pancreata. Data points represent the mean value for each of 48 control and 45 T1D pancreata. (c) Control and (d) T1D Ki-67+ acinar cells (% total) vs. age (years). Data points represent the mean value for each of 59 control and 47 T1D pancreata. (e–f) Ki-67+ acinar cells (% total) vs. duration of ICU stay (days) in (e) control and (f) T1D pancreata. Data points represent the mean value for each of 48 control and 45 T1D pancreata. (g–h) Quantification of Ki-67+ β cells vs. Ki-67+ acinar cells (% total) in (g) control and (h) T1D pancreata. Data points represent the mean value for each of 51 control and 47 T1D pancreata.
Additional concerns have been raised regarding the potential impact of warm or cold ischemia to reduce Ki-67 immunoreactivity and therefore confound measurement of human islet cell turnover (37). However, β-cell proliferation was readily detected in neonatal samples [Fig. 3(a) and 3(g); Supplemental Table 7 (206.5KB, xlsx) ]. Moreover, pancreatic transit time did not correlate with β-cell proliferation [Supplemental Fig. 9(f) and 9(g) (207.2MB, pdf) ; Supplemental Tables 4, 5, and 11 (206.5KB, xlsx) ]. No association was found between acinar cell proliferation and pancreas transit [Supplemental Fig. 9(h) and 9(i) (207.2MB, pdf) ]. Nevertheless, because of the inherent limitations in autopsy studies, we cannot formally rule out the possibility that sample degradation could explain the lack of β-cell turnover within control or T1Ds samples.
No evidence for ductal neogenesis within T1D pancreata with persistent β cells
β-cell neogenesis from ductal progenitors has been widely speculated to contribute to β-cell persistence in T1D, in part due to scattered reports that describe persistent β cells in ducts of T1D pancreata (18, 20). To test this hypothesis, we quantified solitary insulin-positive cells and insulin-positive islets within pancreatic ducts. We carried out our analysis in controls and T1D samples with substantial residual β cells. However, insulin-positive cells within pancreatic ducts were extremely rare in controls and absent in T1D samples (Supplemental Fig. 4 (207.2MB, pdf) ; Supplemental Table 12 (206.5KB, xlsx) ). Solitary insulin-positive cells found within ducts represented 0.20% of insulin-positive clusters found in control pancreata. No multicellular insulin-positive islets were observed in pancreatic ducts of controls or T1Ds; the vast majority of β cells were not associated with pancreatic ducts [99.8% of controls and 100% of T1D samples; Supplemental Fig. 4(m) (207.2MB, pdf) ]. Thus, we find no direct evidence for an association between persistent insulin-positive cells and pancreatic ducts.
No apparent α-cell to β-cell transdifferentiation in T1D
To determine if α-cell to β-cell transdifferentiation is associated with β-cell persistence in T1D pancreata, we tested for insulin and glucagon coexpression within T1Ds with residual β cells and aged-matched controls. Insulin/glucagon coexpression was extremely rare. Although a few α cells and β cells partially overlapped (due to compact three-dimentional islet architecture), no cells perfectly overlapped with a single DAPI nucleus (Supplemental Table 13 (206.5KB, xlsx) ).
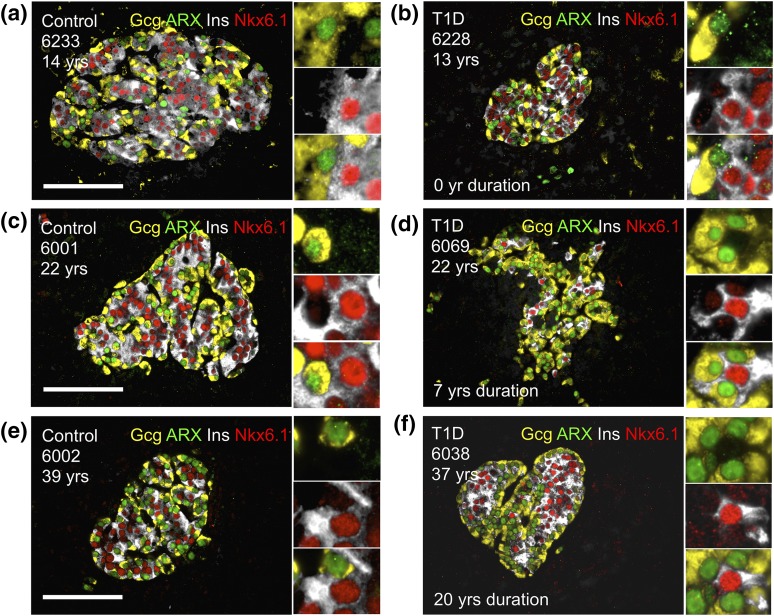
We then simultaneously stained insulin and Nkx6.1 for β cells and glucagon and ARX for α cells. We hypothesized that α- to β-cell or β- to α-cell transdifferentiation would be revealed by a combination of α- and β-cell markers. Of the 42,043 α and β cells observed, there was minimal evidence of α- to β-cell transdifferentiation (Fig. 5; Supplemental Table 14 (206.5KB, xlsx) ). Only 0.01% Gcg+ARX+Nkx6.1+ and 0.02% Gcg+ARX+Nkx6.1+, and 0% Gcg+ARX+Ins+Nkx6.1+ cells, were detected in control pancreata. T1D pancreata did not contain any cells expressing both α- and β-cell markers. We concluded that there was no apparent, or measurable, α- to β-cell transdifferentiation using these techniques.
Figure 5.
No apparent α-cell to β-cell transdifferentiation. (a, c, e) control and (b, d, f) T1D islets stained for glucagon (gcg; yellow), and ARX (green), insulin (ins; white), and Nkx6.1 (red) demonstrate that α- to β-cell transdifferentiation is not the primary mechanism of β-cell persistence. Insets indicate gcg+ARX+ α cells or insulin-positive Nkx6.1+ β cells.
Discussion
By analyzing a large cohort of nPOD pancreata, we found that β cells in longstanding T1D pancreata were rare. Indeed, many T1D pancreata had few residual β cells, if any, regardless of disease duration. We found no relationship between individuals with T1D with persistent β cells and various parameters, including sex, age, ethnicity, disease duration, autoantibodies, and body weight/BMI. In contrast, C-peptide was correlated with β-cell mass in T1D samples.
As expected from studies of recent-onset T1D cases (4, 31) and in longstanding disease (1, 6, 18, 38), β-cell mass declined with disease duration. A few of our T1D samples had substantial β-cell mass. Studies by Keenan et al. (19) suggest that residual β cells are observed in some individuals with T1D after a diabetes duration of 50 years. However, our primary findings of minimal β-cell mass in longstanding T1D are similar to the results of others. Nakanishi and colleagues (38) indicated that β-cell mass in T1D samples was reduced by 90% compared with controls. Similarly, Butler and colleagues (18) reported that β-cell area was nearly undetectable (0.027%) in T1D (n = 42). Diedisheim and colleagues (39) reported that seven T1D samples from nPOD with disease duration of five to 25 years had little (if any) β-cell mass. Finally, Campbell-Thompson and colleagues (6) studied an extensive cohort of 54 T1D nPOD samples and observed that β-cell mass was nearly undetectable in the vast majority of samples. In agreement with these reports, β-cell mass was often undetectable in our T1D samples.
We found that β-cell replication dramatically decreases with age after infancy, confirming previous reports (33). β-cell proliferation did not increase within the few β cells that persisted within adult T1D pancreata. Interestingly, β-cell TUNEL (death) was very low in control and TID samples. To our knowledge, our study represents the largest cohort of T1D pancreata analyzed for β-cell turnover. Additionally, we found no evidence for ductal β-cell neogenesis in T1D pancreata, or α- to β-cell transdifferentiation. Thus, longstanding β-cell function in patients with T1D appears to be largely due to β-cell persistence, without any evidence of attempted β-cell regeneration through increased β-cell proliferation.
The 3D structure of the islet makes it difficult to discern if a cell is truly copositive for insulin and glucagon (or if two cells are lying superior/inferior to one another). Attempts to address whether α- to β-cell transdifferentiation exists have been hindered by the lack of objective tools to identify these cells undergoing transdifferentiation. Using multiple cell-specific markers, high numerical aperture objectives, high-throughput microscopy, and automated image analysis, we searched for α- to β-cell transdifferentiation. No cell conclusively coexpressed glucagon and insulin at the levels of mature α cells or β cells in control or T1D pancreata. However, we found rare indirect evidence of transdifferentiation: a tiny fraction of islet endocrine cells expressed both α- and β-cell markers in controls, but none in T1D. Thus, by the methods used, α- to β-cell transdifferentiation was not readily detected in T1D.
Autopsy tissue studies are complicated by potential confounding variables, including cause and conditions of patient death, tissue harvest and fixation conditions, and other preexisting medical conditions prior to patient death. In a massive study of 363 nondiabetic individuals by Pipeleers and colleagues (36), samples in the top 10% with respect to β-cell proliferation were more likely to be from young patients with prolonged life support. Our cohort was not sufficiently powered to test the impact of ICU stay by stratifying patients according to these criteria. As a result, we cannot directly compare our results with those of Pipeleers and colleagues (36). Thus, although we find no association between ICU stay and β-cell turnover, we cannot formally exclude possible contributions of the length of ICU stay on proliferation of β cells in control and T1D pancreata. Hopefully, future surgical studies will resolve these outstanding issues.
Tissue fixation conditions, including duration and fixative, represent potential confounders of our study. Although overfixed tissues could result in understaining of Ki-67 or other antigens, we were able to readily detect these markers in all pancreatic samples in various tissue compartments. In all of our samples, we detected insulin, synaptophysin, and Ki-67; we also readily detected glucagon, ARX, and Nkx6.1 in a subset of samples (Supplemental Tables 12 and 13 (206.5KB, xlsx) ). We were always able to detect our desired antigen in our samples. Thus, although we do not favor tissue fixation as an explanation for low Ki-67 content in pancreata from aged individuals, we cannot formally rule out this possibility. Although autopsy specimens may have potential limitations for the study of cell turnover of pancreatic exocrine parenchyma, cadaveric pancreata provide a unique window into human β-cell turnover to further our understanding of T1D.
In summary, T1D pancreata show little evidence of ongoing β-cell generation. Our findings are consistent with our prior studies, in which we indicated that there was limited β-cell regenerative capacity in aged mice (40). Thus, future therapies to regenerate β cells from endogenous sources must overcome low rates of β-cell turnover to generate substantial β cells in patients with T1D.
Acknowledgments
The authors thank the pancreas donors and their families. This research was performed with the support of nPOD, a collaborative T1D research project sponsored by the Juvenile Diabetes Research Foundation. Organ procurement organizations, partnering with nPOD to provide research resources, are listed at www.jdrfnpod.org/our-partners.php. The authors thank M. Yang, I. Kusmartseva, A. Pugliese, C. Wasserfall, M. Campbell-Thompson, and M. Atkinson for their generous and essential contributions to this work. The authors also thank C. Blalock, D. Kettlewell, B. Pekkattil, K. Rogers, K. DePrado, T. LaSpina, C. Yee, and S. Vale of the Texas Children's Diabetes and Endocrinology Center for their administrative expertise and support.
Acknowledgments
This study was supported by the Robert and Janice McNair Foundation and the National Institutes of Health (Grant 1R01AG040110), and by the Diabetes Research Center of Baylor College of Medicine (Grant DRC-P30DK079638).
Author contributions: C.J.L., D.R.J., M.M.R., A.R.C., and J.A.K. conceived and designed the experiments. C.J.L., D.R.J., M.M.R., and A.R.C. performed the experiments. C.J.L., D.R.J., M.M.R., A.R.C., and J.A.K. analyzed the data. C.J.L., A.R.C., and J.A.K. wrote the paper. J.A.K. is the guarantor of this work and, as such, had full access to all of the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: J.A.K. has served on the scientific advisory board of Johnson & Johnson and currently serves on an advisory board for Lexicon. The remaining authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- C-peptide
- connecting peptide
- DAPI
- 4′,6-diamidino-2-phenylindole
- ICU
- intensive care unit
- nPOD
- Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes
- OCT
- optimal cutting temperature compound
- T1D
- type 1 diabetes
- TUNEL
- terminal deoxynucleotide transferase-mediated dUTP nick end labeling.
References
- 1.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14(10):619–633. [DOI] [PubMed] [Google Scholar]
- 2.Gepts W, De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes. 1978;27(Suppl 1):251–261. [DOI] [PubMed] [Google Scholar]
- 3.Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4(2):110–125. [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50(11):2323–2331. [DOI] [PubMed] [Google Scholar]
- 5.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10(Suppl 4):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludvigsson J, Heding LG. C-peptide in children with juvenile diabetes. A preliminary report. Diabetologia. 1976;12(6):627–630. [DOI] [PubMed] [Google Scholar]
- 8.Nordwall M, Ludvigsson J. Clinical manifestations and beta cell function in Swedish diabetic children have remained unchanged during the last 25 years. Diabetes Metab Res Rev. 2008;24(6):472–479. [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson J. C-peptide an adequate endpoint in type 1 diabetes. Diabetes Metab Res Rev. 2009;25(8):691–693. [DOI] [PubMed] [Google Scholar]
- 10.Wherrett DK, Chiang JL, Delamater AM, DiMeglio LA, Gitelman SE, Gottlieb PA, Herold KC, Lovell DJ, Orchard TJ, Ryan CM, Schatz DA, Wendler DS, Greenbaum CJ; Type 1 Diabetes TrialNet Study Group . Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: a consensus report. Diabetes Care. 2015;38(10):1975–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanBuecken DE, Greenbaum CJ. Residual C-peptide in type 1 diabetes: what do we really know? Pediatr Diabetes. 2014;15(2):84–90. [DOI] [PubMed] [Google Scholar]
- 12.Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57(1):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maclean N, Ogilvie RF. Observations on the pancreatic islet tissue of young diabetic subjects. Diabetes. 1959;8(2):83–91. [DOI] [PubMed] [Google Scholar]
- 14.Evans DJ. Generalized islet hypertrophy and beta-cell hyperplasia in a case of long-term juvenile diabetes. Diabetes. 1972;21(2):114–116. [DOI] [PubMed] [Google Scholar]
- 15.Löhr M, Klöppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia. 1987;30(10):757–762. [DOI] [PubMed] [Google Scholar]
- 16.Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26(6):456–461. [DOI] [PubMed] [Google Scholar]
- 17.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986;29(5):267–274. [DOI] [PubMed] [Google Scholar]
- 18.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–2228. [DOI] [PubMed] [Google Scholar]
- 19.Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, Kent SC, Hering BJ, West E, Steck A, Bonner-Weir S, Atkinson MA, Coppieters K, von Herrath M, Eisenbarth GS. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia. 2010;53(4):690–698. [DOI] [PubMed] [Google Scholar]
- 21.Gepts W, LeCompte PM, Volk BW, Arquilla ER, eds. The Pathology of Type I (Juvenile) Diabetes. New York, NY: The Diabetic Pancreas Plenum Medical Book Co; 1985:337–360. [Google Scholar]
- 22.Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999;48(5):989–996. [DOI] [PubMed] [Google Scholar]
- 23.Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007;148(11):5136–5144. [DOI] [PubMed] [Google Scholar]
- 24.Ablamunits V, Sherry NA, Kushner JA, Herold KC. Autoimmunity and beta cell regeneration in mouse and human type 1 diabetes: the peace is not enough. Ann N Y Acad Sci. 2007;1103:19–32. [DOI] [PubMed] [Google Scholar]
- 25.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55(12):3238–3245. [DOI] [PubMed] [Google Scholar]
- 26.Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia. 2006;49(8):1838–1844. [DOI] [PubMed] [Google Scholar]
- 27.Granger A, Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Intern Med. 2009;266(4):325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Yang X, Chen B, Xu X. Pancreas β cell regeneration and type 1 diabetes (review). Exp Ther Med. 2015;9(3):653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12(5):817–826. [DOI] [PubMed] [Google Scholar]
- 30.He LM, Sartori DJ, Teta M, Opare-Addo LM, Rankin MM, Long SY, Diehl JA, Kushner JA. Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol Endocrinol. 2009;23(11):1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pipeleers D, Ling Z. Pancreatic beta cells in insulin-dependent diabetes. Diabetes Metab Rev. 1992;8(3):209–227. [DOI] [PubMed] [Google Scholar]
- 32.Campbell-Thompson ML, Kaddis JS, Wasserfall C, Haller MJ, Pugliese A, Schatz DA, Shuster JJ, Atkinson MA. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59(1):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saisho Y, Butler AE, Meier JJ, Monchamp T, Allen-Auerbach M, Rizza RA, Butler PC. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20(8):933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97(9):3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.In’t Veld P, De Munck N, Van Belle K, Buelens N, Ling Z, Weets I, Haentjens P, Pipeleers-Marichal M, Gorus F, Pipeleers D. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59(7):1702–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki-67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human β-cells. Diabetes. 2015;64(5):1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakanishi K, Kobayashi T, Miyashita H, Okubo M, Sugimoto T, Murase T, Kosaka K, Hara M. Relationships among residual beta cells, exocrine pancreas, and islet cell antibodies in insulin-dependent diabetes mellitus. Metabolism. 1993;42(2):196–203. [DOI] [PubMed] [Google Scholar]
- 39.Diedisheim M, Mallone R, Boitard C, Larger E. β-cell mass in nondiabetic autoantibody-positive subjects: an analysis based on the network for pancreatic organ donors database. J Clin Endocrinol Metab. 2016;101(4):1390–1397. [DOI] [PubMed] [Google Scholar]
- 40.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58(6):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]