Abstract
Context:
Although the long-term effects of testosterone on adipose tissue lipid metabolism in men have been defined, the short-term regulation of these effects is not well understood.
Objective:
We examined the effects of acute testosterone withdrawal on subcutaneous abdominal and femoral adipose tissue fatty acid (FA) storage and cellular mechanisms.
Design:
This was a prospective, randomized trial.
Setting:
Mayo Clinic Clinical Research Unit.
Patients or Participants:
Thirty-two male volunteers ages 18 to 50 participated in these studies.
Interventions:
Volunteers were randomized to receive (1) no treatment (control), (2) injections (7.5 mg) of Lupron®, or (3) Lupron and testosterone (L+T) replacement for 49 days, resulting in 4 weeks of sex steroid suppression in the Lupron group.
Main Outcome Measures:
We measured body composition, fat cell size, adipose tissue meal FA and direct free FA storage, lipoprotein lipase (LPL), acyl coenzyme A synthetase (ACS), diacylglycerol acyltransferase activities, and CD36 content.
Results:
Compared with control and L+T groups, acute testosterone deficiency resulted in greater femoral adipose tissue meal FA storage rates, fasting and fed LPL activity, and ACS activity.
Conclusions:
These results suggest that in men, testosterone plays a tonic role in restraining FA storage in femoral adipose tissue via suppression of LPL and ACS activities. FA storage mechanisms in men appear sensitive to short-term changes in testosterone concentrations.
Acute testosterone deficiency in men results in greater femoral adipose lipoprotein lipase and acyl coenzyme A synthetase activities, which correlates with greater meal fat and direct FFA storage.
As men age, decreases in testosterone are associated with body composition shifts toward increasing fat and decreasing muscle mass. The effects of testosterone on body fat distribution in men are especially evident in instances when testosterone concentrations deviate from normal. When testosterone concentrations increase during puberty, peripheral fat decreases relative to trunk fat (1). With Klinefelter syndrome or male eunuchs, chronic testosterone deficiency results in a more female body fat pattern with greater lower-body fat accumulation (2, 3). We have some understanding of the mechanisms by which chronic testosterone exposure (or lack thereof) affects fat distribution (4). How much of testosterone’s effects on adipose tissue is due to short-term effects to stimulate or inhibit these processes is unknown. In the current study, we investigated the effects of acute male sex steroid suppression on storage of dietary and circulating free fatty acids (FFAs) as well as the underlying proteins and enzymes involved in fatty acid (FA) storage.
The FA stored in adipose tissue originate from triglycerides in chylomicrons and very-low-density lipoprotein, as well as the direct FFA reuptake pathway. Although the majority of FA stored originates from triglyceride-rich lipoproteins via hydrolysis by lipoprotein lipase (LPL), the direct FFA pathway appears to contribute to the redistribution of FA between adipose depots (5, 6). Although testosterone treatment is reported to decrease LPL activity (7, 8) we found that chronic testosterone suppression did not have major effects on LPL activity (4). Whether there are acute effects of testosterone suppression on LPL activity is unknown.
FFAs enter the adipocyte via passive (flip-flop) or protein-facilitated diffusion and then undergo a series of enzymatic reactions before being stored as triglycerides (9). In this study, we examined the effects of acute testosterone deficiency on some of the enzymes and proteins at different tiers of the triglyceride storage pathway. At the cell surface, FA transport protein CD36 is a ubiquitously expressed cell-surface glycoprotein that enhances cellular FA uptake when FA concentrations are low (10). Once inside the cell, acyl coenzyme A synthetase (ACS) enzymes activate FA to fatty acyl coenzyme A (11). Diacylglycerol acyltransferase (DGAT) catalyzes the esterification of a FA to a diacylglycerol molecule in the final step of FA-to-triglyceride synthesis (12, 13). In this study, we examined LPL, CD36, ACS, and DGAT in the context of FA storage to better understand the tonic role of testosterone on the steps in adipose tissue triglyceride storage in men.
Materials and Methods
Subjects
Healthy, nonsmoking men ages 18 to 50 years old were recruited and randomized to one of three groups: a nonintervention control group, a group administered leuprolide acetate (Lupron®; AbbVie Inc., North Chicago, IL) to suppress sex steroids, and a group administered Lupron and testosterone (L+T) gel (AndroGel® 7.5 mg/d; AbbVie Inc.). All participants included were required to be healthy and weight stable (±1.0 kg) for >2 months before the study and were not taking any antidepressants or medications that could affect FA metabolism. Men with diabetes or anemia were also excluded. Written informed consent was obtained from all participants. The study was approved by the Institutional Review Board of the Mayo Clinic.
Materials
[l-14C]palmitate and [9,10-3H]triolein were purchased from NEN Life Science Products (PerkinElmer, Boston, MA), and 2H2O and [U-13C]palmitate (both 99 atom % pure) from Isotec (Miamisburg, OH).
Study design
Study visits were conducted at the Mayo Clinical Research Unit. Participants in the Lupron or L+T groups received 49 days of treatment. Both the Lupron and L+T groups received 7.5-mg intramuscular injections of Lupron on days 1 and 21 of the study. Testosterone concentrations were measured on days 1, 21, and 49. Men randomized to the L+T group were provided with a week’s worth of individual 7.5-mg AndroGel® packets and instructed to apply the gel daily throughout the treatment period. Those in the treatment groups filled out a food frequency questionnaire (Viocare Inc, Princeton, NJ) before and at the end of the treatment period to monitor dietary intake. Participants were weighed weekly during the 49-day treatment period, after which they were admitted for the inpatient study. The control group participants underwent the inpatient study immediately.
All volunteers had body composition measured just prior to the inpatient study. Those in the Lupron and L+T groups also had body composition measured prior to the hormone treatment period. Participants were provided with all meals to ensure similar macronutrient intake and weight stability for 5 days prior to the inpatient study stay (4). FA metabolism was measured as previously described (4). Briefly, an experimental meal containing [3H]triolein was consumed at 8:00 am the morning following the first overnight stay. Indirect calorimetry (DeltaTrac, Yorba Linda, CA) measurements were performed just prior to the experimental breakfast meal hourly for 6 hours to measure energy expenditure and substrate oxidation. A 24-hour urine collection was conducted to measure nitrogen and 3H2O excretion. Blood samples were collected just prior to and hourly after the experimental meal for 10 hours. Abdominal and femoral adipose tissue biopsies were performed at 2:00 pm (1 hour after lunch) to measure “fed” LPL activity. The morning after the second overnight stay, FFA tracers were administered intravenously to measure direct FFA storage rates (14). Thirty minutes after the bolus infusion of [1-14C]palmitate, a second set of abdominal and femoral adipose tissue biopsies was performed.
Assays and methods
Plasma triglyceride concentrations and 3H content in chylomicron and nonchylomicron fractions, urinary nitrogen, and plasma hormones were measured as previously described (15). Testosterone was measured via liquid chromatography/mass spectrometry (16).
Body composition
Total and regional fat mass was measured using combined dual-energy x-ray absorptiometry (Lunar iDXA, GE Health Care, Madison, WI) and a single-slice abdominal computed tomography scan at the L2-3 level (17, 18).
Substrate oxidation
Resting energy expenditure (REE) and respiratory exchange ratio were measured over a 30-minute period (basal) and then for 10 minutes every hour for 6 hours (4). Carbohydrate and fat oxidation were calculated at each time point, and total oxidation was determined as area under the curve (19).
FA metabolism studies
Meal FA storage and oxidation were determined as previously described (4). Briefly, at 8:00 am, participants consumed an Ensure Plus meal that provided calories equal to 40% of individually measured REE and contained 50 µCi [9,10-3H]triolein (20). Twenty-four-hour fat oxidation was determined based on the 3H2O concentration in the urine collected after a 24-hour void (4). FFA flux was measured with a continuous infusion of [U-13C]palmitate (21). A bolus infusion of [1-14C]palmitate was given at 8:00 am to measure direct adipose tissue FFA storage rates (22). After lipid extraction, adipose 3H and 14C lipid-specific activity (dpm/g lipid) were measured as previously described (23).
Adipose tissue analysis
Fat cell size was measured using photomicrographs (24), LPL (25), ACS (26), and DGAT (13) activity were determined using enzyme activity assays, and CD36 was measured by capillary Western blot.
Calculations, data analysis, and statistics
Power calculations
These were part of a series of studies to examine the effects of sex steroids on adipose tissue metabolism in humans. In this protocol, we originally planned to study 13 men per group to detect differences in meal FA uptake between control and testosterone-deficient men, with the L+T group serving as a control for a possible independent effect of Lupron. We used our data on the reproducibility of meal FA uptake in men (27) to perform power calculations. Our goal was to have 80% power at an alpha of P = 0.05 to detect a 7% difference in lower meal body FA uptake between groups using an analysis of variance (ANOVA). A 7% difference was selected as an amount that would result in a substantial, detectable shift in body fat distribution if it were to occur over a >6-month time interval. Our subsequent studies revealed that differences in adipose tissue FA metabolism between control and long-term hypogonadal men (4) were much greater than expected. We therefore reduced the number of volunteers we recruited for this study because we didn’t consider it ethically justifiable to study more participants than necessary in this intense protocol to test our primary hypotheses.
We present these data in two ways to accommodate readers with different perspectives. To examine differences in protein content or enzyme activity in one depot compared with another, the per-unit lipid expression is used. To examine the regulation of cellular FA storage, the data per 1000 adipocytes expression are considered. The calculations have been described in detail elsewhere (4, 28). Data that were not normally distributed according to the Shapiro-Wilks test were transformed to fit a normal distribution. Between-group comparison was assessed by one-way ANOVA. Mixed-model ANOVAs were used to determine group differences using time and depot as within-group variables followed by post hoc least-squares means tests. A Spearman rank test was used to determine the relationships between FFA storage and adipogenic proteins. Multivariate regression was conducted to examine the effects of palmitate concentrations on the relationships between ACS and DGAT and palmitate storage. All data are presented as mean ± standard error of the mean (SEM) and were analyzed using JMP Pro 12 (SAS Institute, Cary, NC). Statistical significance was defined as P < 0.05.
Results
Subject characteristics
Participant characteristics are provided in Tables 1 and 2. A total of 32 men were randomized into the study. The food frequency questionnaire data did not show any group × time interactions. The L+T group had more upper-body subcutaneous fat (UBSQ) (P = 0.02) than the Lupron group at week 1, but the difference was no longer significant at week 7 (Table 1).
Table 1.
Body Composition Before (Week 1) and After (Week 7) Treatment in the Lupron and L+T Group
| L+T (n = 9) |
Lupron (n = 12) |
|||
|---|---|---|---|---|
| Week 1 | Week 7 | Week 1 | Week 7 | |
| Weight (kg) | 93.5 ± 4.0 | 93.8 ± 44 | 82.3 ± 3.7 | 83.1 ± 3.6 |
| BMI (kg/m2) | 27.6 ± 0.7 | 27.6 ± 0.8 | 26.0 ± 0.6 | 26.3 ± 0.6 |
| Fat (kg) | 24.6 ± 2.4 | 24.5 ± 2.6 | 19.9 ± 1.5 | 20.8 ± 1.5 |
| Fat-free mass (kg) | 68.1 ± 2.7 | 68.2 ± 2.7 | 61.8 ± 3.2 | 61.1 ± 3.0 |
| UBSQ (kg)a | 14.0 ± 1.4b | 13.7 ± 1.4 | 10.3 ± 0.7 | 10.9 ± 0.7 |
| LBSQ (kg) | 7.7 ± 0.8 | 7.6 ± 0.8 | 6.4 ± 0.5 | 6.6 ± 0.5 |
| Visceral fat (kg) | 3.3 ± 0.6 | 3.2 ± 0.6 | 3.2 ± 0.5 | 3.3 ± 0.5 |
Values are mean ± SEM.
P < 0.05 for group × time interaction.
P < 0.05 at time point between groups.
Table 2.
Subject Characteristics
| Control (n = 11) | L+T (n = 9) | Lupron (n = 12) | |
|---|---|---|---|
| Age | 29 ± 2 | 32 ± 3 | 33 ± 2 |
| Weight (kg) | 90.5 ± 3.4 | 93.8 ± 4.4 | 83.1 ± 3.6 |
| Height (m) | 1.80 ± 0.03 | 1.77 ± 0.03 | 1.84 ± 0.03 |
| BMI (kg/m2) | 28.1 ± 1.0 | 27.6 ± 0.8 | 26.3 ± 0.6 |
| Fat (%) | 26.1 ± 1.6 | 26.1 ± 2.2 | 25.6 ± 1.4 |
| Fat (kg) | 23.1 ± 1.7 | 24.5 ± 2.6 | 20.8 ± 1.5 |
| UBSQ (kg) | 11.9 ± 1.1 | 13.7 ± 1.4 | 10.9 ± 0.7 |
| LBSQ (kg) | 8.4 ± 0.6 | 7.6 ± 0.8 | 6.6 ± 0.5 |
| Visceral fat (kg) | 2.8 ± 0.5 | 3.2 ± 0.6 | 3.3 ± 0.5 |
| Abdominal fat cell size (μg lipid/cell) | 0.71 ± 0.08 | 0.71 ± 0.12 | 0.66 ± 0.06 |
| Femoral fat cell size (μg lipid/cell) | 0.76 ± 0.10 | 0.73 ± 0.12 | 0.80 ± 0.08 |
| Testosterone (ng/dL) | |||
| Week 0 | NA | 481 ± 63 | 532 ± 38a |
| Week 3 | NA | 607 ± 158 | 52 ± 7a |
| Week 7/Control | 506 ± 33b | 985 ± 17b | 20 ± 5a,b |
| Fasting insulin (μU/L) | 5.9 ± 1.0 | 5.6 ± 1.1 | 6.4 ± 1.4 |
| Fasting plasma glucose (mg/dL) | 85 ± 2 | 87 ± 2 | 87 ± 2 |
| Plasma epinephrine (pg/mL) | 19 ± 4 | 14 ± 3 | 21 ± 4 |
| Plasma norepinephrine (pg/mL) | 165 ± 12 | 142 ± 18 | 219 ± 42 |
| Plasma palmitate (μmol/L) | 108 ± 8 | 102 ± 8 | 104 ± 8 |
| Palmitate flux (μmol/min) | 110 ± 12 | 99 ± 11 | 80 ± 9 |
Values are mean ± SEM. Testosterone concentrations were not random variables and therefore not subject to statistical testing.
Abbreviations: NA, not applicable.
P < 0.05 across time.
P < 0.05 between groups.
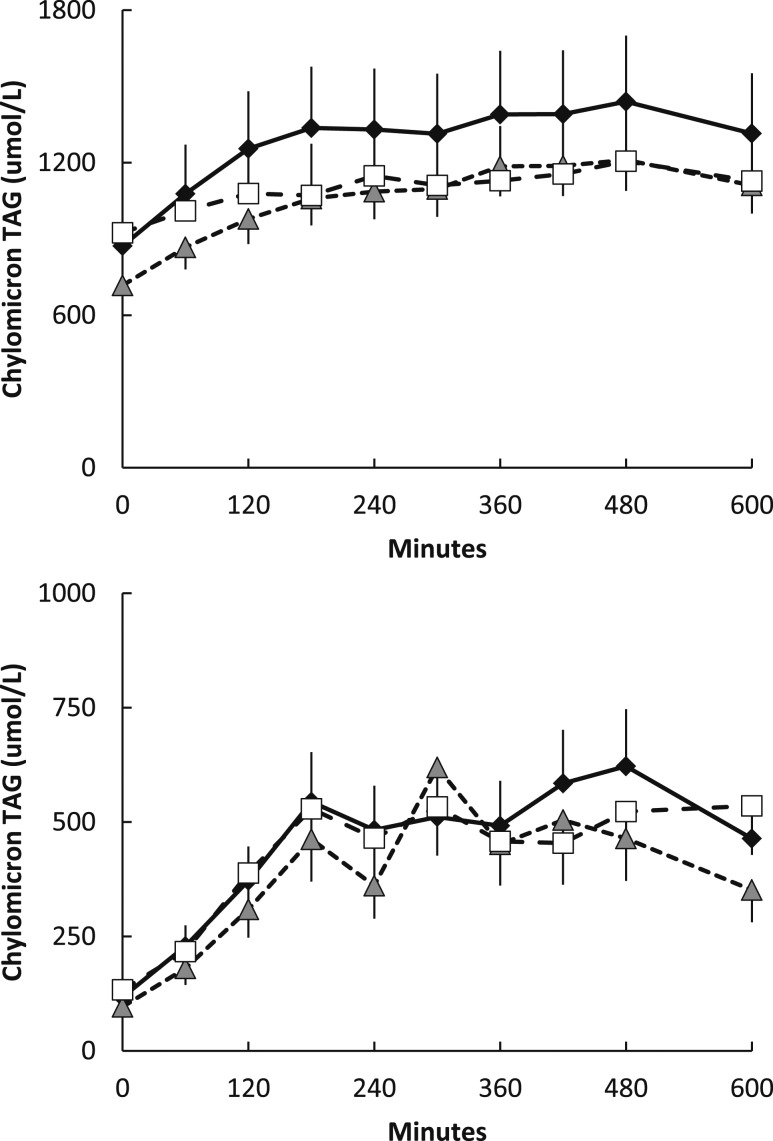
Based upon the assessments at the time of the FA storage studies, the three groups of men were well matched for age, body mass index (BMI), and body composition (Table 2). Concentrations of serum testosterone were greater in the L+T and control than the Lupron group by design. The average of the week-3 and week-7 serum testosterone concentrations in the L+T men was 726 ± 120 ng/dL. Baseline epinephrine, norepinephrine, insulin, and glucose were not different between groups. Postabsorptive plasma palmitate concentrations and flux were not different between groups (Table 2). There were also no differences in plasma chylomicron and nonchylomicron triglyceride concentrations (Fig. 1).
Figure 1.
Daytime plasma triglyceride concentrations. Plasma nonchylomicron (top) and chylomicron triglyceride concentrations (bottom) during the first 10 hours of the experimental meal day. Gray triangles indicate Lupron men, black diamonds indicate control men, and white squares indicate L+T men. There were no significant between-group differences in the area under the curve of the nonchylomicron and chylomicron triglyceride concentrations. TAG, triglyceride.
Substrate and meal FA oxidation
The total amount of FAs oxidized over the 6 hours after breakfast (indirect calorimetry) and the 24-hour meal FA oxidation (3H2O generation) were not different between groups (Table 3). Basal metabolic rate and respiratory exchange ratios after the overnight stay were not different between groups. Collectively, these findings indicate that the three groups were metabolically similar with respect to energy/substrate oxidation.
Table 3.
Energy, FA Metabolism, and Adipogenic Factors
| Control (n = 11) | L+T (n = 9) | Lupron (n = 12) | ||||
|---|---|---|---|---|---|---|
| REE (kcal/d) | 2074 ± 102 | 1984 ± 97 | 1932 ± 99 | |||
| Baseline respiratory exchange ratio | 0.81 ± 0.01 | 0.86 ± 0.05 | 0.79 ± 0.02 | |||
| 6-hour substrate oxidation | ||||||
| Carbohydrate (g) | 89 ± 8 | 89 ± 9 | 65 ± 12 | |||
| Fat (g) | 29 ± 4 | 28 ± 5 | 31 ± 4 | |||
| Protein (g) | 39 ± 4 | 40 ± 4 | 41 ± 4 | |||
| 24-hour meal FA oxidation | ||||||
| (g) | 10.0 ± 0.7 | 8.8 ± 0.8 | 10.6 ± 1.3 | |||
| (%) | 41 ± 3 | 42 ± 5 | 36 ± 3 | |||
| Abdominal | Femoral | Abdominal | Femoral | Abdominal | Femoral | |
| 24-hour meal FA storagea (mg × g lipid–1) | 0.70 ± 0.14b,c | 0.38 ± 0.08c | 0.44 ± 0.09c | 0.32 ± 0.06c | 0.72 ± 0.10c | 0.74 ± 0.07c |
| Rate of direct FFA-palmitate storage | ||||||
| (μmol × kg adipose tissue–1 × min–1)a | 0.29 ± 0.05b | 0.19 ± 0.04 | 0.23 ± 0.03 | 0.18 ± 0.03 | 0.32 ± 0.09 | 0.32 ± 0.08 |
| ×10–4 (pmol × 1000 cells–1 × min–1) | 1.89 ± 0.35 | 1.36 ± 0.32 | 1.50 ± 0.25 | 1.36 ± 0.35 | 2.29 ± 0.78 | 2.28 ± 0.41 |
| Adipose tissue FA storage (%) | UBSQ | LBSQ | UBSQ | LBSQ | UBSQ | LBSQ |
| Meal FAa | 30 ± 4b | 12 ± 2c | 22 ± 4b | 10 ± 2c | 31 ± 3b | 19 ± 2c |
| Direct FFA-palmitate | 3.1 ± 0.5b | 1.5 ± 0.2 | 3.0 ± 0.3b | 1.4 ± 0.2 | 4.3 ± 0.8b | 2.5 ± 0.4 |
| LPL activity (μmol × g tissue–1 × h–1) | Abdominal | Femoral | Abdominal | Femoral | Abdominal | Femoral |
| Fasteda | 0.41 ± 0.06d | 0.45 ± 0.05c | 0.36 ± 0.07d | 0.42 ± 0.06c | 0.56 ± 0.10b,d | 0.95 ± 0.16c,d |
| Feda | 0.87 ± 0.13 | 0.67 ± 0.10c | 0.80 ± 0.16 | 0.76 ± 0.07c | 0.84 ± 0.09 | 1.53 ± 0.30c |
| Adipocyte factors | ||||||
| ACSa (pmol × mg lipid–1 × min–1) | 88 ± 13 | 76 ± 10c | 81 ± 15 | 95 ± 11c | 102 ± 7 | 119 ± 5c |
| DGAT (pmol × mg lipid–1 × min–1) | 11.2 ± 2.5 | 8.5 ± 1.6 | 7.5 ± 1.5 | 6.3 ± 1.1 | 12.7 ± 2.7 | 12.2 ± 1.9 |
| CD36 (relative units/mg lipid) | 13.5 ± 1.0 | 12.4 ± 0.8 | 11.0 ± 1.3 | 11.1 ± 1.8 | 14.3 ± 1.4 | 15.9 ± 1.7 |
| ACS (pmol × 1000 cells–1 × min–1) | 59 ± 8 | 54 ± 7c | 49 ± 5 | 72 ± 18c | 66 ± 8 | 94 ± 10c |
| DGAT (pmol × 1000 cells–1 × min–1) | 7.6 ± 1.8 | 5.8 ± 1.1 | 4.4 ± 0.4 | 4.1 ± 0.6 | 8.9 ± 2.4 | 8.9 ± 1.1 |
| CD36 (relative unit × 1000 cells–1) | 10.0 ± 1.5 | 9.3 ± 1.3 | 7.9 ± 1.7 | 8.4 ± 2.1 | 11.3 ± 1.8 | 13.4 ± 2.8 |
Values are mean ± SEM. Six-hour substrate oxidation refers to the indirect calorimetry data collected beginning just before and for 6 hours following the experimental breakfast meal. Twenty-four-hour meal FA storage and oxidation refers to isotope-measured 3H-triolein disposal into adipose tissue (as assessed by biopsies and generation of 3H2O) 24 hours after consumption of the experimental breakfast meal. Direct adipose FFA-palmitate storage rates are based on isotope dilution measures using adipose biopsies and palmitate kinetics.
P < 0.05 for group × depot interaction.
P < 0.05 between depots within a group.
P < 0.05 within depot between groups.
P < 0.05 across time (i.e., fasted vs fed).
Regional meal and direct FA (palmitate) storage
There was a group × depot (P = 0.01) interaction indicating that the differences between the abdominal and femoral depot were different between groups (Table 3). Meal FA storage (mg meal fat × g adipose lipid–1) was greater (P = 0.0006) in the abdominal than femoral region in the control group and approximately 35% greater (P = 0.10) in abdominal than femoral fat in the L+T group as well. In contrast, meal FA storage was nearly identical in abdominal and femoral fat in the Lupron group, and femoral adipose meal FA storage in the Lupron group was over double that in the control and L+T groups. In abdominal fat, meal FA storage was greater (P = 0.03) in the Lupron than the L+T group but not different from the control group. With regards to the proportion of meal FA stored, there was a group × depot (P = 0.04) interaction indicating that the difference in the proportion of meal FA stored in UBSQ vs lower-body subcutaneous fat (LBSQ) was different between groups. The proportion of meal FA stored in LBSQ in the Lupron group was greater (P = 0.02 and 0.0005, respectively) than in the control and L+T groups. The proportion of meal FA stored in UBSQ was greater (P < 0.001 for all) than in LBSQ for all three groups.
Rates of FFA palmitate storage are presented as µmol × kg adipose tissue–1 × min–1 for physiological interpretation and pmol × 1000 cells–1 × min–1 to examine mechanisms at the cellular level. There was a group × depot interaction (P = 0.03) indicating that the differences in the rate of direct palmitate FFA storage (µmol × kg adipose tissue–1 × min–1) in the abdominal vs femoral region was different between groups (Table 3). The rates of direct palmitate FFA storage (µmol × kg adipose tissue–1 × min–1) were greater (P = 0.001 and 0.055, respectively) in the abdominal vs femoral region in the control and L+T groups but not the Lupron group, which had nearly identical rates of storage in the two regions. There was no effect of group, depot, or group × depot on rates of direct FFA storage at the cellular level (pmol × 1000 cells–1 × min–1). There was an effect of depot (P < 0.0001) on the proportion of direct palmitate FFA storage indicating greater abdominal vs femoral storage for all groups.
Adipose-specific effectors of FA storage
LPL activity
There was a group × depot interaction (P = 0.03) for fasting LPL activity. In the fasting state, LPL activity in the femoral region of the Lupron group was more than double that of the control and L+T groups. Femoral adipose tissue fasting LPL activity in the Lupron group was almost twice (P = 0.0002) that in the abdominal region (Table 3), whereas fasting LPL activity was not different between abdominal and femoral fat in the control and L+T groups. Fasting femoral LPL activity was greater (P < 0.001 for both) in the Lupron than the control and L+T groups. There was also a group × depot interaction (P = 0.01) for fed-state LPL activity. Although there was no depot effect (P = 0.26), the significant group × depot interaction indicates that the difference in fed LPL activity between the depots was significant between groups. In both the control and L+T groups, fed LPL activity was higher in abdominal than femoral fat, whereas in the Lupron group, fed LPL activity was greater in the femoral than abdominal fat. Additionally, fed femoral LPL activity in the Lupron group was approximately double (P = 0.0005 and 0.003, respectively) that in the control and L+T groups. Whereas LPL activity in the abdominal region increased (P < 0.001 for all) from the fasting to fed state in all groups, in the femoral region, the LPL activity increase from the fasting to fed state was significant (P = 0.003) in the Lupron group only.
LPL activity and meal FA storage
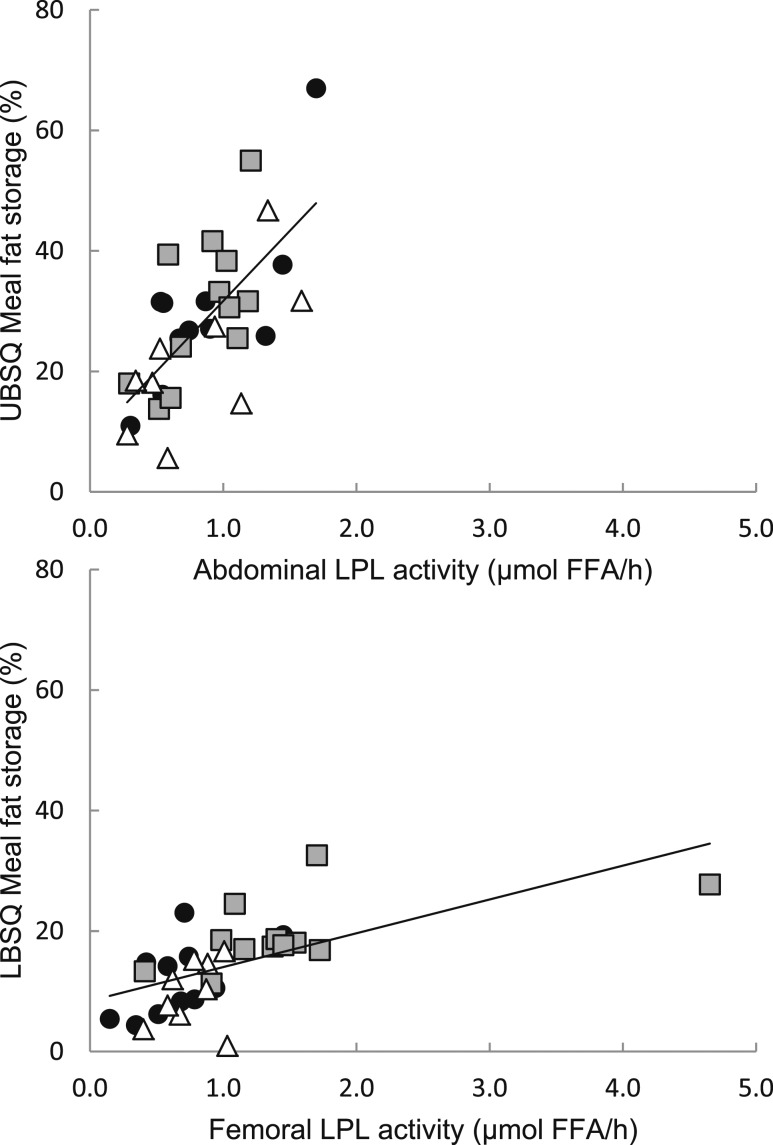
For both the abdominal and femoral fat, fed LPL activity and the proportion of meal FA stored were significantly correlated in the three groups combined (Fig. 2; ρ = 0.68, P < 0.0001 and ρ = 0.72, P < 0.0001, respectively).
Figure 2.
Regional LPL activity vs regional meal FA storage. Fed LPL activity in the abdominal (top) and femoral (bottom) is plotted vs the proportion of meal FAs stored in UBSQ and LBSQ adipose tissue. Gray squares indicate Lupron men, black circles indicate control men, and white triangles indicate L+T men. For the three groups combined, there were significant correlations between fed LPL activity and the proportion of meal FA stored in both the abdominal and femoral depot (ρ = 0.68, P < 0.0001 and ρ = 0.72, P < 0.0001, respectively).
Adipocyte FA storage factors
At the cellular level, femoral ACS activity was greater (P = 0.002 and 0.04, respectively) in the Lupron group than in the control and L+T groups. At the physiological level (per mg lipid), there was a group × depot interaction (P = 0.04). There was also a group effect (P = 0.04) where femoral ACS activity was greater (P = 0.002) in the Lupron than the control group. There was no group × depot, group, or depot effect in DGAT activity or CD36 at either the cellular or physiological level.
Adipocyte proteins vs direct FFA palmitate storage rates
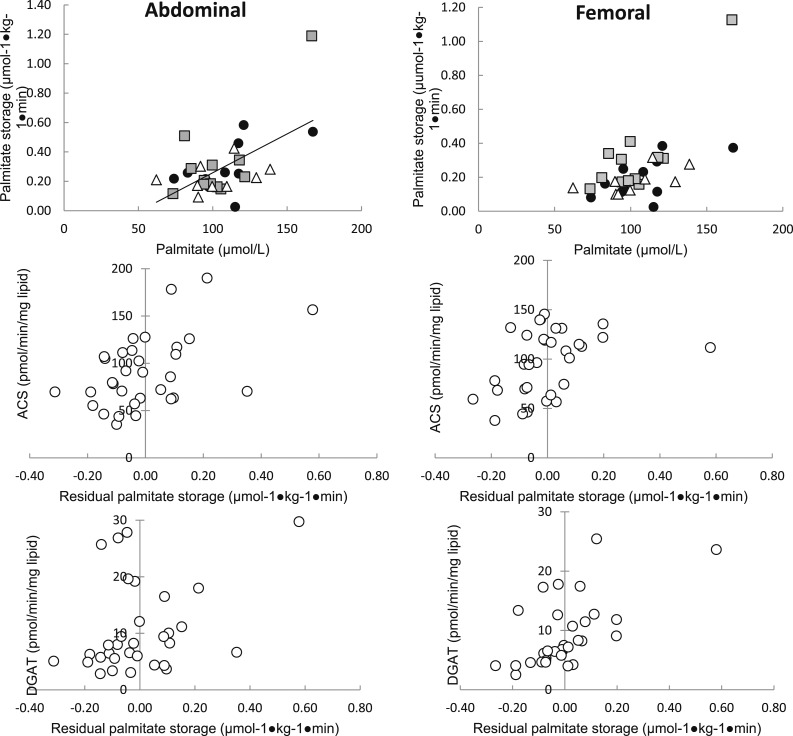
Consistent with our previous observations, palmitate concentrations were the best predictor of palmitate storage rates in abdominal [r2 = 0.35, P = 0.0003; Fig. 3(left upper panel)] and femoral [r2 = 0.38, P < 0.0002; Fig. 3(right upper panel)] fat for the overall group. If ACS and DGAT activities were included in a multivariate regression with palmitate concentrations, they were predictive of storage, independent of palmitate. To better depict this effect, the association between the residual variance in adipose tissue palmitate storage and palmitate concentration is plotted vs abdominal and femoral ACS [Fig. 3(left middle panel) and 3(right middle panel)] and abdominal and femoral DGAT [Fig. 3(left lower panel) and 3(right lower panel)]. Neither abdominal nor femoral adipose tissue CD36 content were significant contributors to predicting palmitate storage using multivariate regression analysis when added to plasma palmitate concentrations.
Figure 3.
Relationships between abdominal and femoral adipose tissue FA storage factors vs direct FFA storage rates expressed relative to tissue. Regional rates of FFA storage (µmol × kg fat–1 × min–1) vs lipogenic protein activity expressed per mg lipid/min. Gray squares indicate Lupron men, black circles indicate control men, and white triangles indicate L+T men. The line represents a relationship between activity and rate of FFA storage for all participants. The activity of ACS and DGAT was significantly correlated with the rate of FA storage in the femoral region of women in the Lupron group (r = 0.72, P = 0.008 and r = 0.75, P = 0.005, respectively) but not control or L+T groups. No associations were found between adipocyte proteins (per mg lipid) and rate of FA storage (per kg adipose tissue) in the abdominal region in any group.
Relationship between adipocyte FA storage factors
In the three groups combined, we found relationships between abdominal and femoral ACS and DGAT activity (per mg lipid) (ρ = 0.78, P < 0.0001 and ρ = 0.43, P = 0.01, respectively). In the individual groups, abdominal ACS and DGAT activity was correlated (ρ = 0.68, P = 0.02; ρ = 0.85, P = 0.004; and ρ = 0.74, P = 0.006) in the control, L+T, and Lupron groups. Abdominal ACS and CD36 were also correlated (ρ = 0.68, P = 0.02) in the control group. Femoral ACS and DGAT activity was correlated (ρ = 0.75, P = 0.009) in the control group only.
Discussion
The underlying mechanisms by which testosterone affects body fat distribution are incompletely understood. To our knowledge, this is the first study that examines the effects of acute suppression of testosterone on both measured adipose tissue FA storage as well as some of the underlying cellular mechanisms. By randomizing healthy men to either a control group, a Lupron group (acute testosterone deficiency), or L+T (testosterone-replete) group, we could study the effects of short-term sex steroid suppression. Our results support the notion that testosterone withdrawal, and by inference, testosterone itself, has short/intermediate acting effects, most prominently on lower-body adipose tissue FA storage. We found that men with acute testosterone deficiency had sufficiently high meal FA storage and direct FFA storage rates in femoral fat so as to eliminate the “normal” abdominal > femoral FA storage patterns observed in testosterone-sufficient groups (control and L+T). Interestingly, fasting and fed LPL activity was greatest in the femoral region of hypogonadal men, as was ACS activity. Collectively, these findings suggest that testosterone may act as a tonic suppressor of femoral LPL and ACS activity, which serves to limit local FA storage.
Although we observed lesser fat oxidation in chronically hypogonadal than eugonadal men (4), in this study, we found no evidence of differences in fat oxidation between the three groups. Other longer-term testosterone suppression studies have found reduced fat oxidation in men (29, 30). Thus, it appears that the effects of testosterone on fat oxidation occur over periods of time longer than those that modulate lower-body adipose tissue FA storage. This may represent testosterone’s effects on skeletal muscle FA metabolism.
Our findings of greater meal FA and direct FFA storage in femoral fat with acute testosterone deficiency are consistent with what we observed with chronic testosterone deficiency (4). In a way, these results are a mirror image of the finding that testosterone supplementation to men with low biologically available testosterone concentrations increases meal FA storage in the abdominal vs femoral region (31). In light of these more recent findings, our previous result (31) may have been due to inhibitory effects of testosterone on femoral fat than positive effects on abdominal subcutaneous fat.
We also investigated the effects of acute testosterone suppression on adipocyte storage factors. Based on our results, it appears that ACS, DGAT, and palmitate concentrations contribute coordinately to direct FFA storage. Acute testosterone suppression modulates this relationship, especially in the femoral depot. At both the cellular and physiological level, femoral ACS activity in the testosterone-deficient men was greater than that in the men from the testosterone-sufficient groups. We also found greater femoral ACS in chronically hypogonadal men (4). These combined data more strongly suggest that the greater ACS activity with acute or chronic testosterone deficiency accounts for greater femoral adipose tissue FFA storage.
A major strength of this study is that we had a testosterone-replete (L+T) group as a positive control so that we could assure any effects of acute hypogonadism using Lupron were indeed due to testosterone suppression. Because the results for the L+T and control groups were the same, it’s very likely that the main effect was that of testosterone suppression, even though Lupron suppresses other sex hormones in men. However, we acknowledge that our study addresses only the effects of acute and profound testosterone withdrawal; we don’t know the dose response or threshold effect of testosterone. In addition, the effects observed in this study might be different from that which occurs in response to the milder and more gradual decline of testosterone often occurring in aging men. We didn’t measure sex hormone binding globulin (SHBG) in these volunteers, and changes in SHBG could affect the relationship between total and free testosterone. However, previous studies that have measured SHBG in normal-weight men given gonadatropin releasing hormone agonist treatment found no significant change in the concentration after 24 weeks (32, 33). Thus, we suspect that there were no meaningful changes in SHBG in our volunteers.
In summary, acute testosterone deficiency in men increases femoral adipose tissue FA storage, probably by allowing greater femoral adipose LPL and ACS activities. The changes occur before changes in body fat, metabolic rate, or fat oxidation can be observed. As such, our findings suggest these pathways are proximally involved in some of the changes in fat storage with declining testosterone concentrations in males. It would be of interest to understand whether the changes in adipose enzymes/fat storage with aging are similar to those we observed, as well as whether estrogen plays any role in this process, perhaps by studies using an aromatase inhibitor. Finally, it will be important to understand whether adipose tissue FA storage pathways in visceral fat in men are also sensitive to changes in testosterone concentrations.
Acknowledgments
We thank the research volunteers for their participation, Barbara Norby and Carley Vrieze for assistance with nursing care and adipose biopsies, and Christy Allred, Debra Harteneck, Darlene Lucas, and Lendia Zhou from the Mayo Clinic for assay development and performance.
Acknowledgments
This work was supported by National Center for Research Resources 1UL1 RR024150, National Institutes of Health Grants DK45343, DK40484, and DK50456, American Diabetes Association Grant 7-06-DCS-03, the Natural Sciences and Engineering Research Council of Canada (to S.S.), and the Canadian Diabetes Association (to S.S.).
Author contributions: S.S. contributed to study design, performed the studies, researched data, and wrote the manuscript. N.C.B. performed the studies, researched data. and edited the manuscript. M.D.J. designed and oversaw the study, reviewed the data, edited the manuscript, and is the guarantor of the work.
Clinical trial registry: ClinicalTrials.gov no. NCT01160328 (registered 8 July 2010).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACS
- acyl coenzyme A synthetase
- ANOVA
- analysis of variance
- BMI
- body mass index
- DGAT
- diacylglycerol acyltransferase
- FA
- fatty acid
- FFA
- free fatty acid
- L+T
- Lupron and testosterone
- LBSQ
- lower-body subcutaneous fat
- LPL
- lipoprotein lipase
- REE
- resting energy expenditure
- SEM
- standard error of the mean
- SHBG
- sex hormone binding globulin
- UBSQ
- upper-body subcutaneous fat.
References
- 1.Wells JCK. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21(3):415–430. [DOI] [PubMed] [Google Scholar]
- 2.Berenguer B, de la Cruz L, de la Plaza R. The role of lipoaspiration in defeminization of Klinefelter syndrome: a case report. Ann Plast Surg. 1999;43(3):306–308. [DOI] [PubMed] [Google Scholar]
- 3.Pomerantz HZ, Sheiner N. Myocardial infarction associated with hypercholesterolaemia in a young eunuch. Can Med Assoc J. 1959;80(5):362–365. [PMC free article] [PubMed] [Google Scholar]
- 4.Santosa S, Jensen MD. Effects of male hypogonadism on regional adipose tissue fatty acid storage and lipogenic proteins. PLoS One. 2012;7(2):e31473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santosa S, Jensen MD. Why are we shaped differently, and why does it matter? Am J Physiol Endocrinol Metab. 2008;295(3):E531–E535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol (Lausanne). 2015;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mårin P, Odén B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80(1):239–243. [DOI] [PubMed] [Google Scholar]
- 8.Mårin P. Testosterone and regional fat distribution. Obes Res. 1995;3(Suppl 4):609S–612S. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 1999;48(12):2255–2269. [DOI] [PubMed] [Google Scholar]
- 10.Sfeir Z, Ibrahimi A, Amri E, Grimaldi P, Abumrad N. Regulation of FAT/CD36 gene expression: further evidence in support of a role of the protein in fatty acid binding/transport. Prostaglandins Leukot Essent Fatty Acids. 1997;57(1):17–21. [DOI] [PubMed] [Google Scholar]
- 11.Mashek DG, Li LO, Coleman RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007;2(4):465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen C-LE, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49(11):2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity (Silver Spring). 2009;17(6):1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes. 2011;60(8):2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab. 2000;279(2):E455–E462. [DOI] [PubMed] [Google Scholar]
- 16.Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ III, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19(10):1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen MD, Kanaley JA, Roust LR, O’Brien PC, Braun JS, Dunn WL, Wahner HW. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68(9):867–873. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61(2):274–278. [DOI] [PubMed] [Google Scholar]
- 19.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. [DOI] [PubMed] [Google Scholar]
- 20.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in human adipose tissue: technical and experimental design issues. Am J Physiol Endocrinol Metab. 2000;279(2):E447–E454. [DOI] [PubMed] [Google Scholar]
- 21.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51(9):2761–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56(5):1369–1375. [DOI] [PubMed] [Google Scholar]
- 23.Mårin P, Rebuffé-Scrive M, Björntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest. 1990;20(2):158–165. [DOI] [PubMed] [Google Scholar]
- 24.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res. 2003;44(9):1795–1801. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976;17(5):536–541. [PubMed] [Google Scholar]
- 26.Hall AM, Smith AJ, Bernlohr DA. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem. 2003;278(44):43008–43013. [DOI] [PubMed] [Google Scholar]
- 27.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab. 2005;288(3):E547–E555. [DOI] [PubMed] [Google Scholar]
- 28.Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol. 1949;2(1):1–15. [DOI] [PubMed] [Google Scholar]
- 29.Høst C, Gormsen LC, Christensen B, Jessen N, Hougaard DM, Christiansen JS, Pedersen SB, Jensen MD, Nielsen S, Gravholt CH. Independent effects of testosterone on lipid oxidation and VLDL-TG production: a randomized, double-blind, placebo-controlled, crossover study. Diabetes. 2013;62(5):1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83(6):1886–1892. [DOI] [PubMed] [Google Scholar]
- 31.Koutsari C, Ali AH, Nair KS, Rizza RA, O’Brien P, Khosla S, Jensen MD. Fatty acid metabolism in the elderly: effects of dehydroepiandrosterone and testosterone replacement in hormonally deficient men and women. J Clin Endocrinol Metab. 2009;94(9):3414–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eri LM, Haug E, Tveter KJ. Effects on the endocrine system of long-term treatment with the luteinizing hormone-releasing hormone agonist leuprolide in patients with benign prostatic hyperplasia. Scand J Clin Lab Invest. 1996;56(4):319–325. [DOI] [PubMed] [Google Scholar]
- 33.Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13(1):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]