Abstract
Objectives:
We aimed to compare the associations of directly measured plasma free 25-hydroxyvitamin D [25(OH)D] and total 25(OH)D concentrations with insulin sensitivity (SI) and β-cell function in nondiabetic Hispanics and African Americans. We hypothesized that directly measured free 25(OH)D would be more strongly associated with these measures of glucose homeostasis and that associations would differ by race.
Design:
We studied 1189 nondiabetic participants in the Insulin Resistance Atherosclerosis Study Family Study using data from baseline examinations from 2000 to 2002. SI, acute insulin response, and disposition index (DI) were determined from frequently sampled intravenous glucose tolerance tests. Plasma free and total 25(OH)D concentrations were measured by enzyme-linked immunosorbent assay and radioimmunoassay, respectively.
Results:
The median concentrations of plasma free 25(OH)D were 3.46 pg/mL for Hispanics and 2.17 pg/mL for African Americans (P < 0.0001), whereas the median concentrations of plasma total 25(OH)D were 16 ng/mL for Hispanics and 10 ng/mL for African Americans (P < 0.0001). Plasma free and total 25(OH)D were both positively associated with SI and DI in generalized estimating equations adjusted for demographic and lifestyle factors. After further adjustment with body mass index, the associations were no longer statistically significant, except for a significant association between plasma free 25(OH)D and SI. There was no effect modification by ethnicity on any of the exposure-outcome associations.
Conclusions:
Our data showed that plasma free 25(OH)D had a slightly stronger association with SI compared with plasma total 25(OH)D, although the difference was modest and there were no marked differences in the associations between Hispanics and African Americans.
A cross-sectional analysis showed a slightly stronger association of directly measured plasma free 25(OH)D with detailed measures of insulin sensitivity compared with plasma total 25(OH)D.
It is well established that insulin resistance and pancreatic β-cell dysfunction are central pathophysiological mechanisms underlying type 2 diabetes (T2DM). Experimental evidence in animal models and cell lines has suggested that vitamin D metabolites may exert beneficial effects on β-cell function and insulin sensitivity (SI) through a variety of mechanisms, subsequently modifying risk for T2DM development (1). However, epidemiologic research on the association between plasma total 25-hydroxyvitamin D [25(OH)D] and incident T2DM has been inconsistent. Although meta-analyses of prospective cohort studies have reported significant associations of low plasma total 25(OH)D concentrations with incident T2DM (2, 3), findings have been less consistent among African Americans (4), a phenomenon that may be due to lower plasma concentrations of total 25(OH)D in this population (5). In addition, randomized controlled trials testing the efficacy of vitamin D supplementation on improving SI or β-cell function, or preventing T2DM, have been highly inconsistent to date (6).
A large proportion of 25(OH)D in the circulation is bound to vitamin D–binding protein and albumin, with a very small fraction circulating in the unbound, or free, form (7). Under the free hormone hypothesis, it has been proposed that it is the free fraction of hormones that exerts biological activity (8). Indeed, emerging data from studies of bone mineral density have suggested that plasma free 25(OH)D may be a superior marker of vitamin D metabolism compared with measures of the total metabolite (9–11). In addition, a recent study reported similar plasma concentrations of free 25(OH)D in white and African American subjects, despite significantly lower plasma concentrations of total 25(OH)D in the latter group (12). These observations are of interest given inconsistencies in the literature on vitamin D in T2DM etiology, especially among African Americans. However, to our knowledge, the association of plasma free 25(OH)D with T2DM and its underlying abnormalities has not been reported. In addition, most previous studies have not directly measured free 25(OH)D, instead relying on estimates derived from published formulae that include total 25(OH)D, albumin, and vitamin D–binding protein (9–11).
In this context, we compared the associations of directly measured plasma free and total 25(OH)D concentrations with SI, acute insulin response (AIR), disposition index (DI), and homeostatic model assessment of insulin resistance (HOMA-IR). We used data from a large, well-characterized cohort of Hispanics and African Americans with direct measurements of insulin metabolism (secretion and sensitivity) and adipose tissue depots (visceral and subcutaneous) in the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. We hypothesized that plasma free 25(OH)D concentrations would show stronger associations with glucose homeostasis outcomes compared with plasma total 25(OH)D concentrations and that plasma free 25(OH)D would be particularly useful in understanding the vitamin D-T2DM link among African Americans.
Methods and Materials
Study population
This study used data from the IRAS Family Study, which recruited large families between 2000 and 2002 at study centers in San Antonio, Texas (Hispanics), San Luis Valley, Colorado (Hispanics), and Los Angeles, California (African Americans), with probands identified from the parent study (IRAS) and the general population. Details of the study population and methods have been published (13). The institutional review boards approved the study protocol, and all participants provided written informed consent. A total of 1856 participants attended baseline examinations. After excluding those with prevalent diabetes at baseline (n = 229) and those with missing values of directly measured plasma free 25(OH)D, total 25(OH)D, SI, AIR, DI, or HOMA-IR at baseline (n = 9), the sample size of the current report was 1189 participants.
Data collection
Demographic data (e.g., age, sex, and ethnicity) and lifestyle factors (e.g., smoking and alcohol consumption) were collected on standardized questionnaires by self-report (13). Physical activity was self-reported in a 1-year recall using a modified version of a validated instrument that incorporated activities common among participants in the IRAS Family Study. Total energy expenditure (in kcal/kg/y) was the sum of all activities at home, work, or leisure time, estimated energy expenditure from sleep, and estimated energy expenditure from light activities (13, 14). Season of blood draw was determined from the date of the baseline visit. Blood draws taken from December to May were categorized as winter/spring and those taken from June to November were categorized as summer/fall (15). Anthropometric (e.g., height, weight, and waist circumference) and blood pressure measurements were taken using standardized procedures. Body mass index (BMI) was calculated as weight in kg divided by height in m2 (13). Duplicate measurements were made, and averages were used in the analyses.
Biochemical analyses
Participants provided a fasting blood draw at each examination. Plasma insulin was measured by dextran-charcoal radioimmunoassay (16). Plasma glucose was determined on an auto-analyzer using the glucose oxidase method. Prevalent diabetes at baseline was defined as fasting glucose ≥126 mg/dL or use of antidiabetic medications by self-report.
Measurement of plasma 25(OH)D concentrations
Plasma free 25(OH)D was measured by a two-step enzyme-linked immunosorbent assay (DIASource, Louvain-La-Neuve, Belgium) in blood samples collected at baseline. During the first incubation step, free 25(OH)D binds to the monoclonal anti–vitamin D in the microtiter plate. The microtiter plate is then washed, and a fixed amount of biotinylated 25(OH) is added to allow it to react with the remaining antibody in the second incubation. This incubation is followed by another wash and quantitation of the absorbance, which is inversely proportional to the level of free 25(OH)D in the samples. The interassay coefficient of variation for plasma free 25(OH)D was 24%. Plasma total 25(OH)D was measured by a two-step procedure involving a rapid extraction of 25(OH)D and other hydroxylated metabolites from plasma and a radioimmunoassay with a 25(OH)D-specific antibody (DIASorin, Stillwater, MN) with an interassay coefficient of variation <8% (17).
Measurement of SI and insulin secretion
SI was determined using a frequently sampled intravenous glucose tolerance test, with two modifications to the original protocol (18). First, insulin, instead of tolbutamide, was injected to ensure adequate levels of plasma insulin for accurate calculation of SI across a broad range of glucose tolerance (19). Second, a reduced sampling protocol, using 12 instead of 30 samples, was used because of the large number of participants (20). SI, expressed as the SI, was calculated using minimal model analysis (21). HOMA-IR was calculated according to the method by Matthews et al. (22). Insulin secretion was measured by AIR, defined as the average increase in plasma insulin above the basal level in the first 8 minutes after infusing glucose (23). DI was defined as SI multiplied by AIR (24). DI differs from SI and HOMA-IR in that it is an integrated measure of insulin secretion that takes account of the background SI. Thus it is thought to be a more appropriate measure of β-cell response.
Measurement of adipose tissue depots
Abdominal fat mass was measured by computed tomography using a standardized protocol at each clinical center. The computed tomography scans were read centrally at the University of Colorado Health Sciences Center, Department of Radiology, Bio-Imaging Research Laboratory. Images obtained at the L4/L5 vertebral regions were used to determine the area of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). Bowel fat was subtracted from the measurements of VAT (25, 26).
Statistical analyses
We described the characteristics of participants at baseline (overall and separately by ethnicity), stratified by thirds of the distribution of plasma free 25(OH)D, using medians and interquartile ranges for continuous variables and percentages for categorical variables. We used analysis of variance, Kruskal-Wallis tests, and χ2 tests to determine whether continuous and categorical variables differed across the distribution of plasma free 25(OH)D. We used Spearman correlation to determine the relationship between plasma free and total 25(OH)D concentrations.
Because the distributions of SI, AIR, and DI were skewed, we applied the following transformations to achieve normality for subsequent analyses: signed square root of AIR and DI and natural log-transformation of SI. Because of the presence of 0 values for SI, we added a constant of 1 to all values of SI before the log-transformation. We used unadjusted and multivariable-adjusted generalized estimating equations (GEEs), which accounted for the family structure of the cohort, to examine the cross-sectional associations of plasma free and total 25(OH)D with SI, AIR, DI, and HOMA-IR.
We presented the regression coefficients (β) for GEE along with their 95% confidence intervals (CIs) per 1 standard deviation (SD) increase in plasma free or total 25(OH)D. We included covariates in multivariable-adjusted models if they were associated with both the exposure and the outcome, or if there was a priori clinical relevance. Potential confounders included age, sex, ethnicity, smoking, season of blood draw, and BMI. We tested for effect modification by ethnicity and BMI on the associations of plasma free and total 25(OH)D on the outcomes of SI, AIR, DI, and HOMA-IR. We used the quasi-likelihood under the independence model criterion (QIC) program in STATA to determine whether plasma free 25(OH)D or total 25(OH)D was a better model fit for the outcomes. The lower the QIC value, the better the model fit. A P value of <0.05 for the interaction term suggested a significant effect modification.
In sensitivity analyses, we adjusted for VAT, SAT, or waist circumference instead of BMI in GEE models to explore whether there was any difference in the magnitudes of association after considering different adiposity measures as covariates. We also additionally adjusted for fasting blood glucose to determine its impact on the overall association in all outcomes. All statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX).
Results
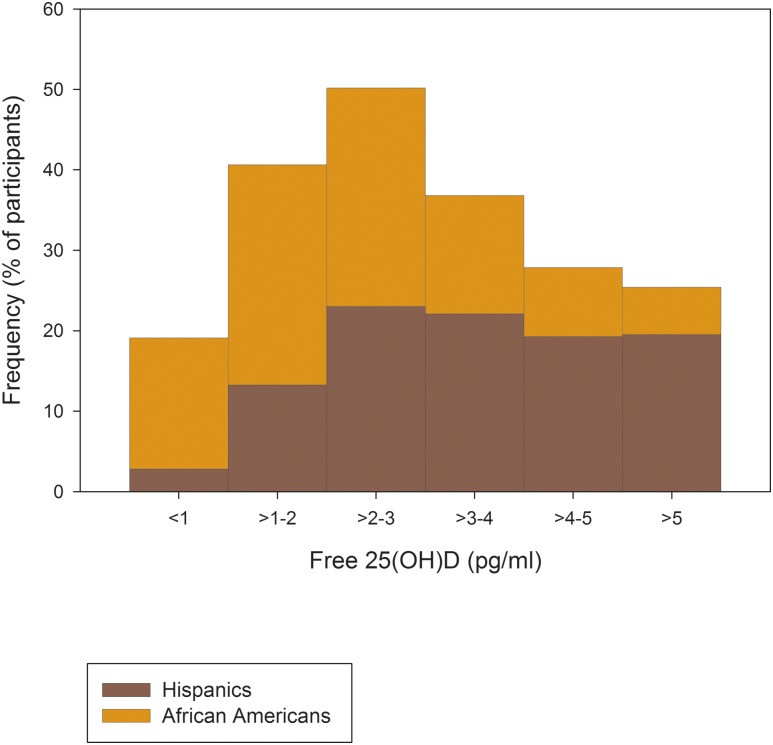
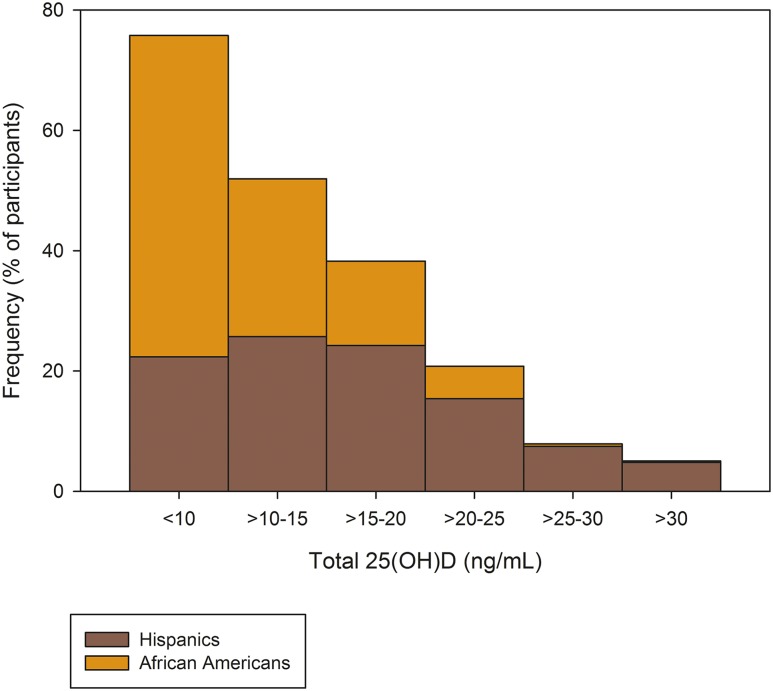
Of the 1189 nondiabetic participants in this analysis, 63% were Hispanics (n = 747), 37% were African Americans (n = 442), and, overall, 57% were women. The median age of these participants was 39 years (interquartile range, 30 to 47 years). The median concentrations of plasma free 25(OH)D were 2.98 pg/mL (interquartile range, 1.93 to 4.21 pg/mL) for the entire cohort, 3.46 pg/mL (interquartile range, 2.45 to 4.71 pg/mL) for Hispanics, and 2.17 pg/mL (interquartile range, 1.43 to 3.23 pg/mL) for African Americans (ethnic difference, P < 0.0001). The median concentrations of plasma total 25(OH)D were 13 ng/mL (interquartile range, 9 to 19 ng/mL) for the entire cohort, 16 ng/mL (interquartile range, 11 to 21 ng/mL) for Hispanics, and 10 ng/mL (interquartile range, 7 to 14 ng/mL) for African Americans (ethnic difference, P < 0.0001). The frequency distributions of plasma free and total 25(OH) vitamin D according to ethnicity are shown in Figs. 1 and 2, respectively.
Figure 1.
Distribution of plasma free 25(OH)D by ethnicity. Data in percentage of participants.
Figure 2.
Distribution of plasma total 25(OH)D by ethnicity. Data in percentage of participants.
Participants who had higher plasma levels of free 25(OH) vitamin D were more likely to be male and have lower BMI, waist circumference, and SAT and higher energy expenditure. These participants also had higher SI and DI as well as lower fasting blood glucose concentrations and HOMA-IR (Table 1). These patterns were largely similar when Hispanics and African Americans were examined separately (Supplemental Tables 1 and 2 (25.9KB, docx) ). Plasma free and total 25(OH)D were significantly correlated with each other in the whole cohort (ρ = 0.77, P < 0.0001) and by ethnicity (ρ = 0.70 for Hispanics and 0.75 for African Americans; both P < 0.0001). After adjusting for BMI, plasma free and total 25(OH)D remained significantly correlated with each other in the whole study population (ρ = 0.76, P < 0.0001).
Table 1.
Baseline Characteristics of Nondiabetic Participants in the IRAS Family Study Stratified by Thirds of the Distribution of Plasma Free 25(OH)D
| Thirds of Plasma Free 25(OH)D Distributiona |
P Value | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| n | 384 | 388 | 417 | |
| Free 25(OH)D (pg/mL) | 1.4 ± 0.55 | 2.9 ± 0.40 | 5.2 ± 1.4 | <0.0001 |
| Total 25(OH)D (ng/mL) | 8.5 ± 3.7 | 13.5 ± 4.6 | 21.2 ± 6.77 | <0.0001 |
| Age (y) | 38.9 ± 12.6 | 39.4 ± 13.1 | 40.3 ± 13.0 | 0.34 |
| Sex (% female) | 70.3 | 55.7 | 46.0 | <0.001 |
| Ethnicity (%) | ||||
| African Americans | 60.2 | 32.7 | 20.1 | |
| Hispanics | 39.8 | 67.3 | 79.9 | <0.001 |
| Seasons (%) | ||||
| Summer | 30.6 | 38.7 | 57.8 | |
| Winter | 69.4 | 61.3 | 42.2 | <0.001 |
| BMI (kg/m2) | 30.8 ± 7.1 | 28.7 ± 5.9 | 27.1 ± 4.9 | <0.0001 |
| Waist circumference (cm) | 92.2 ± 15.1 | 88.9 ± 13.7 | 86.0 ± 13.2 | <0.0001 |
| VAT area (cm2) | 97.6 ± 53.6 | 97.9 ± 56.6 | 92.9 ± 55.2 | 0.37 |
| SAT area (cm2) | 389 ± 185 | 339 ± 163 | 283 ± 136 | <0.0001 |
| Energy expenditure (kcal/kg/d) | 38.8 (35.6–46.7) | 40.7 (37.0–49.0) | 42.2 (37.5–51.1) | 0.0001 |
| SI (×104/min/µU/mL) | 1.4 (0.74–2.2) | 1.5 (0.80–2.5) | 1.9 (1.1–3.0) | 0.0001 |
| DI (SI × AIR) | 1028 (404–1820) | 1074 (441–1813) | 1156 (642–2073) | 0.0098 |
| AIR (pmol/mL/min) | 719 (417–1208) | 657 (398–1169) | 616 (355–1009) | 0.028 |
| HOMA-IR | 2.9 (1.9–4.7) | 2.8 (1.8–4.6) | 2.4 (1.6–3.7) | <0.0001 |
| Fasting blood glucose (mg/dL) | 94.4 ± 9.6 | 94.1 ± 9.6 | 92.8 ± 9.0 | 0.022 |
Data presented are means ± SD, medians (interquartile ranges), or percentages.
SI was positively correlated with plasma free and total 25(OH) vitamin D (ρ = 0.18 and 0.13, respectively), whereas indexes of adiposity [i.e., BMI (ρ = –0.22 and –0.19, respectively), waist circumference (ρ = –0.16 and –0.14, respectively), and SAT (ρ = –0.26 and –0.022, respectively)] were negatively correlated with plasma free and total 25(OH)D (all P < 0.0001). VAT was negatively correlated with plasma free 25(OH)D (ρ = –0.059, P < 0.05) but not with plasma total 25(OH)D (ρ = –0.022). DI was positively correlated with plasma free 25(OH)D (ρ = 0.092, P < 0.05) but not with plasma total 25(OH)D (ρ = 0.058). Both AIR and HOMA-IR were negatively correlated with plasma free 25(OH)D (ρ = –0.098 and –0.15, respectively) and total 25(OH)D (ρ = –0.065 and –0.11, respectively) (all P < 0.05) (Supplemental Table 3 (25.9KB, docx) ).
In unadjusted GEE models, plasma free 25(OH)D was positively associated with SI and negatively associated with HOMA-IR. The significant associations remained after adjusting for age, sex, ethnicity, smoking, energy expenditure, season of blood draw, and BMI. Plasma free 25(OH)D was positively associated with DI in unadjusted GEE models and after adjusting for demographic and lifestyle factors. The association was attenuated after adjustment of BMI but remained statistically significant. There was no association between plasma free 25(OH)D and AIR and SI-adjusted AIR in both unadjusted and multivariable-adjusted GEE models (Table 2).
Table 2.
Estimated Regression Coefficients (95% CIs) on the Association Between Plasma Free 25(OH)D and Components of Insulin Metabolism
| Standardized β-Coefficients (95% CI) per 1 SD Increase in Plasma Free 25(OH)D | P Value | |
|---|---|---|
| SI | ||
| Unadjusted | 0.19 (0.13, 0.25) | <0.001 |
| Adjusted forge, sex, ethnicity (model 1) | 0.19 (0.13, 0.25) | <0.001 |
| Model 1 + smoking, energy expenditure, season (model 2) | 0.21 (0.14, 0.27) | <0.001 |
| Model 2 + BMI | 0.082 (0.027, 0.14) | 0.003 |
| DI | ||
| Unadjusted | 0.12 (0.056, 0.17) | <0.001 |
| Adjusted for age, sex, ethnicity (model 1) | 0.15 (0.087, 0.21) | <0.001 |
| Model 1 + smoking, energy expenditure, season (model 2) | 0.076 (0.042, 0.11) | <0.001 |
| Model 2 + BMI | 0.039 (0.0055, 0.073) | 0.023 |
| AIR | ||
| Unadjusted | −0.056 (–0.12, 0.0029) | 0.062 |
| Adjusted for age, sex, ethnicity (model 1) | −0.035 (–0.095, 0.025) | 0.25 |
| Model 1 + smoking, energy expenditure, season (model 2) | −0.054 (–0.12, 0.077) | 0.086 |
| Model 2 + BMI | 0.00015 (–0.062, 0.062) | 1.0 |
| SI-adjusted AIR | ||
| Unadjusted | −0.0034 (–0.061, 0.054) | 0.91 |
| Adjusted for age, sex, ethnicity (model 1) | 0.029 (–0.029, 0.086) | 0.33 |
| Model 1 + smoking, energy expenditure, season (model 2) | 0.011 (–0.048, 0.070) | 0.73 |
| Model 2 + BMI | 0.022 (–0.039, 0.083) | 0.48 |
| HOMA-IR | ||
| Unadjusted | −0.16 (–0.22, –0.10) | <0.001 |
| Adjusted for age, sex, ethnicity (model 1) | −0.20 (–0.26, –0.14) | <0.001 |
| Model 1 + smoking, energy expenditure, season (model 2) | −0.20 (–0.26, –0.13) | <0.001 |
| Model 2 + BMI | −0.074 (–0.13, –0.016) | 0.013 |
Results presented per 1 SD (1.794) in plasma free 25(OH)D.
Plasma total 25(OH)D was positively associated with SI and DI and negatively associated with HOMA-IR in GEE models that were adjusted for demographic and lifestyle factors. Further adjustment of BMI attenuated the associations to nonsignificance. Plasma total 25(OH)D was not associated with AIR or SI-adjusted AIR (Table 3). The QIC values for plasma free 25(OH) and total 25(OH)D on SI were 782 and 792, suggesting that plasma free 25(OH)D model had a better fit for the outcome. We found a significant interaction with BMI on the association between free 25(OH)D and SI (P < 0.001 for interaction), with a significant association in the upper half of the BMI distribution [standardized β per 1 SD increase in free 25(OH)D, 0.096 (0.022, 0.17); P = 0.011] but not the lower half [0.047 (–0.018, 0.11), P = 0.16]. There was no significant interaction with BMI on the association of total 25(OH)D with SI. There was no effect modification by ethnicity on any of the exposure-outcome associations. In sensitivity analyses, replacing BMI in the fully adjusted models for alternate measures of adiposity, including waist circumference, VAT, or SAT, did not impact the results materially (Supplemental Tables 4 and 5 (25.9KB, docx) ). Additional adjustment for fasting blood glucose in multivariable-adjusted GEEs did not change the overall association for any of the outcomes.
Table 3.
Estimated Regression Coefficients (95% CIs) on the Association Between Plasma Total 25(OH)D and Components of Insulin Metabolism
| Standardized β-Coefficients (95% CI) per 1 SD Increase in Plasma Total 25(OH)D | P Value | |
|---|---|---|
| SI | ||
| Unadjusted | 0.14 (0.077, 0.20) | <0.001 |
| Adjusted for age, sex, ethnicity (model 1) | 0.14 (0.077, 0.20) | <0.001 |
| Model 1 + smoking, energy expenditure, season (model 2) | 0.15 (0.086, 0.22) | <0.001 |
| Model 2 + BMI | 0.031 (–0.026, 0.14) | 0.29 |
| DI | ||
| Unadjusted | 0.069 (0.0084, 0.13) | 0.026 |
| Adjusted for age, sex, ethnicity (model 1) | 0.016 (0.0073, 0.024) | <0.001 |
| Model 1 + smoking, energy expenditure, season (model 2) | 0.013 (0.0046, 0.022) | 0.003 |
| Model 2 + BMI | 0.052 (–0.0035, 0.014) | 0.24 |
| AIR | ||
| Unadjusted | −0.060 (–0.12, 0.00053) | 0.052 |
| Adjusted for age, sex, ethnicity (model 1) | −0.021 (–0.082, 0.041) | 0.51 |
| Model 1 + smoking, energy expenditure, season (model 2) | −0.045 (–0.11, 0.020) | 0.18 |
| Model 2 + BMI | 0.0081 (–0.058, 0.074) | 0.81 |
| SI-adjusted AIR | ||
| Unadjusted | –0.024 (–0.082, 0.06) | 0.43 |
| Adjusted for age, sex, ethnicity (model 1) | 0.025 (–0.034, 0.081) | 0.41 |
| Model 1 + smoking, energy expenditure, season (model 2) | 0.0013 (–0.061, 0.064) | 0.97 |
| Model 2 + BMI | 0.016 (–0.048, 0.081) | 0.62 |
| HOMA-IR | ||
| Unadjusted | −0.12 (–0.18, –0.061) | <0.001 |
| Adjusted for age, sex, ethnicity (model 1) | −0.15 (–0.22, –0.092) | <0.001 |
| Model 1 + smoking, energy expenditure, season (model 2) | −0.15 (–0.22, –0.083) | <0.001 |
| Model 2 + BMI | −0.033 (–0.094, 0.029) | 0.30 |
Results presented per 1 SD (7.396) in plasma total 25(OH)D.
Discussion
Recent studies have reported that free 25(OH)D levels are more strongly associated with measures of bone mineral density and metabolism than levels of total 25(OH)D (9–11), observations that are consistent with the free hormone hypothesis (8). In light of these findings, as well as the inconsistencies in published results regarding associations of total 25(OH)D with diabetes-related traits (2, 3), especially among African Americans (4), who have been reported to have similar concentrations of free 25(OH)D as whites despite lower total 25(OH)D concentrations (12), our overarching hypothesis in the current study was that free 25(OH)D concentrations would be more strongly associated than total concentrations with SI and β-cell function. We addressed this hypothesis in a cohort of African Americans and Hispanics who had been extensively characterized, including direct assessment of SI and secretion from the frequently sampled intravenous glucose tolerance test. An additional unique aspect of this work was the use of a recently developed assay from DIASource, which allows for the direct measurement of free 25(OH)D concentrations (27). The majority of previous studies assessing the impact of free 25(OH)D have estimated concentrations of this form of the metabolite using published formulae (9–12).
Our results show that both free and total 25(OH)D concentrations were significantly associated with SI (SI and HOMA-IR) and β-cell function (DI) but not with insulin secretion (AIR) in models adjusted for age, sex, ethnicity, smoking, energy expenditure, and season. The associations with free 25(OH)D were slightly stronger in magnitude compared with total 25(OH)D, despite the larger coefficient of variation for the free 25(OH)D assay (Tables 2 and 3), and the model with free 25(OH)D had a lower QIC compared with a similar model containing total 25(OH)D. After further adjustment for BMI, only the association of free 25(OH)D with indices of SI (including SI, DI, and HOMA-IR) remained significant. The inverse correlation of total 25(OH)D with measures of adiposity may be due to the fact that vitamin D is sequestered in adipose tissues, making it less bio-available (28). Our findings are therefore modestly supportive of a stronger impact of free 25(OH)D on SI and β-cell dysfunction, independent of BMI, although additional studies are clearly required. In the current study, we observed that concentrations of both total and free 25(OH)D were significantly lower in African Americans vs Hispanics. The lower concentration of total 25(OH)D is consistent with the majority of previous studies of African Americans, in which vitamin D status, as measured using total 25(OH)D, has been documented to be generally poorer in this population (5). A recent study from Powe et al., using data from the HANDLS cohort (12), estimated free 25(OH)D concentrations using a published formula that incorporates concentrations of total 25(OH)D as well as levels of albumin and vitamin D–binding protein. The authors reported that free 25(OH)D levels were similar in whites and African Americans despite significantly lower concentrations of total 25(OH)D in the latter group (12). This study is not directly comparable to ours for a number of reasons, including differences in the composition of the study populations, most notably the inclusion of Hispanics in our study. In addition, the methods for measuring free 25(OH)D were different between the two studies. We used a recently developed assay that directly measures free 25(OH)D (27), whereas Powe et al. estimated free 25(OH)D concentrations using a published equation (12). Estimation of free 25(OH)D using these formulae has generated considerable discussion in the literature recently in light of uncertainties regarding the impact of different vitamin D–binding protein assays (monoclonal vs polyclonal), genetic variation in vitamin D–binding protein and its impact on binding affinities of the protein, and assumptions used in the calculations regarding vitamin D–binding protein affinity constants (29–34). In addition, the directly measured free 25(OH)D assay had a high coefficient of variation. It is clear that a substantial amount of additional research is needed to clarify optimal approaches for the measurement of free 25(OH)D.
The strengths of the IRAS Family Study include the well-characterized prospective cohort of Hispanics and African Americans, two ethnic groups that are at high risk of developing diabetes, the detailed measurements of SI, insulin secretion, and adiposity, and the availability of directly measured (vs calculated) plasma free 25(OH)D. Nevertheless, several potential limitations should also be considered. Without oral glucose tolerance tests, some individuals with elevated postprandial glucose levels may have been misclassified as not having diabetes. Plasma total 25(OH)D was measured by radioimmunoassay instead of the gold standard of liquid chromatography-tandem mass spectrometry. The low precision of the radioimmunoassay method may have impacted the magnitude of associations observed. In addition, the cross-sectional design of this study does not allow us to exclude the possibility of residual confounding. Finally, the results of this study may not be generalizable to other populations.
In conclusion, plasma free 25(OH)D was significantly associated with SI in the IRAS Family Study cohort. Both free and total 25(OH)D were low in African Americans based on direct measurement. Although the free form of plasma 25(OH)D appeared to have a modestly stronger association vs the total form of plasma 25(OH)D, additional research is needed to confirm this observation.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health Grants HL-60944-02, HL-61210-02, HL-61019-02, HL-60894, and HL-60931-02 and the Dairy Research Cluster Initiative (Dairy Farmers of Canada, Agriculture and Agri-Food Canada, the Canadian Dairy Network, and the Canadian Dairy Commission).
Author contributions: L.E.W. and S.M.H. designed the study. C.C.L. and A.J.H. wrote, reviewed, and edited the manuscript. All authors reviewed and edited the manuscript and contributed to discussion. A.J.H. is the guarantor of this study and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 25(OH)D
- 25-hydroxyvitamin D
- AIR
- acute insulin response
- BMI
- body mass index
- CI
- confidence interval
- DI
- disposition index
- GEE
- generalized estimating equation
- HOMA-IR
- homeostatic model assessment of insulin resistance
- IRAS
- Insulin Resistance Atherosclerosis Study
- QIC
- quasi-likelihood under the independence model criterion
- SAT
- subcutaneous adipose tissue
- SD
- standard deviation
- SI
- insulin sensitivity
- T2DM
- type 2 diabetes
- VAT
- visceral adipose tissue.
References
- 1.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):205–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59(2):381–391. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. [DOI] [PubMed] [Google Scholar]
- 8.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10(3):232–274. [DOI] [PubMed] [Google Scholar]
- 9.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almås B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest. 2014;74(3):177–183. [DOI] [PubMed] [Google Scholar]
- 12.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henkin L, Bergman RN, Bowden DW, Ellsworth DL, Haffner SM, Langefeld CD, Mitchell BD, Norris JM, Rewers M, Saad MF, Stamm E, Wagenknecht LE, Rich SS. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Ann Epidemiol. 2003;13(4):211–217. [DOI] [PubMed] [Google Scholar]
- 14.Sidney S, Jacobs DR Jr, Haskell WL, Armstrong MA, Dimicco A, Oberman A, Savage PJ, Slattery ML, Sternfeld B, Van Horn L. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133(12):1231–1245. [DOI] [PubMed] [Google Scholar]
- 15.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93(9):3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25(10):1375–1384. [DOI] [PubMed] [Google Scholar]
- 17.Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, Norris JM. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94(9):3306–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6(1):45–86. [DOI] [PubMed] [Google Scholar]
- 19.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71(6):1508–1518. [DOI] [PubMed] [Google Scholar]
- 20.Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes. 1993;42(2):250–256. [DOI] [PubMed] [Google Scholar]
- 21.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23(2):113–122. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 23.Hanley AJ, Wagenknecht LE, Norris JM, Bryer-Ash M, Chen YI, Anderson AM, Bergman R, Haffner SM. Insulin resistance, β cell dysfunction and visceral adiposity as predictors of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetologia. 2009;52(10):2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010;33(9):2098–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hairston KG, Vitolins MZ, Norris JM, Anderson AM, Hanley AJ, Wagenknecht LE. Lifestyle factors and 5-year abdominal fat accumulation in a minority cohort: the IRAS Family Study. Obesity (Silver Spring). 2012;20(2):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52(10):2490–2496. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D(3) in fat tissue. Endocrine. 2008;33(1):90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouillon R, van Baelen H, de Moor P. The measurement of the vitamin D-binding protein in human serum. J Clin Endocrinol Metab. 1977;45(2):225–231. [DOI] [PubMed] [Google Scholar]
- 30.Winters SJ, Chennubhatla R, Wang C, Miller JJ. Influence of obesity on vitamin D-binding protein and 25-hydroxy vitamin D levels in African American and white women. Metabolism. 2009;58(4):438–442. [DOI] [PubMed] [Google Scholar]
- 31.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47(4):753–756. [PubMed] [Google Scholar]
- 32.Bouillon R, van Baelen H, de Moor P. Comparative study of the affinity of the serum vitamin D-binding protein. J Steroid Biochem. 1980;13(9):1029–1034. [DOI] [PubMed] [Google Scholar]
- 33.Walsh PG, Haddad JG. “Rocket” immunoelectrophoresis assay of vitamin D-binding protein (Gc globulin) in human serum. Clin Chem. 1982;28(8):1781–1783. [PubMed] [Google Scholar]
- 34.Henderson CM, Lutsey PL, Misialek JR, Laha TJ, Selvin E, Eckfeldt JH, Hoofnagle AN. Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D-binding protein in blacks and whites. Clin Chem. 2016;62(1):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]