Abstract
Context:
Somatic mutations in the ubiquitin-specific protease 8 (USP8) gene have been recently identified as the most common genetic alteration in patients with Cushing disease (CD). However, the frequency of these mutations in the pediatric population has not been extensively assessed.
Objective:
We investigated the status of the USP8 gene at the somatic level in a cohort of pediatric patients with corticotroph adenomas.
Design and Methods:
The USP8 gene was fully sequenced in both germline and tumor DNA samples from 42 pediatric patients with CD. Clinical, biochemical, and imaging data were compared between patients with and without somatic USP8 mutations.
Results:
Five different USP8 mutations (three missense, one frameshift, and one in-frame deletion) were identified in 13 patients (31%), all of them located in exon 14 at the previously described mutational hotspot, affecting the 14-3-3 binding motif of the protein. Patients with somatic mutations were older at disease presentation [mean 5.1 ± 2.1 standard deviation (SD) vs 13.1 ± 3.6 years, P = 0.03]. Levels of urinary free cortisol, midnight serum cortisol, and adrenocorticotropic hormone, as well as tumor size and frequency of invasion of the cavernous sinus, were not significantly different between the two groups. However, patients harboring somatic USP8 mutations had a higher likelihood of recurrence compared with patients without mutations (46.2% vs 10.3%, P = 0.009).
Conclusion:
Somatic USP8 gene mutations are a common cause of pediatric CD. Patients harboring a somatic mutation had a higher likelihood of tumor recurrence, highlighting the potential importance of this molecular defect for the disease prognosis and the development of targeted therapeutic options.
Mutations in the USP8 gene have been identified in one third of patients from a cohort of 42 pediatric patients with Cushing disease, in association with increased likelihood of disease recurrence.
As in adults, adrenocorticotropic hormone (ACTH)-producing pituitary adenomas are the most common cause of endogenous hypercortisolemia in children, especially after the age of 7 years (1, 2). Childhood Cushing disease (CD) is characterized by deceleration of the growth velocity with concomitant weight gain, along with other classic clinical features of CD (skin changes such as facial plethora, striae, acne, hirsutism, and easy bruising, disturbed mood, decreased bone mineral density, myopathy, and weakness) (3–5). The treatment of choice for CD is transsphenoidal surgical resection (TSS) of the pituitary adenoma, with a success rate of 65% to >95%, depending on the treatment center (6–8). Patients with recurrence or failure to cure may be treated with repeat surgery, radiation, or medications (ketoconazole, metyrapone, mifepristone, or pasireotide).
The molecular background of the corticotroph adenomas has been largely unknown until recently. Mutations in genes that cause syndromes associated with pituitary adenomas, such as multiple endocrine neoplasia type 1 (MEN1), multiple endocrine neoplasia type 4 (CDKN1B), familial isolated pituitary adenoma (AIP), McCune–Albright syndrome (GNAS1), Carney complex (PRKAR1A), pituitary adenoma with paraganglioma and pheochromocytoma [3PAs-collectively the succinate dehydrogenase complex, subunits A, B, C and D genes (SDHx), and pituitary blastoma (DICER1), do not account for the majority of the corticotropinomas (9–11).
A recent study by Reincke et al. reported that somatic mutations of the ubiquitin-specific protease 8 (USP8; Ensembl: ENSG00000138592) gene are found in 40% of corticotroph adenomas (12). The USP8 gene codes for a protein with deubiquitinase activity that inhibits the lysosomal degradation of the epidermal growth factor receptor (EGFR) (13). The gain-of-function somatic mutations impair binding of the inhibitory 14-3-3 proteins, resulting in increased deubiquitination of EGFR and induction of proopiomelanocortin (POMC) transcription and ACTH secretion (12).
The detection of somatic USP8 gene mutations in corticotroph adenomas has been replicated by other groups, but only one study included pediatric patients (14, 15). A possibly lower frequency of this genetic defect in the pediatric population (17%) was suggested, but because of the small number of identified pediatric patients, their clinical and biochemical profile was not described separately from the adult population (15). We have enlarged this population, included the previously studied subgroup, and analyzed the frequency of somatic USP8 gene mutations in the largest cohort of pediatric patients with CD available to date.
Subjects and Methods
Subjects
We screened our cohort of pediatric patients with pathologically confirmed corticotroph adenomas who presented at the National Institutes of Health (NIH) Clinical Research Center between 1997 and 2015, to identify patients with CD who had undergone TSS and for whom germline DNA and tissue from the resected tumor were available for further studies. Of the 139 pediatric patients who were diagnosed with and treated for CD at the NIH during this time, we identified 42 patients for whom both blood and tumor samples were available for molecular studies. Twenty-four of the study subjects were part of the original cohort published by Perez-Rivas et al. (15). Their data have never before been analyzed separately. In addition, one patient was sequenced at the germline level in a previous study (16). For the patients who underwent repeat TSS for relapse of CD, we had available tumor samples only from one surgical intervention, and for that reason we were not able to compare the genetic profile of the initial and relapsed tumor.
The diagnosis of CD was based on previously described criteria based on elevated levels of urinary free cortisol (UFC) and midnight serum cortisol level (9). ACTH-dependent CD was further confirmed by basal plasma ACTH >30 pg/mL, suppression of serum cortisol >69% during a high-dose dexamethasone suppression test (dose: 120 μg/kg, maximum 8 mg), and increase of ACTH and cortisol levels by >35% and 20% compared with baseline, respectively, after corticotropin-releasing hormone administration (corticotropin-releasing hormone test). All patients underwent magnetic resonance imaging of the pituitary, and if a tumor was not visible on magnetic resonance imaging, bilateral inferior petrosal sinus sampling was performed. They all underwent TSS performed by a neurosurgeon with expertise in pituitary disorders, and ACTH-producing tumor was confirmed histologically after resection. Sociodemographic, clinical, imaging, and biochemical data before and after surgery were collected for all patients.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board approved this study. Written informed consent from the parents and assent from the minor patients were obtained at the time of the first visit (protocol 1997-CH-0076).
USP8 sequence analysis in peripheral and tumor DNA samples
DNA was extracted from peripheral blood leukocytes and from microdissected tumor tissues with the Quick-DNA™ Universal Kit, according to manufacturer protocols (Zymo, Irvine, CA). For the 24 patients who were previously included in the study by Perez-Rivas et al. (Supplemental Table 1 (1.1MB, docx) ), repeat DNA extraction was performed from microdissected sections of the tumor after confirmation that they originated from the adenoma (diffuse staining for ACTH, with breakdown of the reticulin) and not from the adjacent normal pituitary. The complete USP8 coding sequence of these blood and tumor samples was analyzed either by bidirectional Sanger sequencing (5 patients) or with whole exome sequencing (37 patients). All the variants identified by whole exome sequencing were confirmed with Sanger sequencing.
Fourteen patients, from the original cohort of 139 patients, were previously sequenced by Sanger sequencing for known genes related to CD (including MEN1, AIP, PRKAR1A, CDKN1B, and CDKN2C) (16). Of those 14 patients, just 1 had resected tissue available and was included in this study. This patient was positive for a MEN1 mutation (p.Arg4158) (16) (Supplemental Table 1 (1.1MB, docx) ). Sanger sequencing for candidate pituitary tumor–causing genes was not performed for the remaining patients. However, all 41 remaining patients included in this manuscript are part of an ongoing whole exome sequencing project, and they are negative for mutations in the aforementioned known genes and other known candidate genes (data not shown). Part of these data were recently published (17, 18)
Immunohistochemistry
Pituitary tissues from one patient bearing the USP8 mutation p.Ser718Pro and one patient with no USP8 mutation were used for immunostaining. The tissues were obtained during surgery, fixed in formalin, and embedded in paraffin, and sections (5 µm) were mounted onto 3-aminopropyl-triethoxylasine coated slides (Sigma Chemical Co., St. Louis, MO). Routine staining with hematoxylin and eosin (Histoserv Inc, Germantown, MD) was performed on several sections across each sample. Unstained slides were used for immunostaining as follows: paraffin-embedded tumor slides were deparaffinized with Histoclear (National Diagnostics, Atlanta, GA) and rehydrated with ethanol gradient. The epitopes were retrieved by boiling for 20 minutes in Antigen Unmasking Solution (pH 6; Vector Laboratories, Burlingame, CA). For double coimmunofluorescence with ACTH and USP8, USP8 was visualized with rabbit anti-USP8 (sc-376130; Santa Cruz Biotechnology, Dallas, TX), which was detected with antirabbit secondary antibody coupled to biotin (1:500; Jackson ImmunoResearch, West Grove, PA). Biotin was complexed with streptavidin peroxidase conjugate (1:500; Jackson ImmunoResearch), and horseradish peroxidase activity was detected with TSA-Alexa 488 fluorescently labeled horseradish peroxidase conjugate (Molecular Probes, Eugene, OR). After this, rabbit anti-ACTH (1:500; National Hormone and Peptide Program, UCLA, Torrance, CA) was directly detected by donkey antirabbit Alexa 594 (Life Technologies, Carlsbad, CA). USP8/ACTH costaining was analyzed under the Zeiss Axioplan microscope.
Statistical analysis
Continuous variables were compared between groups via Student t test, the Mann–Whitney U test, or the Kruskal–Wallis test, as appropriate. Measurements are reported as mean ± standard deviation (SD). Categorical variables were compared via χ2 or Fisher’s exact test, as appropriate. An exact, two-tailed P <0.05 was considered statistically significant. Analysis was performed in the statistical software package SPSS, version 20.0 (IBM SPSS Statistics, Armonk, NY).
Results
Patient characteristics
In our cohort of 42 patients, there was a predominance of female patients (27 vs 15), with a mean age at diagnosis of 13.7 ± 3.4 years and an average duration of symptoms before diagnosis of 2.1 ± 1.5 years. The mean midnight serum cortisol and 24-hour UFC levels were 18.9 ± 13.5 μg/dL and 446 ± 610 μg/24 hours, respectively. The mean size of the identified pituitary adenomas was 6.2 ± 6.0 mm, and 4 of the 42 patients (9.5%) presented with evidence of cavernous sinus invasion (Table 1).
Table 1.
Demographic Data of our Cohort of Patients
| n | Minimum | Maximum | Mean ± SD | |
|---|---|---|---|---|
| Age at diagnosis, y | 42 | 6.1 | 18.7 | 13.7 ± 3.4 |
| Female sex, n (%) | 27 (64.3) | |||
| Ethnicity, n (%) | ||||
| Asian | 1 (2.4) | |||
| African American | 4 (9.5) | |||
| Unknown | 6 (14.3) | |||
| Caucasian | 31 (73.8) | |||
| Adenoma size, mm | 42 | 1.00 | 28.00 | 6.2 ± 6.0 |
| Invasion to cavernous sinus, n (%) | 4 (9.5) | |||
| Needed further management, n (%) | 12 (28.6) | |||
| Midnight plasma cortisol, μg/dL | 42 | 2.1 | 89.9 | 18.9 ± 13.5 |
| Plasma ACTH, pg/mL | 42 | 5.0 | 512.0 | 71.5 ± 81.3 |
| 24 hour UFC, μg/24 h | 42 | 19 | 2851 | 446 ± 610 |
| SBP, mm Hg | 42 | 91 | 158 | 125 ± 16 |
| SBP z score | 42 | −0.6 | 4.6 | 2.0 ± 1.3 |
| DBP, mm Hg | 42 | 53 | 100 | 73 ± 11 |
| DBP z score | 42 | −1.3 | 3.3 | 1.0 ± 1.0 |
| BMI, kg/m2 | 42 | 19.2 | 51.3 | 31.2 ± 8.6 |
| BMI z score | 42 | −0.6 | 3.1 | 2.0 ± 0.8 |
| Time from first symptom to diagnosis, y | 42 | 0.5 | 7.0 | 2.1 ± 1.5 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Postoperative follow-up was available in 35 patients (73.8%), with mean follow-up of 34.7 ± 37.9 months and median follow-up of 17 months. Thirty-eight of the patients (90.4%) achieved remission after surgery. Of the 4 patients who did not achieve remission after surgery, 1 underwent sellar radiation therapy, 2 were managed with ketoconazole, and 1 underwent both sellar radiation and ketoconazole treatment. Five of the 31 patients with initial remission developed recurrent CD over the course of the study, with a mean time to recurrence 46.4 ± 11.8 months (range 34–66 months). One of them was treated with repeat TSS, one underwent repeat pituitary surgery in addition to pituitary radiation therapy, and three are awaiting repeat TSS. Overall, 4 patients failed to achieve remission immediately after surgery, and 5 patients had recurrence of disease after initial remission. No significant difference was found when the percentage of patients with cavernous sinus invasion who harbored somatic USP8 mutations (2 of 13, or 15.4%) was compared with those with invasion who did not have USP8 mutations (2 of 29, or 6.9%; P = 0.4) (Table 2).
Table 2.
Clinical and Biochemical Characteristics of the Patients With and Without Somatic USP8 Mutations
| Somatic USP8 Mutation, n = 13 (31.0%) | No Somatic USP8 Mutation, n = 29 (50.0%) | P | |
|---|---|---|---|
| Mean age at diagnosis, y | 15.1 ± 2.1 | 13.1 ± 3.6 | 0.03 |
| Female sex, n (%) | 11 (84.6) | 16 (55.2) | 0.07 |
| Follow up duration, mo | 21.1 ± 22.4 | 27.7 ± 4.8 | 0.6 |
| Ethnicity | |||
| Asian | 0 | 1 (3.4) | 0.1 |
| African American | 2 (15.4) | 2 (6.9) | |
| Unknown | 4 (30.8) | 2 (6.9) | |
| Caucasian | 7 (53.8) | 24 (82.8) | |
| Anthropometric data | |||
| BMI z score | 1.5 ± 1.0 | 2.1 ± 0.7 | 0.02 |
| BMI z score ≥2, n (%) | 5 (38.5) | 22 (75.9) | 0.02 |
| Height z score | −1.1 ± 1.2 | −1.0 ± 1.1 | 0.9 |
| Height z-score ≤−0.5, n (%) | 10 (76.9) | 21 (72.4) | 0.8 |
| Tumor characteristics | |||
| Diameter, mm | 8.0 ± 7.0 | 5.4 ± 5.4 | 0.2 |
| Diameter ≥5 mm, n (%) | 9 (69.2) | 13 (44.8) | 0.1 |
| Invasion of cavernous sinus, n (%) | 2 (15.4) | 2 (6.9) | 0.4 |
| Biochemical data | |||
| Midnight cortisol, μg/dL | 19.5 ± 7.4 | 18.7 ± 15.7 | 0.9 |
| Plasma ACTH, pg/mL | 47.7 ± 30.1 | 82.2 ± 94.3 | 0.2 |
| 24 hour UFC, μg/24 h | 396 ± 466 | 468 ± 671 | 0.7 |
| Time from first symptoms to diagnosis | |||
| Time, y | 2.1 ± 1.2 | 2.1 ± 1.7 | 0.9 |
| ≥3 y, n (%) | 4 (30.8) | 6 (20.7) | 0.5 |
| Treatment | |||
| Recurrence or failure to cure, n (%) | 6 (46.2) | 3 (10.3) | 0.009 |
Frequency of USP8 gene mutations
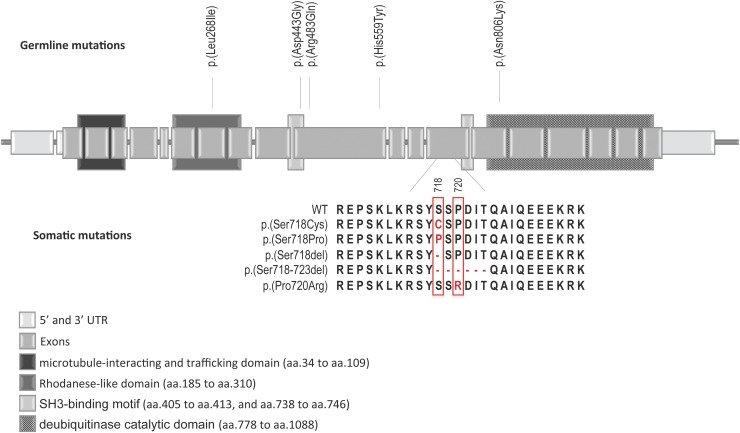
We identified five different somatic mutations (Fig. 1) in 13 patients, resulting in a frequency of 31% in our cohort. All the mutations were heterozygous, and they were all located in exon 14 of the USP8 gene. They consisted of three missense mutations (p.Ser718Pro–rs672601307, p.Ser718Cys–rs672601308, p.Pro720Arg–rs672601311) and two deletions of 1 (p.Ser718del) and 6 (p.Ser719_724del) amino acids. All these mutations are located at the hotspot of the gene at the 14-3-3 binding motif (Fig. 1) (12, 14, 15). None of the germline DNA samples were found to have USP8 hotspot mutations.
Figure 1.
Schematic representation of the USP8 gene showing the somatic mutations identified. Vertical lines show the variant position. aa, amino acids. UTR, untranslated region.
Clinical and biochemical characteristics of the patients with somatic USP8 mutations
Patients harboring somatic USP8 gene mutations had a later mean age at diagnosis of 15.1 ± 2.1 years, compared with 13.1 ± 3.6 years for those without USP8 mutations (P = 0.03). They also had lower body mass index (BMI) z scores, with fewer patients having a BMI z score >2 SD above the mean (38.5% vs 75.9%; P = 0.02), whereas the height z score was similar in the two groups (Table 2).
The biochemical profile of the two groups was similar, with no differences in the level of midnight serum cortisol, UFC, or ACTH before treatment. The size of the identified tumors was not significantly different in patients with USP8 mutations compared with the patients without USP8 mutations (diameter 8.0 ± 7.0 mm vs 5.4 ± 5.4 mm, respectively; P = 0.2), and the adenoma volumes were comparable in the two groups (200 ± 234 mm3 vs 191 ± 186 mm3; P = 0.9). There was also no significant difference in the frequency of invasion of the cavernous sinus between the two groups (15.4% vs 6.9%; P = 0.4).
Outcomes
Of the four patients with initial nonremission after pituitary surgery, one patient harbored a somatic USP8 mutation, and three did not. An analysis of these patients revealed that all patients had large, invasive adenomas with extension to the cavernous sinus and substantial tumor remnants after surgery. This result is expected because tumors harboring USP8 mutations may have smaller volume in general (12). Interestingly, however, all recurrences after initial remission (n = 5) occurred in tumors harboring somatic USP8 mutations. The proportion of patients with recurrence among those with somatic USP8 mutations was 5 in 13 (38.5%), compared with 0 in the remaining group (0 in 29; P = 0.0015).
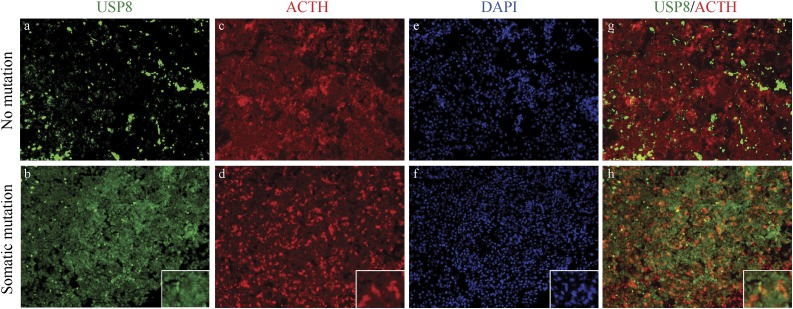
Immunohistochemical analysis
To investigate more specifically the effect of one of the somatic mutations detected in our cohort, we analyzed USP8 expression by immunohistochemistry (Fig. 2). USP8 was exclusively expressed in the cytoplasm of the nonmutant tumors, whereas its expression was more nuclear in the mutated tumors, consistent with findings in previous reports (12, 15).
Figure 2.
Immunohistochemistry staining of pituitary tumors with no USP8 mutation (a, c, e, g) and with the somatic USP8 gene mutation p.Ser718Pro (b, d, f, h) for USP8 (a, b), ACTH (c, d), and DAPI (e, f) showing the nuclear localization of USP8.
Discussion
In this study, we report our experience with the identification of somatic USP8 mutations in pediatric patients seen at the NIH. To our knowledge this is the largest pediatric cohort of patients with CD that has been screened for this genetic defect. A subset of these patients (n = 24) were previously reported in Perez-Rivas et al. (15); however, we performed repeat DNA extraction from the resected tissue after confirming that the selected cut of the tissue originated exclusively from the corticotroph adenoma, to exclude the possibility of mixing the tumor DNA sample with DNA from normal cells.
We identified 13 patients with somatic mutations demonstrating a high frequency of USP8 gene defects (31%) in our cohort. This finding is similar to the reported frequency in the adult population (around one-third of all adult patients with CD) and is higher than the low frequency (17%) previously documented in the pediatric patients with CD (12, 14, 15). A recent study by Ma et al. reported a frequency of somatic mutations in their adult population of ≤62.5%, suggesting that it is possible that the genetic background of corticotropinomas in children differs from that in adults (14), although the difference probably lies in the fact that the smaller size of pediatric ACTH-producing pituitary adenomas does not allow for full capture of tumor DNA and permits admixture of DNA from the surrounding normal cells.
Notably, we documented a higher rate of subsequent recurrence of CD in our patients with USP8 mutations. Previous reports suggested that the USP8 mutated tumors are not as aggressive, with higher rates of remission (19). The demonstration of a higher rate of recurrence in our pediatric population with USP8 defects might suggest that CD in these children may have a different course than that in adults. However, it could also reflect the fact that only larger microadenomas and possibly more aggressive tumors were available for analysis in children with CD.
The comparison of demographic, clinical, and biochemical data of patients with and without USP8 mutations revealed an older age at diagnosis for patients with a USP8 defect in the tumor. Although some previous studies showed that patients with USP8 mutations had different levels of plasma ACTH compared with patients without mutations, this finding was not confirmed in other studies nor by our group; patients with USP8 mutations had lower ACTH levels (Table 2) but the difference was not statistically significant (47.7 ± 30.1 vs. 82.2 ± 94.3, P = 0.2) (14, 19). Additionally, conflicting data on tumor size have been reported: Perez Rivas et al. reported that tumors in female patients with USP8 somatic mutations were larger than those in the nonmutant group or in male patients with USP8 defects, whereas Ma et al. and Hayashi et al. found that pituitary tumors were smaller in patients with USP8 mutations (14, 15, 19). In our population the size of the tumor in the two groups was similar; however, the available samples were usually from larger tumors, because those had sufficient material to be processed for research, which may have biased our results.
Physiologically, the USP8 protein is a deubiquitinase involved in the ubiquitination pathway of EGFR degradation, resulting in its “rescue” from ubiquitination (13, 20, 21). The inhibition of USP8 action is usually mediated by binding to 14-3-3 proteins after phosphorylation of the amino acid serine 718 (Ser718) (22, 23).
All identified somatic mutations in our population are located in exon 14, at amino acids 718 to 723, which are highly conserved across species. They result in loss of 14-3-3 protein binding and catalytic cleavage of a 40-kDa fragment of the protein, leading to elevated deubiquitinase activity, “rescue” of EGFR from degradation, and elevated intracellular levels of EGFR (19, 24). EGFR has been shown in the past to induce POMC expression, with mediation of Erk1/2 (25). Thus the high levels of EGFR, along with a possible independent effect of the mutated USP8 protein, result in increased POMC promoter activation and ACTH production by the corticotroph cells (12, 14, 15).
The identification of a common molecular etiology explaining the pathogenesis of a large number of ACTH-producing pituitary adenomas is of foremost importance, especially because it can provide new treatment targets. The rate of recurrence of CD or failure to cure after the first TSS has been estimated as ≤25% in the pediatric population, and the probability of impairment of normal pituitary function after repeat surgery increases significantly (6–8, 26). In children, where final growth and puberty have not yet occurred, preservation of pituitary function and the possibility of less invasive treatments are critical.
Recently Hayashi et al. reported that USP8-mutated tumors bear more somatostatin receptors subtype 5, which can be a target for pharmacologic treatment with specific somatostatin analogs such as pasireotide (19). Additionally, EGFR-targeting agents, including tyrosine kinase inhibitors and monoclonal antibodies, are other promising options (27). For example, gefitinib, an EGFR tyrosine kinase inhibitor, was shown to decrease the expression of POMC, inhibiting ACTH production and corticotroph tumor cell growth in canine and mouse cells (25). However, studies of this medication in humans have not produced consistently good outcomes when tested on other tumors (28). A clinical trial is being conducted by Zhang et al. to evaluate its effect specifically in CD (ClinicalTrials.gov, identifier NCT02484755). USP8 protein–targeting medications could be used in this subgroup of patients, and inhibitors of the protein have already been designed for other malignancies (29).
A limitation of this study is its small sample size. However, our goal was to elucidate the genetics of pediatric CD, which is a rare condition, and to our knowledge, our cohort is the largest of its kind, with patients of exclusively pediatric age who have been screened for USP8 mutations. Another limitation is a possible bias in the selection of the patients included in the study: Because we had to include only patients with both blood and tumor tissue available, we excluded 97 patients for whom tumor DNA was missing who in these last few years have also presented with CD and were treated with TSS at the NIH. Thus, the patients we finally included in this study may represent those who had larger tumors at detection (although by imaging there was no difference, as presented in our results). These patients may have had a more aggressive tumors to begin with, and the detection of the USP8 defect in them may be a coincidence rather than causative. In addition, “contamination” with normal cells is a big problem whenever one looks for a somatic mutation in a small specimen; although we took great care to detect such admixture, it is possible that we missed the presence of the mutation in patients with smaller tumors, making those with more aggressive lesions appear more frequently as USP8-mutation bearing. Additional studies are needed to define the effect of a USP8 defect in the biologic behavior of an ACTH-producing tumor in both children and adults.
Conclusion
In almost one-third (31%) of tumors from patients with pediatric CD, we identified somatic USP8 gene mutations. All defects were present at the hotspot location of exon 14 of the gene; mutations there appear to cause decreased EGFR degradation, which in turn leads to increased POMC expression and ACTH production. Pediatric patients with USP8 mutations had more severe overall disease, with more failures of primary surgical resection and increased rate of recurrences. This study is part of a larger one aiming at defining the genetic causes of CD in children and adults (17, 18).
Acknowledgments
We thank Dr. Reincke and his group for collaboration on the USP8 project. We are grateful to our patients and the clinicians who support our study with referrals from around the nation and the world.
Acknowledgments
This study was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (to C.A.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 3PAs
- pituitary adenoma with paraganglioma/pheochromocytoma association
- ACTH
- adrenocorticotropic Hormone
- AIP
- aryl hydrocarbon receptor-interacting protein gene
- BMI
- body mass index
- CD
- Cushing disease
- CDKN1B
- cyclin-dependent kinase inhibitor 1B gene
- CDKN2C
- cyclin-dependent kinase inhibitor 2C
- DICER1
- dicer, drosophila, homolog of, 1 gene
- EGFR
- epidermal growth factor receptor gene
- MEN1
- multiple endocrine neoplasia, type I gene
- NIH
- National Institutes of Health
- POMC
- proopiomelanocortin gene
- PRKAR1A
- protein kinase, camp-dependent, regulatory, type I, alpha gene
- SD
- standard deviation
- SDHx
- collectively the succinate dehydrogenase complex, subunits A, B, C and D genes
- TSS
- transsphenoidal surgical resection
- UFC
- urinary free cortisol
- USP8
- ubiquitin-specific protease 8 gene.
References
- 1.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913–927. [DOI] [PubMed] [Google Scholar]
- 2.Storr HL, Chan LF, Grossman AB, Savage MO. Paediatric Cushing’s syndrome: epidemiology, investigation and therapeutic advances. Trends Endocrinol Metab. 2007;18(4):167–174. [DOI] [PubMed] [Google Scholar]
- 3.Afshari A, Ardeshirpour Y, Lodish MB, Gourgari E, Sinaii N, Keil M, Belyavskaya E, Lyssikatos C, Chowdhry FA, Chernomordik V, Anderson AA, Mazzuchi TA, Gandjbakhche A, Stratakis CA. Facial plethora: modern technology for quantifying an ancient clinical sign and its use in Cushing syndrome. J Clin Endocrinol Metab. 2015;100(10):3928–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratakis CA, Mastorakos G, Mitsiades NS, Mitsiades CS, Chrousos GP. Skin manifestations of Cushing disease in children and adolescents before and after the resolution of hypercortisolemia. Pediatr Dermatol. 1998;15(4):253–258. [DOI] [PubMed] [Google Scholar]
- 5.Lodish MB, Hsiao HP, Serbis A, Sinaii N, Rothenbuhler A, Keil MF, Boikos SA, Reynolds JC, Stratakis CA. Effects of Cushing disease on bone mineral density in a pediatric population. J Pediatr. 2010;156(6):1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batista DL, Oldfield EH, Keil MF, Stratakis CA. Postoperative testing to predict recurrent Cushing disease in children. J Clin Endocrinol Metab. 2009;94(8):2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage MO, Storr HL. Pediatric Cushing’s disease: management issues. Indian J Endocrinol Metab. 2012;16(suppl 2):S171–S175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler WF, Barkan AL, Hollon T, Sakharova A, Sack J, Brahma B, Schteingart DE. Outcome of transsphenoidal surgery for Cushing disease: a single-center experience over 32 years. Neurosurgery. 2016;78(2):216–223. [DOI] [PubMed] [Google Scholar]
- 9.Stratakis CA. Diagnosis and clinical genetics of Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2016;45(2):311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sbiera S, Deutschbein T, Weigand I, Reincke M, Fassnacht M, Allolio B. The new molecular landscape of Cushing’s disease. Trends Endocrinol Metab. 2015;26(10):573–583. [DOI] [PubMed] [Google Scholar]
- 11.Xekouki P, Szarek E, Bullova P, Giubellino A, Quezado M, Mastroyannis SA, Mastorakos P, Wassif CA, Raygada M, Rentia N, Dye L, Cougnoux A, Koziol D, Sierra MdeL, Lyssikatos C, Belyavskaya E, Malchoff C, Moline J, Eng C, Maher LJ III, Pacak K, Lodish M, Stratakis CA. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J Clin Endocrinol Metab. 2015;100(5):E710–E719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, Tanaka K, Wieland T, Graf E, Saeger W, Ronchi CL, Allolio B, Buchfelder M, Strom TM, Fassnacht M, Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31–38. [DOI] [PubMed] [Google Scholar]
- 13.Alwan HA, van Leeuwen JE. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J Biol Chem. 2007;282(3):1658–1669. [DOI] [PubMed] [Google Scholar]
- 14.Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, Mao Y, Li YM, Hu RG, Zhang ZY, Ye HY, Shen M, Shou XF, Li ZQ, Peng H, Wang QZ, Zhou DZ, Qin XL, Ji J, Zheng J, Chen H, Wang Y, Geng DY, Tang WJ, Fu CW, Shi ZF, Zhang YC, Ye Z, He WQ, Zhang QL, Tang QS, Xie R, Shen JW, Wen ZJ, Zhou J, Wang T, Huang S, Qiu HJ, Qiao ND, Zhang Y, Pan L, Bao WM, Liu YC, Huang CX, Shi YY, Zhao Y. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Rivas LG, Theodoropoulou M, Ferraù F, Nusser C, Kawaguchi K, Stratakis CA, Faucz FR, Wildemberg LE, Assié G, Beschorner R, Dimopoulou C, Buchfelder M, Popovic V, Berr CM, Tóth M, Ardisasmita AI, Honegger J, Bertherat J, Gadelha MR, Beuschlein F, Stalla G, Komada M, Korbonits M, Reincke M. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J Clin Endocrinol Metab. 2015;100(7):E997–E1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, Verma S, Daly AF, Raygada M, Keil MF, Papademetriou J, Drori-Herishanu L, Horvath A, Tsang KM, Nesterova M, Franklin S, Vanbellinghen JF, Bours V, Salvatori R, Beckers A. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78(5):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Ramírez LC, Tatsi C, Lodish MB, Faucz FR, Pankratz N, Chittiboina P, Lane J, Kay DM, Valdés N, Dimopoulos A, Mills JL, Stratakis CA. Corticotropinoma as a component of Carney complex [published online ahead of print May 30, 2017]. J Endocr Soc. doi:10.1210/js.2017-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Ramírez LC, Gam R, Valdés N, Lodish M, Pankratz N, Balsalobre A, Gauthier Y, Faucz FR, Trivellin G, Chittiboina P, Lane J, Kay DM, Dimopoulou A, Gaillard S, Neou M, Bertherat J, Assié G, Villa C, Mills JL, Drouin J, Stratakis CA. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease [published online ahead of print May 22, 2017]. Endocr Relat Cancer. doi:10.1530/ERC-17-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi K, Inoshita N, Kawaguchi K, et al. . The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur J Endocrinol. 2016;174(2):213–226. [DOI] [PubMed] [Google Scholar]
- 20.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123(5):773–786. [DOI] [PubMed] [Google Scholar]
- 21.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuno E, Kitamura N, Komada M. 14-3-3-dependent inhibition of the deubiquitinating activity of UBPY and its cancellation in the M phase. Exp Cell Res. 2007;313(16):3624–3634. [DOI] [PubMed] [Google Scholar]
- 23.Meijer IM, Kerperien J, Sotoca AM, van Zoelen EJ, van Leeuwen JE. The Usp8 deubiquitination enzyme is post-translationally modified by tyrosine and serine phosphorylation. Cell Signal. 2013;25(4):919–930. [DOI] [PubMed] [Google Scholar]
- 24.Theodoropoulou M, Reincke M, Fassnacht M, Komada M. Decoding the genetic basis of Cushing’s disease: USP8 in the spotlight. Eur J Endocrinol. 2015;173(4):M73–83. [DOI] [PubMed] [Google Scholar]
- 25.Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, Melmed S. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121(12):4712–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH. Outcome of surgical treatment of 200 children with Cushing’s disease. J Clin Endocrinol Metab. 2013;98(3):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wondisford FE. A new medical therapy for Cushing disease? J Clin Invest. 2011;121(12):4621–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Greene MI. Mechanisms of resistance to ErbB-targeted cancer therapeutics. J Clin Invest. 2008;118(7):2389–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byun S, Lee SY, Lee J, et al. . USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin Cancer Res. 2013;19(14):3894–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]