Abstract
Context:
Remission failure following transsphenoidal surgery in Cushing disease (CD) from pituitary corticotroph tumors (CtTs) remains clinically challenging. Histone deacetylase inhibitors (HDACis) are antitumor drugs approved for clinical use, with the potential to affect adrenocorticotropin hormone (ACTH) hypersecretion by inhibiting pro-opiomelanocortin (POMC) transcription.
Objective:
Testing the efficacy of suberoylanilide hydroxamic acid (SAHA) on human and murine ACTH-secreting tumor (AtT-20) cells.
Design:
Cell viability, ACTH secretion (enzyme-linked immunosorbent assay), apoptosis, and gene expression profile were investigated on AtT-20 cells. In vivo efficacy was examined in an athymic nude mouse AtT-20 xenograft model. SAHA efficacy against human-derived corticotroph tumor (hCtT) (n = 8) was tested in vitro.
Setting:
National Institutes of Health.
Intervention:
SAHA (0.5 to 8 µM).
Main Outcome Measures:
AtT-20 and hCtT cell survival, in vitro/in vivo ACTH measurements.
Results:
SAHA (1 µM) reduced AtT-20 viability to 75% at 24 hours, 43% at 48 hours (analysis of variance; P = 0.002). Apoptosis was confirmed with elevated BAX/Bcl2 ratio and FACS. Intriguingly, early (3-hour) significant decline (70%; P < 0.0001) of secreted ACTH and diminished POMC transcription was observed with SAHA (1 µM). Microarray analysis revealed a direct association between liver X receptor alpha (LXRα) and POMC expression. Accordingly, SAHA reduced LXRα in AtT-20 cells but not in normal murine corticotrophs. Xenografted nude-mice tumor involution (126 ± 33/160 ± 35 vs 337 ± 49 mm3; P = 0.0005) was observed with 5-day intraperitoneal SAHA, with reversal of elevated ACTH (P < 0.0001). SAHA did not affect serum ACTH in nontumor mice. Lastly, we confirmed that SAHA (1 µM/24 h) decreased hCtT survival (78.92%; P = 0.0007) and ACTH secretion (83.64%; P = 0.03).
Conclusion:
Our findings demonstrate SAHA’s efficacy in reducing survival and ACTH secretion in AtT-20 and hCtT cells, providing a potential intervention for recurrent/unremitting CD.
SAHA efficacy was tested on human/murine ACTH-secreting tumor cells. It successfully reduced survival and ACTH secretion, offering potential novel treatment of recurrent/unremitting Cushing disease.
Cushing disease (CD) is characterized by a state of hypercortisolism driven by an adrenocorticotropin hormone (ACTH)-secreting adenoma and is associated with two- to fivefold increased mortality (1, 2). Although curative treatments return mortality risk to baseline, substantial morbidity persists (1, 3). Successful resection of a CD-associated pituitary adenoma can lead to immediate and lasting biochemical remission in 80% to 90% of patients (1, 3–5). Consequently, transsphenoidal surgery is considered the initial preferred intervention in the treatment of CD.
Remission failure from pituitary corticotroph tumors (CtTs) following transsphenoidal surgery for CD remains clinically challenging. Definitive therapy for recurrent and unremitting CD is limited to radiation and medical/surgical adrenalectomy (4). Medical therapy, including ketoconazole and surgical adrenalectomy, offer good control of hypercortisolemia; however, these therapies come with a major burden of adverse effects. Moreover, pharmacotherapies (comprising cabergoline and pasireotide) aimed at the adenohypophysis remain mostly ineffective (6, 7). The antitumor agent retinoic acid targets the underlying CtTs (8) but has limited effectiveness in the clinical setting (9, 10).
Histone deacetylase inhibitors (HDACis) are compounds with promising antineoplastic properties that have generated growing interest for the treatment of different types of cancers (11, 12). Suberoylanilide hydroxamic acid (SAHA; Vorinostat) is an oral pan-HDACi approved by the Food and Drug Administration (FDA) that has induced growth arrest and increased cell death in pituitary adenoma–derived cells using clinically achievable concentrations (13). Nonetheless, the effects of HDACi on ACTH-secreting adenomas and thus the potential for biochemical remission have remained unexplored. We investigated the effect of HDACis on survival, ACTH release, and gene expression in murine and human ACTH-producing tumors.
This study demonstrated that SAHA reduced survival and ACTH release from ACTH-secreting tumor (AtT-20) and human-derived corticotroph tumor (hCtT) cells. We showed that this effect was mediated by transcriptional downregulation of pro-opiomelanocortin (POMC) via suppression of the nuclear liver X receptor alpha (LXRα). Further, we showed that SAHA differentially regulated LXRα in tumors and normal murine corticotrophs. We hypothesized that the differential regulation of LXRα may underlie the selective effect of SAHA on ACTH secretion limited to CtTs but not normal corticotrophs. These findings support the potential use of SAHA in the management of recurrent/unremitting CD.
Material and Methods
Cell culture and tissue sample collection
AtT-20/D16/16 murine CtT cells (a generous gift from Dr. Steven L. Sabol at the National Heart, Lung and Blood Institute) were cultured in T75 flasks with Dulbecco’s modified Eagle medium (Gibco), 10% fetal bovine serum (Gibco, Gaithersburg, MD), and 100 U/mL of penicillin-streptomycin (Invitrogen, Carlsbad, CA) in 5% CO2/95% air atmosphere at 37°C. Fresh (<30 minutes posteuthanasia) mouse pituitary cells were digested and homogenized with collagenase (Sigma, St. Louis, MO; 1 mg/mL) for 30 minutes and cultured as previously described. All animal studies were approved by the Institutional Animal Care and Use Committee of the National Institutes of Health (NIH). Human pituitary tumor tissues were obtained from eight nonconsecutive patients who underwent transsphenoidal surgery for CD at the NIH from 2013 to 2016 under a protocol (NIH 03-N-0164, NCT00060541) approved by the Combined Neuroscience Institutional Review Board of the National Institute of Neurological Disorders and Stroke at the NIH. Written informed consent was obtained from each patient for research study participation, and the study was conducted according to the standards set by the institutional review board.
Animal studies
NCRNU-F sp/sp nude mice (females, aged 6 to 8 weeks; Taconic Biosciences, Hudson, NY) were randomized to five groups of five mice each: (1) saline-without tumor; (2) SAHA 25 mg/kg without tumor; (3) SAHA 25 mg/kg with tumor; (4) SAHA 50 mg/kg with tumor; and (5) saline with tumor. Animals in the tumor groups received subcutaneous injections of AtT-20 cells as described previously (14). Treatment with intraperitoneal SAHA or saline was initiated (day zero) when xenografts reached a minimum size of 0.5 ± 0.1 cm. Thereafter, tumor volume was calculated from the following formula: tumor volume = length × width2 × π/6. After 7-day treatment, facial vein blood samples were collected for ACTH analysis, and tumors and skin were collected for histological examination.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
After exposure to different concentrations of HDACi, including SAHA (0.5 μM, 1.0 μM, and 4.0 μM) for 3 to 72 hours and valproate (4 mM) and panobinostat (200 nM) for 24 hours, the cell proliferation and metabolic activity of AtT-20/D16/16 cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as reported (15).
Fluorescence-activated sorting of murine corticotrophs and detection of apoptosis
Following dissociation and culture of pooled mouse pituitary cells (16), corticotrophs (murine normal pituitary corticotrophs) were isolated by flow cytometry using anti–corticotropin-releasing hormone receptor 1 antibody (Invitrogen) followed by Alexa Fluor-555 conjugated antibody (Thermo Fisher, Waltham, MA). For detection of apoptosis, AtT-20 cells were exposed to SAHA (0.5 μM, 1.0 μM, and 4.0 μM) for 24 hours and then treated with Hoechst blue (Thermo Fisher) for 30 minutes followed by propidium iodide (Thermo Fisher) for 5 minutes. Lastly, cell sorting and analysis were carried out using a MoFlo Astrios cell sorter and Summit acquisition and analysis software (Beckman Coulter, Brea, CA).
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was isolated with the RNeasy Mini Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions, and synthesis of complementary DNA was performed using the SuperScript III First-Strand Synthesis SuperMix kit (Invitrogen). Quantitative real-time transcription polymerase chain reaction (qRT-PCR) was performed with the CXF384 Real-Time System (Bio-Rad, Hercules, CA) and SYBR Green master mix (Applied Biosystems, Foster City, CA). Gene expression was quantified with the CFX384 Real-time System (Bio-Rad) (see Supplemental Table 1 (123.5KB, pdf) for primer sets).
Western blot and protein complex immunoprecipitation
For protein extraction, whole-cell lysates were collected in radioimmunoprecipitation buffer containing a protease inhibitor cocktail. Proteins were electrophoretically separated on 4% to 12% Bis-Tris gels (Invitrogen) and subsequently electroblotted onto polyvinylidene difluoride membranes with the Trans-Blot Transfer Turbo System (Bio-Rad). Following blocking, membranes were probed with anti-LXRα (Abcam, UK), anti-retinoid X receptor α (RXRα; Invitrogen), anti-poly (adenosine 5′-diphosphate-ribose) polymerase (PARP; Cell Signaling, Danvers, MA). Immunoreactive signal was detected with the ChemiDoc MP Imaging System (Bio-Rad), and densitometric analysis was performed using Image Laboratory Software (Bio-Rad). Protein complex immunoprecipitation was performed on AtT-20 cells with anti-LXRα (Invitrogen) and anti-RXRα (Abcam, UK). Immunoprecipitates were fractionated, transferred to polyvinylidene difluoridemembranes, and immunoblotted as described previously.
ACTH enzyme-linked immunosorbent assay analysis
ACTH concentrations in CtT cells were determined by enzyme-linked immunosorbent assay (ELISA). Measurements of ACTH were performed with ACTH ELISA kits (MD Bioproducts, Hercules, CA) according to the manufacturer’s instructions.
Luciferase POMC reporter assays
AtT-20/D16/16 cells (1 × 105) were placed on 24-well plates with Dulbecco’s modified Eagle medium growth medium containing serum without antibiotics until a 70% to 80% confluency was reached. POMC gene reporter assays were subsequently performed with a POMC Gene Promoter Reporter Vector Kit (Affymetrix, Santa Clara, CA) as recommended by the manufacturer. Once transfection was performed, cells were exposed to different concentrations of SAHA for 3 hours. Subsequently, lysis buffer (Luciferase Assay System; Promega, Madison, WI) was added, and lysates were mixed with Luciferase Assay Reagent (Promega) and incubated for 10 minutes at room temperature. Spectrophotometric analysis was carried out with the IVIS Lumina II Imaging System (PerkinElmer, Waltham, MA).
Gene expression microarray analysis
Following SAHA exposure, whole-genome microarray analysis on the murine AtT-20/D16/16 cell line was performed with the Agilent Array platform. Sample preparation and microarray hybridization were performed according to the manufacturer’s standard protocols Briefly, total RNA from each sample was amplified and transcribed into fluorescent complementary RNA using Agilent’s Quick Amp Labeling protocol (version 5.7; Agilent Technologies, Santa Clara, CA). Labeled complementary RNA was hybridized onto a Whole Mouse Genome Oligo microarray (4 × 44K; Agilent Technologies). Arrays were then scanned by the Agilent Scanner G2505C, and Agilent Feature Extraction software (v11.0.1.1) was used to analyze the acquired images.
Statistical analyses
Following normalization of the raw microarray data, an unpaired t test comparison between control and experimental samples was performed using GeneSpring GX v12.1 software (Agilent Technologies). Differentially expressed genes (DEGs) with a P value ≤0.05 and fold-change ≥1.5 were selected for subsequent functional analysis.
Statistical significance was determined by one-factor analysis of variance (ANOVA) using the GraphPad Prism v6.0 software program (GraphPad, La Jolla, CA) for in vitro and in vivo results. A P value ≤0.05 was used to determine statistical significance.
Functional analyses
The list of DEGs that reached the preselected statistical threshold (P ≤ 0.05 and fold-change ≥1.5) was further examined by the Gene Functional Classification tool in the Database for Annotation, Visualization and Integrated Discovery (v6.8) (17, 18). Functional clusters of DEGs were calculated by the modified Fisher exact test [Expression Analysis Systematic Explorer (EASE score)], with a score of zero being the highest enrichment achievable. Biological functions that were significantly enriched (based on EASE scores) were further analyzed using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (v10.0) (19) to assess any functional association between DEG products and our proteins of interest. Identified associations with high confidence scores were further investigated.
Results
SAHA reduced AtT-20 cell line survival by inducing apoptosis
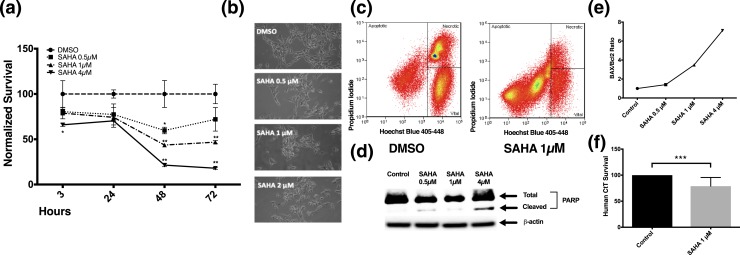
Clinically achievable concentrations of SAHA (0.5 to 4 µM) significantly reduced the viability of AtT-20 cells in concentration and time-dependent manners following a 3- to 72-hour treatment, as assessed by MTT. When compared with that of the control group, the lowest tested concentration of SAHA (0.5 µM) reduced AtT-20 survival to 60% ± 3.6% at 48 hours (ANOVA P = 0.002) [Fig. 1(a)]. SAHA (1 µM) reduced AtT-20 survival to 44% ± 0.3% at 48 hours (ANOVA P = 0.002) and 47% ± 1.7% at 72 hours (ANOVA P = 0.002) [Fig. 1(a)]. Similarly, maximal concentration of SAHA (4 µM) reduced AtT-20 cellular viability to 66% ± 1.7% at 24 hours (ANOVA P = 0.002), 21% ± 1.7% at 48 hours (ANOVA P = 0.002), and 18% ± 1.1% at 72 hours (ANOVA P = 0.002) vs control [Fig. 1(a)]. Subsequently, we assessed the efficacy of two additional HDACis, valproate and panobinostat, which have been recognized as useful therapeutic agents for a broad range of central nervous system disorders (20). In our study, however, valproate (4 mM) did not reduce AtT-20 cell survival at 24 hours (Supplemental Fig. 1A (123.5KB, pdf) ), supporting prior clinical experience (21).
Figure 1.
SAHA reduced AtT-20 cell survival by apoptotic induction. Clinically achievable concentrations of SAHA (0.5 to 4 µM) significantly reduced the viability of transformed AtT-20 cells in concentration- and time-dependent manners following 3- to 72- hour treatment. (a) Time course of their survival, as assessed by MTT, was plotted. (b) Representative live microscopy images of AtT-20 cells exposed to various doses of SAHA for 24 hours. (c) FACS analysis revealed increased apoptosis in AtT-20 cells treated with SAHA (1 µM) for 24 hours. (d) Western blot analysis revealed an increase in cleaved PARP, a hallmark of apoptotic cell death, when AtT-20 cells were exposed for 24 hours to SAHA (0.5 to 4 µM). (e) SAHA (0.5 to 4 µM) resulted in an increase in the Bax/Bcl2 ratio at 24 hours in a dose-dependent manner in AtT-20 cells, suggesting that SAHA activates proapoptotic pathways. The Bax/Bcl2 ratio curve was generated from two independent experiments. (f) SAHA (1 µM) reduced cell survival at 24 hours in hCtT cells (n = 7). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 compared with corresponding control values. Horizontal bars represent mean ± standard deviation. DMSO, dimethyl sulfoxide.
MTT measures mitochondrial activity but cannot differentiate between a reduction in cellular proliferation and/or an increase in cell death. Consistent with known proapoptotic effects in other pituitary-derived cells (13), SAHA (1 to 4 µM) resulted in an increase of Bax/Bcl2 ratio at 24 hours [Fig. 1(e)]. Immunoblotting revealed an increase in cleaved PARP, a hallmark of apoptotic cell death, when AtT-20 cells were exposed to SAHA (1 to 4 µM) [Fig. 1(d)].
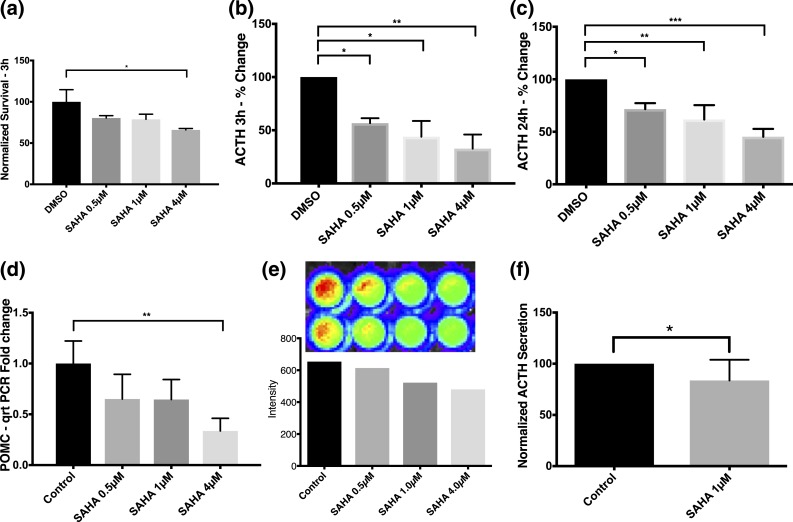
SAHA reduced secreted ACTH and POMC transcription in AtT-20 cells
Unexpectedly, within 3 hours of exposure to SAHA, doses of 0.5 µM, 1 µM, and 4 µM reduced levels of ACTH secretion by AtT-20 cells (60% ± 0.4%, 54% ± 1.9%, and 42% ± 0.4%, respectively; ANOVA P = 0.04) compared with the control group [Fig. 2(b)]. This effect of SAHA on ACTH in media continued at 24 hours (73% ± 7.5% [0.5 µM], 73% ± 5% [1 µM], 51% ± 5.3% [4 µM]; ANOVA P = 0.001) [Fig. 2(c)]. Because the decrease in secreted ACTH occurred before significant apoptosis [Fig. 2(a)], we then examined the mechanism by which SAHA may affect ACTH. First, qRT-PCR revealed decreased transcription of POMC with physiologically available concentrations of SAHA. A trend toward decreased POMC expression was seen with lower doses of SAHA (65% ± 0.1% for both 0.5 and 1 µM) [Fig. 2(d)]. At a 4-µM dose of SAHA, a significant decrease in expression was confirmed (33% ± 0.07%; ANOVA P = 0.02) [Fig. 2(d)]. Suppression of POMC promoter (8) with SAHA (1 µM at 3 hours) with a POMC promoter luciferase transfection study was corroborated [Fig. 2(e)].
Figure 2.
Before apoptotic death, SAHA evoked robust and sustained declines in ACTH release and POMC transcription in AtT-20 cells. (a) Early (within 3 hours) exposure to SAHA (4 µM) significantly decreased the survival of AtT-20 cells, as observed in MTT assays. Percentage changes in ACTH secretion by AtT-20 cells upon exposure to increasing concentrations of SAHA for (b) 3 hours and (c) 24 hours; ACTH levels significantly decreased by clinically achievable concentrations of SAHA (0.5 to 4 µM) in both time groups (b, c). Each sample was run in duplicate in every experiment, and each experiment was repeated three times for the ELISA assay at 3 and 24 hours (b, c). (d) Physiologically available concentrations of SAHA led to transcriptional downregulation of POMC in AtT-20 cells after 24-hour exposure. (e) As inferred by the luciferase transfection study, the aforementioned effect was likely caused by suppression of the POMC promoter. (f) Expectedly, SAHA led to a significant decrease in ACTH levels after 24 hours of exposure on hCtT cells. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 compared with corresponding control values. Horizontal bars represent mean ± standard deviation.
SAHA reduced human tumor survival and ACTH secretion ex vivo
Human tumors causing CD are genetically heterogeneous (22). Therefore, we confirmed the effect of SAHA on hCtTs derived from surgical extirpation in eight patients with CD (8). SAHA (1 µM; 24 hours) decreased hCtT survival (78.92%; P = 0.0007) [Fig. 1(f)] and ACTH secretion (83.64%; P = 0.03) [Fig. 2(f)]. Following Sanger sequencing (Supplemental Materials and Methods (123.5KB, pdf) ), we identified two adenomas with the same USP8 gene mutation (p.Arg715Gly) within the previously reported hot spot (22). These adenomatous cells behaved similarly to wild-type cells in vitro.
SAHA had pleiotropic effects on gene expression in AtT-20 cells
To characterize the changes in gene expression exerted by HDACi exposure, AtT-20 cells were treated with SAHA (1 µM), valproic acid (4 mM), and panobinostat (200 nM) for 3 hours and subsequently investigated by whole-genome microarray analysis. With SAHA treatment, 2657 DEGs were identified (P ≤ 0.05 and fold-change ≥1.5) [Fig. 3(a)]. Functional analysis of statistically significant DEGs revealed the effects of SAHA in two relevant clusters, graphically represented in a heatmap [Fig. 3(c)]. First, a cluster of closely correlated genes (EASE score = 0.47) of zinc finger nuclear receptors (Nr1d2, Nr1h3, Nr2f1, and Nr2f2) was downregulated. From our list of DEGs, SAHA induced a 2.3-fold downregulation of the nuclear receptor subfamily 1 group H member 3 (Nr1h3) gene, also known as LXRα (P = 0.027) (23). Notably, a direct correlation (confidence score = 0.706) was found between POMC and LXRα in the STRING database. In the second pathway, genes of the mitochondria-mediated death pathway (Bid, Dffa, Casp6, Casp9) were upregulated (EASE score = 0.67). A robust increase in expression of the BH3-interacting domain death agonist (Bid) gene was noted (2.7-fold; P = 0.02). Lastly, to understand the global interactions among DEGs, the Kyoto Encyclopedia of Genes and Genomes was used. Correspondingly, 34 pathways were affected following SAHA exposure [Fig. 3(b)].
Figure 3.
SAHA exerts pleiotropic effects on AtT-20 cell gene expression. Following SAHA (1 µM) treatment, the gene expression profile of AtT-20 cells was investigated by whole-genome microarray analysis. (a) Unpaired t test comparison of microarray data identified 2657 DEGs that reached the preselected statistical threshold and are depicted in a volcano plot. (b) From our list of DEGs, 34 KEGG transduction pathways were found to be affected by SAHA. (c) Functional analyses of DEGs with the DAVID resources revealed that SAHA altered the expression of two relevant gene clusters: nuclear hormone receptor and apoptosis; a heat map illustrates the differential expression patterns of these two clusters when AtT-20 cells were exposed to different HDACis. *Bonferroni adjusted. cGMP-PKG, cGMP-dependent protein kinase; HTLV, human T-lymphotropic virus; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPAR, peroxisome proliferator-activated receptors.
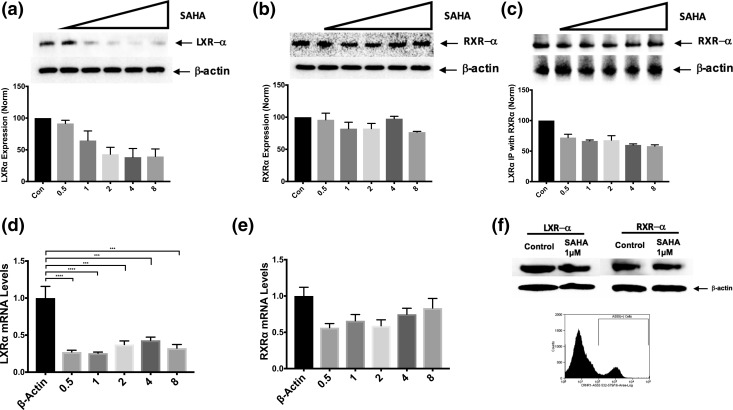
SAHA downregulated LXRα expression in AtT-20 cells but not in normal corticotrophs
Because a direct association was discovered between POMC and LXRα in the STRING database, we proceeded to examine the effects of SAHA on LXRα and its obligate heterodimer, RXRα. When AtT-20 cells were exposed to increasing concentrations of SAHA (0.5 to 8 µM) for 3 hours, LXRα protein expression was reduced in a dose-dependent fashion, as indicated by immunoblot and densitometric analyses [Fig. 4(a)]. qRT-PCR demonstrated concomitantly reduced LXRα messenger RNA (mRNA) levels [Fig. 4(b)]. RXRα protein expression, however, was not altered by SAHA [Fig. 4(a)]. Expectedly, RXRα mRNA was insignificantly reduced with SAHA exposure (>0.5-fold for all SAHA concentrations tested) [Fig. 4(b)]. In addition, quantification of LXRα/RXRα heterodimers was performed with protein-complex coimmunoprecipitation. As expected, an inverse relationship between LXRα/RXRα heterodimer formation and SAHA concentration was noted [Fig. 4(c)]. In clinical use (24) and in in vivo testing, SAHA has not resulted in adrenal insufficiency (or depressed plasma ACTH level). Hence, the effect on LXRα by SAHA in sorted murine normal pituitary corticotrophs was explored. Accordingly, no reduction in LXRα or RXRα with 3-hour exposure to SAHA (1 µM) was detected [Fig. 4(f)].
Figure 4.
Selective degradation and transcriptional downregulation of LXRα by SAHA occurred in tumors but not in normal corticotrophs. (a) After 3 hours of exposure to increasing doses of SAHA (0.5 to 8 µM), AtT-20 cells displayed decreasing LXRα protein levels in a dose-dependent fashion. (b) RXRα protein levels, however, did not seem to be affected by SAHA. (c) Because of decreased levels of LXRα protein, the effects of SAHA were also assessed on the formation of LXRα/RXRα heterodimers using protein-complex immunoprecipitation methods. LXRα was immunoprecipitated and subjected to immunoblotting with an anti-RXRα antibody. A reduction in heterodimerization was observed with increasing doses of SAHA (0.5 to 8 µM) after 3 hours. (d, e) Furthermore, to evaluate the correlation between protein and messenger levels, increasing doses of SAHA were used on AtT-20 cells, and fold-change message levels were measured by qRT-PCR for (d) LXRα and (e) RXRα. (d) A significant reduction in LXRα messenger RNA (mRNA) fold-change after 3 hours of SAHA treatment (0.5 to 8 µM) was demonstrated. (e) Although message levels for RXRα seem to be reduced with SAHA, statistical significance was not reached. (f) To validate the limited action of SAHA on normal cells, LXRα and RXRα protein levels were also measured in corticotroph cells harvested from healthy nude-mice. Flow-cytometric isolation of anti–corticotropin-releasing hormone receptor 1–positive cells, as shown by the double peak, were subjected to a single dose of SAHA (1 µM) for 3 hours; consequently, no change in LXRα and RXRα protein expression was observed. The results are representative of two independent experiments. ***P ≤ 0.001; ****P ≤ 0.0001 compared with corresponding control values. Horizontal bars represent mean ± standard deviation. x-Axis on densitometric and polymerase chain reaction analyses: Con, control: 0.5 to 0.5 µM, 1 to 1 µM, 2 to 2 µM, 4 to 4 µM, and 8 to 8 µM. IP, immunoprecipitation.
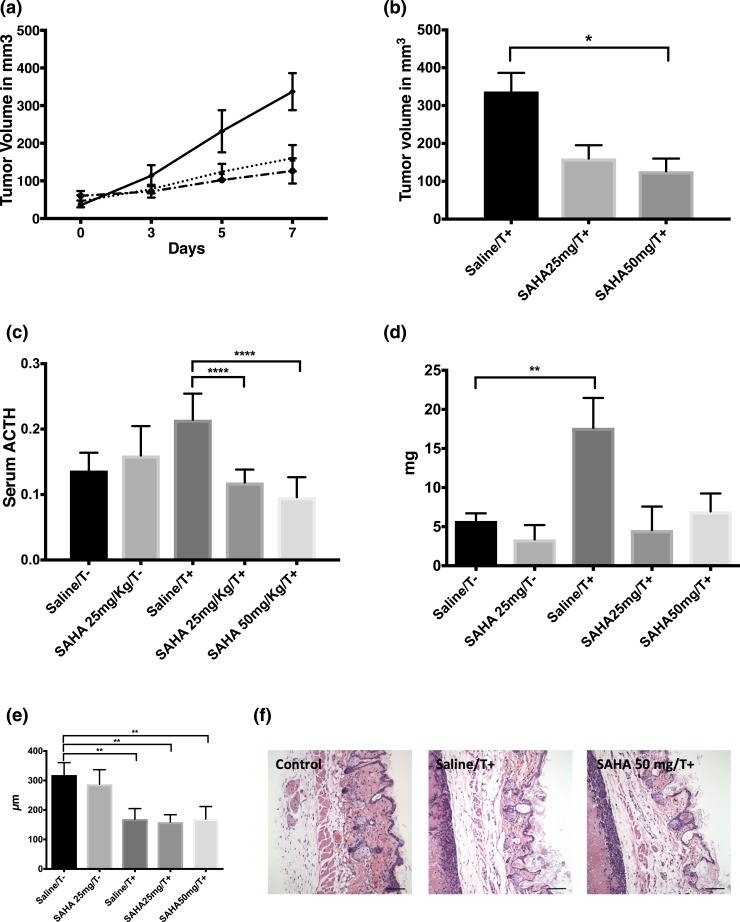
SAHA caused tumor involution and ACTH normalization in an AtT-20 flank tumor model
In vivo testing revealed that SAHA was effective in abrogating tumor growth in a nude-mice xenograft AtT-20 model. Mice treated for 5 days with intraperitoneal SAHA had significant tumor involution compared with controls (126 ± 33 mm3 for 50 mg/kg over 24 hours, 160 ± 35 mm3 for 25 mg/kg over 24 hours vs 337 ± 49 mm3; P = 0.0005) [Fig. 5(a) and 5(b)]. In addition, serum ACTH levels were assessed to corroborate the effects of SAHA on hormonal hypersecretion seen in vitro. ACTH levels drastically decreased in mice harboring tumors treated with either SAHA 25 or 50 mg/kg vs tumor mice that received only saline (P < 0.0001) [Fig. 5(c)].
Figure 5.
SAHA arrested tumor growth and reversed ACTH hypersecretion in vivo. (a) SAHA was effective in abrogating tumor growth in a nude-mice AtT-20 xenograft model. (b) Tumor volume was similarly suppressed up to 70% with SAHA (50 mg/kg). (c) In addition, plasma ACTH levels were quantified following 5 days of SAHA treatment; ACTH levels significantly decreased in both treatment groups (25 and 50 mg/kg). (d) Although adrenal gland dimensions were greater in tumor-implanted animals, statistical significance was not reached compared with treatment animals. (e) Similarly, reversal of skin thinning in grafted mice was nonstatistically significant with SAHA treatment. (f) Pictures of skin thickness illustrate cutaneous thinning in tumor groups (scale bars = 10 µm). *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001, compared with corresponding control values. Horizontal bars represent mean ± standard deviation.
Reduction in plasma ACTH level can be attributed to tumor involution; therefore, we tested the early effects of SAHA on serum ACTH levels in control mice. SAHA, valproate, or panobinostat had no effects on basal serum ACTH levels at 3 hours in healthy nude-mice, confirming the selectivity of HDACi toward tumor cells observed in vitro (Supplemental Fig. 1B (123.5KB, pdf) ).
We then evaluated the effect of 5-day SAHA treatment on chronic effects of excess ACTH in the flank tumor model. The increased adrenal gland weight seen in tumor groups did not change significantly among treatment groups [Fig. 5(d)]. Similarly, reversal of skin thinning in tumor-implanted animals was nonstatistically significant with SAHA treatment [Fig. 5(e) and 5(f)].
Discussion
Most effective pharmacological treatments for CD are aimed at the endocrine organs but come with major adverse effects (4). The ones targeting the pituitary gland, such as dopamine agonists, have been largely ineffective, requiring lifelong administration and poor rates of compliance (6). Tumor-targeting therapies, including retinoic acid (8, 9), unfortunately did not perform well in the clinical setting (10). Previous evidence demonstrated that the antiproliferative effects of retinoic acid were hindered in AtT-20 cells expressing chicken ovalbumin upstream promoter transcription factor 1 (COUP-TF1) (8). Immunohistochemistry demonstrated the ubiquitous expression of COUP-TF1 in normal human corticotroph cells, suggesting an underlying mechanism of resistance for normal cells (8). ACTH-secreting cell lines are heterogeneous, and we found that in the AtT-20/D16/16 cell line, COUP-TF1 was abundantly expressed (Supplemental Fig. 1D (123.5KB, pdf) ), making this an unlikely target for retinoic acid. Unlike retinoic acid, SAHA appears to overcome COUP-TF1−mediated resistance in AtT-20 cells.
Acetylation of the nucleosomal core histones regulates numerous biological processes, including cell cycle regulation, cellular proliferation, and apoptosis, suggesting a potential role in regulating tumor progression (25). In this study, we corroborated the antiproliferative effect of the FDA-approved pan-HDACi SAHA in the corticotroph adenoma cell line AtT-20/D16/16 and subsequently confirmed this in hCtT cells. Explicitly, clinically achievable doses of SAHA reduced the viability of AtT-20 in concentration- and time-dependent manners following 3- to 72-hour treatment, as evaluated by MTT assay [Fig. 1(a)]. SAHA increased proteolysis of PARP, a hallmark of apoptotic cell death catalyzed by the activation of caspase-3 (26), along with an increase in the Bax/Bcl2 ratio consistent with a proapoptotic effect and in agreement with previous evidence [Fig. 1(d) and 1(e)] (13).
SAHA can trigger apoptosis via the mitochondrial/intrinsic (27) and death receptor/extrinsic pathways (27). Furthermore, SAHA may trigger mitochondrial-mediated death through increased activity and/or expression of proapoptotic genes such as Bid, Bim, Bmf, Puma, and Noxa (28). In agreement, our large-scale gene expression analysis substantiated activation of the mitochondrial death pathway via Bid upregulation. Translocation of cleaved Bid to the mitochondria triggered cytochrome c release and consequently initiated the apoptotic program (29).
Although ACTH-producing adenomas are characterized by a relatively slow rate of growth compared with that of other malignant brain tumors, a chronic state of hypercortisolism contributes to high patient morbidity. Our study demonstrated that within 3 hours of exposure, SAHA decreased levels of ACTH secretion by AtT-20 cells, with the effect being maintained at the 24-hour mark [Fig. 2(b) and 2(c)]. This was confirmed ex vivo, where ACTH reduction by 20% after 24 hours from CtT adenoma cells in humans was observed [Fig. 2(f)]. Furthermore, in vivo testing using a xenograph model led to ACTH reduction in mice treated with SAHA [Fig. 5(c)]. These early effects of SAHA may be due to significant downregulation of POMC expression, as demonstrated by qRT-PCR [Fig. 2(d)], and suppression of the POMC promoter, as seen in the luciferase transfection study, leading in turn to a reduction in ACTH release [Fig. 2(e)]. These results compare favorably with the effects of pasireotide in the reduction of ACTH secretion (approximately 10% at 24 hours) (30).
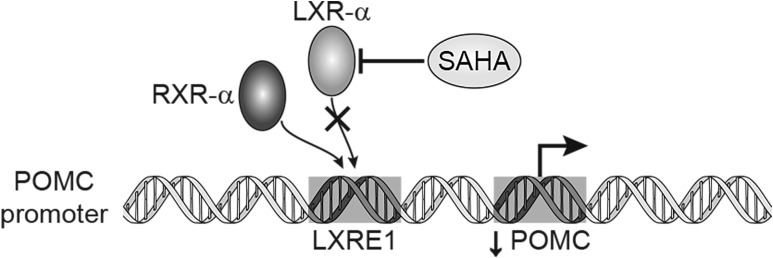
Whole-genome analysis revealed that SAHA induced a 2.3-fold downregulation of the LXRα gene. Using the STRING database to assess functional protein association networks, we identified a direct correlation between POMC and LXRα. LXRα and β are ligand-activated transcription factors belonging to the nuclear receptor superfamily (23), for which activity is dependent on the formation of obligate heterodimers with the RXRs (23). In the absence of ligand activation, the RXR/LXR heterodimer is thought to remain bound to the promoter region in complex with corepressors, thereby blocking the activation of target genes (23). LXRs play a critical role in the control of gene transcription involved in lipid and carbohydrate metabolism (23, 31, 32). Moreover, recent studies have shed light on the possible roles of LXRs in the hypothalamic-pituitary-adrenal axis, where the use of a synthetic LXR ligand (TO901317) induced POMC gene expression in mice pituitary glands via binding of LXRα to the LXR response element 1 found within the promoter region of POMC (Fig. 6) (33). This suggests that LXRα positively regulates the POMC gene at the transcriptional level.
Figure 6.
Proposed mechanism for suppression of POMC transcription by SAHA. Schematic representation of the effects of SAHA on LXRα leading to transcriptional downregulation of POMC. By selectively degrading LXRα in tumor cells, heterodimerization with RXRα at the LXR response element 1 site was decreased; therefore, downstream transcription was reduced.
Interestingly, in our study, SAHA reduced both the protein and mRNA levels of LXRα but did not seem to have a significant effect on RXRα [Fig. 4(f)]. Expectedly, immunoprecipitation showed that LXRα/RXRα heterodimerization was reduced with increasing doses of SAHA, in part because of reduced LXRα availability [Fig. 4(c)]. Our work provides evidence that SAHA suppressed expression of the prohormone POMC, abolishing ACTH hypersecretion. LXRα degradation seems to be the predominant mechanism for this response, though the details remain to be elucidated (Fig. 6).
Consistent with previous studies validating the beneficial effects of SAHA on malignant cell lines (34, 35), clinically relevant concentrations were effective in abrogating tumor growth in a nude-mice AtT-20 xenograft model, and tumor volume was suppressed up to 70% [Fig. 5(a) and 5(b)]. Preliminary data from this study demonstrate that SAHA significantly decreased ACTH levels in our xenograft model [Fig. 5(c)]. The ACTH levels were likely affected by both direct effect of SAHA on ACTH secretion (via LXR suppression) and tumor involution (by apoptosis induction). Remarkably, expression of LXRα in normal corticotrophs [Fig. 4(f)] and serum ACTH levels from healthy nude-mice were not affected following acute (Supplemental Fig. 1B (123.5KB, pdf) ) or chronic [Fig. 5(c)] treatment with SAHA, highlighting its targeted effects on tumors cells. Studies demonstrate that histone acetylation increased in both normal and tumor cells after treatment with SAHA (36), but the underlying mechanisms for differential LXRα remain unknown.
In humans, CD is caused by adenomas that are quite heterogeneous, both in terms of underlying genetic mutations (22) and gene expressions. We found that SAHA was also effective against hCtT (n = 7) [Fig. 1(e) and 1(f)]. A clinical trial is planned to evaluate the effects of SAHA on ACTH and cortisol levels in addition to the expected antitumor effect. HDACis are also effective radiosensitizers (37, 38), raising the possibility of coadjuvant SAHA and radiation for recurrent CD. Lastly, epidermal growth factor receptor signaling is affected by USP8 mutation (22) and may in turn lead to POMC upregulation (39). SAHA may affect epidermal growth factor receptor signaling through its effects on cytoplasmic HDAC6 (40).
Conclusion
In this study, the proapoptotic effect of the FDA-approved HDACi SAHA in ACTH-secreting cells was observed. Decreased cellular viability and elevated markers of proapoptotic cell death were demonstrated. In vivo testing revealed tumor involution and decreased ACTH levels in an AtT-20 xenograft model. These findings provide evidence of the mechanisms by which SAHA leads to decreased ACTH secretion and support its potential role in the treatment of CD.
Acknowledgments
We thank Dr. Steven L. Sabol at the National Heart, Lung and Blood Institute for his generous gift of AtT-20 cells. We also thank Dr. Constantine A. Stratakis for insightful discussions.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Neurologic Diseases and Stroke, Bethesda, Maryland. It was supported also by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH. For a complete list, please visit the Foundation website at https://fnih.org/what-we-do/current-education-and-training-programs/mrsp.
Clinical trial registry: ClinicalTrials.gov no. NCT00060541 (registered 7 May 2003).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- ANOVA
- analysis of variance
- AtT-20
- ACTH-secreting tumor
- Bid
- BH3-interacting domain death agonist
- CD
- Cushing disease
- COUP-TF1
- chicken ovalbumin upstream promoter transcription factor 1
- CtT
- corticotroph tumor
- DEG
- differentially expressed gene
- EASE
- Expression Analysis Systematic Explorer
- ELISA
- enzyme-linked immunosorbent assay
- FACS
- fluorescence-activated cell sorting
- FDA
- US Food and Drug Administration
- HDACi
- histone deacetylase inhibitor
- hCtT
- human-derived corticotroph tumor
- LXRα
- liver X receptor alpha
- mRNA
- messenger RNA
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NIH
- National Institutes of Health
- PARP
- poly (adenosine 5′-diphosphate-ribose) polymerase
- POMC
- pro-opiomelanocortin
- qRT-PCR
- quantitative real-time polymerase chain reaction
- RXR
- retinoid X receptor
- SAHA
- suberoylanilide hydroxamic acid
- STRING
- Search Tool for the Retrieval of Interacting Genes/Proteins.
References
- 1.Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96(3):632–642. [DOI] [PubMed] [Google Scholar]
- 2.Dekkers OM, Horváth-Puhó E, Jørgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, Pereira AM, Sørensen HT. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277–2284. [DOI] [PubMed] [Google Scholar]
- 3.Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur J Endocrinol. 2012;167(3):311–326. [DOI] [PubMed] [Google Scholar]
- 4.Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, Laws ER Jr. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab. 2008;93(2):358–362. [DOI] [PubMed] [Google Scholar]
- 6.Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. 2009;94(1):223–230. [DOI] [PubMed] [Google Scholar]
- 7.Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BMK; Pasireotide B2305 Study Group . A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012;366(10):914–924. [DOI] [PubMed] [Google Scholar]
- 8.Páez-Pereda M, Kovalovsky D, Hopfner U, Theodoropoulou M, Pagotto U, Uhl E, Losa M, Stalla J, Grübler Y, Missale C, Arzt E, Stalla GK. Retinoic acid prevents experimental Cushing syndrome. J Clin Invest. 2001;108(8):1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo V, Giacomini D, Páez-Pereda M, Stalla J, Labeur M, Theodoropoulou M, Holsboer F, Grossman AB, Stalla GK, Arzt E. Retinoic acid as a novel medical therapy for Cushing’s disease in dogs. Endocrinology. 2006;147(9):4438–4444. [DOI] [PubMed] [Google Scholar]
- 10.Pecori Giraldi F, Ambrogio AG, Andrioli M, Sanguin F, Karamouzis I, Corsello SM, Scaroni C, Arvat E, Pontecorvi A, Cavagnini F. Potential role for retinoic acid in patients with Cushing’s disease. J Clin Endocrinol Metab. 2012;97(10):3577–3583. [DOI] [PubMed] [Google Scholar]
- 11.Kuendgen A, Schmid M, Schlenk R, Knipp S, Hildebrandt B, Steidl C, Germing U, Haas R, Dohner H, Gattermann N. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all-trans retinoic acid in patients with acute myeloid leukemia. Cancer. 2006;106(1):112–119. [DOI] [PubMed] [Google Scholar]
- 12.Adler JT, Hottinger DG, Kunnimalaiyaan M, Chen H. Combination therapy with histone deacetylase inhibitors and lithium chloride: a novel treatment for carcinoid tumors. Ann Surg Oncol. 2009;16(2):481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangeetha SR, Singh N, Vender JR, Dhandapani KM. Suberoylanilide hydroxamic acid (SAHA) induces growth arrest and apoptosis in pituitary adenoma cells. Endocrine. 2009;35(3):389–396. [DOI] [PubMed] [Google Scholar]
- 14.Leung CKH, Paterson JA, Imai Y, Shiu RPC. Transplantation of ACTH-secreting pituitary tumor cells in athymic nude mice. Virchows Arch A Pathol Anat Histol. 1982;396(3):303–312. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. [DOI] [PubMed] [Google Scholar]
- 16.Ma Q-Y, Zhang X-N, Jiang H, Wang Z-Q, Zhang H-J, Xue L-Q, Chen M-D, Song H-D. Mimecan in pituitary corticotroph cells may regulate ACTH secretion and the HPAA. Mol Cell Endocrinol. 2011;341(1-2):71–77. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(D1):D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7(10):854–868. [DOI] [PubMed] [Google Scholar]
- 21.Colao A, Pivonello R, Tripodi FS, Orio F Jr, Ferone D, Cerbone G, Di Somma C, Merola B, Lombardi G. Failure of long-term therapy with sodium valproate in Cushing’s disease. J Endocrinol Invest. 1997;20(7):387–392. [DOI] [PubMed] [Google Scholar]
- 22.Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, Tanaka K, Wieland T, Graf E, Saeger W, Ronchi CLC, Allolio B, Buchfelder M, Strom TM, Fassnacht M, Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31–38. [DOI] [PubMed] [Google Scholar]
- 23.Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13(6):433–444. [DOI] [PubMed] [Google Scholar]
- 24.Duvic M, Olsen EA, Breneman D, Pacheco TR, Parker S, Vonderheid EC, Abuav R, Ricker JL, Rizvi S, Chen C, Boileau K, Gunchenko A, Sanz-Rodriguez C, Geskin LJ. Evaluation of the long-term tolerability and clinical benefit of vorinostat in patients with advanced cutaneous T-cell lymphoma. Clin Lymphoma Myeloma. 2009;9(6):412–416. [DOI] [PubMed] [Google Scholar]
- 25.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–784. [DOI] [PubMed] [Google Scholar]
- 26.Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares AH, Smulson ME. Transient poly(ADP-ribosyl)ation of nuclear proteins and role of poly(ADP-ribose) polymerase in the early stages of apoptosis. J Biol Chem. 1998;273(22):13703–13712. [DOI] [PubMed] [Google Scholar]
- 27.Matthews GM, Newbold A, Johnstone RW. Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity. Adv Cancer Res. 2012;116:165–197. [DOI] [PubMed] [Google Scholar]
- 28.Wiegmans AP, Alsop AE, Bots M, Cluse LA, Williams SP, Banks K-M, Ralli R, Scott CL, Frenzel A, Villunger A, Johnstone RW. Deciphering the molecular events necessary for synergistic tumor cell apoptosis mediated by the histone deacetylase inhibitor vorinostat and the BH3 mimetic ABT-737. Cancer Res. 2011;71(10):3603–3615. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–490. [DOI] [PubMed] [Google Scholar]
- 30.Murasawa S, Kageyama K, Sugiyama A, Ishigame N, Niioka K, Suda T, Daimon M. Inhibitory effects of SOM230 on adrenocorticotropic hormone production and corticotroph tumor cell proliferation in vitro and in vivo. Mol Cell Endocrinol. 2014;394(1-2):37–46. [DOI] [PubMed] [Google Scholar]
- 31.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–219. [DOI] [PubMed] [Google Scholar]
- 32.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116(3):607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto S, Hashimoto K, Yamada M, Satoh T, Hirato J, Mori M. Liver X receptor-alpha regulates proopiomelanocortin (POMC) gene transcription in the pituitary. Mol Endocrinol. 2009;23(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, Rocha K, Wang H-G, Richon V, Bhalla K. Activity of suberoylanilide hydroxamic acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005;11(17):6382–6389. [DOI] [PubMed] [Google Scholar]
- 35.Mitsiades CS, Poulaki V, McMullan C, Negri J, Fanourakis G, Goudopoulou A, Richon VM, Marks PA, Mitsiades N. Novel histone deacetylase inhibitors in the treatment of thyroid cancer. Clin Cancer Res. 2005;11(10):3958–3965. [DOI] [PubMed] [Google Scholar]
- 36.Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60(18):5165–5170. [PubMed] [Google Scholar]
- 37.Munshi A, Tanaka T, Hobbs ML, Tucker SL, Richon VM, Meyn RE. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther. 2006;5(8):1967–1974. [DOI] [PubMed] [Google Scholar]
- 38.Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang S-MM, Harari PM. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62(1):223–229. [DOI] [PubMed] [Google Scholar]
- 39.Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, Melmed S. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121(12):4712–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Fan LX, Zhou X, Sweeney WE Jr, Avner ED, Li X. HDAC6 regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation in renal epithelial cells. PLoS One. 2012;7(11):e49418. [DOI] [PMC free article] [PubMed] [Google Scholar]