Abstract
Context:
Thyroid immune-related adverse events (irAEs) in patients treated with programmed death receptor-1 (PD-1) blockade are increasingly recognized as one of the most common adverse effects. Our aim was to determine the incidence and examine the potential mechanisms of anti-PD-1–induced thyroid irAEs.
Design:
Single-center, retrospective cohort study.
Patients and Measurements:
We studied 93 patients with advanced cancer (ages 24 to 82 years; 60% males) who received at least one infusion of pembrolizumab. Thyroid test results and thyroid imaging modalities were reviewed. Comprehensive 10-color flow cytometry of peripheral blood was performed.
Results:
Thirteen (14%) thyroid irAEs were observed. Thyroiditis occurred in seven patients (54%), from which four recovered. New onset of hypothyroidism overt/subclinical developed in three patients. Levothyroxine dosing required doubling in three patients with a known history of hypothyroidism. Thyroperoxidase antibodies were positive in the minority of the patients [4/13 (31%)] and diffuse increased 18fludeoxyglucose uptake of the thyroid gland was observed in the majority [7/11 (64%)] of patients. We observed more circulating CD56+CD16+ natural killer (NK) cells and an elevated HLA-DR surface expression in the inflammatory intermediate CD14+CD16+ monocytes in anti-PD-1–treated patients.
Conclusions:
Thyroid dysfunction is common in cancer patients treated with pembrolizumab. Reversible destructive thyroiditis and overt hypothyroidism are the most common clinical presentations. The mechanism of thyroid destruction appears independent of thyroid autoantibodies and may include T cell, NK cell, and/or monocyte-mediated pathways. Because the thyroid is a frequent target of anti-PD-1 therapies, patients with therapeutically refractory thyroid cancer may be ideal candidates for this treatment.
We studied 93 pembrolizumab-treated patients and found that destructive thyroiditis was the most common clinical presentation, possibly triggered by T cell, NK cell, and/or monocyte-mediated pathways.
Harnessing the immune system to fight cancer has now well-proven efficacy. The immune check point inhibitors pembrolizumab, nivolumab, and ipilimumab represent a class of immune-directed antineoplastic therapies, first approved in metastatic melanoma (1).These fully humanized, monoclonal antibodies block the negative regulatory receptors, cytotoxic T-lymphocyte associated protein 4 (CTLA-4) or programmed death receptor -1 (PD-1) on T cells, resulting in a de-repression and/or reactivation of cytotoxic T cell function.
Ipilimumab, the first immune check point inhibitor that targeted CTLA-4, resulted in durable tumor responses and an improvement in overall survival in metastatic melanoma patients (1, 2). Subsequent clinical trials with pembrolizumab, targeting the PD-1 receptor, generated great enthusiasm after demonstrating potent durable responses in patients with melanoma and with comparably less toxicity (3, 4). Of greatest interest, combination therapies with antibodies to CTLA-4 and PD-1 produced unprecedented clinical activities in advanced melanoma patients, with response rates as high as 40% (5). Not unexpectedly, the rates of grade 3 or 4 immune-related adverse events (irAEs) were also markedly higher compared with monotherapies (54% vs 24%) (6).
Currently, the indications for ipilimumab, pembrolizumab, and/or nivolumab have expanded to include unresectable or metastatic melanoma (7, 8), metastatic non–small cell lung carcinoma (9, 10), small cell lung cancer (11), Hodgkin lymphoma (12), head and neck squamous cell carcinoma (13), advanced Merkel cell carcinoma (14), and advanced clear cell renal cell carcinoma (15). Wider application of these immunotherapies has also resulted in the emergence of a unique array of irAEs, several of which are rather different within the oncology practices. The successes of these therapies across these broad types of cancer patients mandates the development of a keen clinical acumen focused on prompt identification and management of irAEs so that patients can achieve the maximum benefit from these potentially lifesaving therapies.
Endocrinopathies affecting the pituitary and thyroid are emerging as particularly unique, often symptomatic irAEs (16). Pembrolizumab-induced thyroid irAEs have been reported to range from 3.2% to 10.1% from limited data of phase 2 and 3 clinical trials (4, 17). Such studies are limited by the lack of standardized diagnostic criteria and terminology used to define thyroid irAEs. Recent studies have begun characterizing the incidence and clinical course of thyroid-related irAEs following immune therapy (18–20). There remains limited knowledge of the pathogenesis and the underlying cellular subtypes involved in the development of these irAEs in cancer patients treated with pembrolizumab. A better understanding and characterization of the clinical presentation of thyroid-related abnormalities as well as their potential mechanisms will improve clinical care of these patients and will help identify patients at risk for developing these irAEs and enable ongoing therapy with these highly efficacious treatments. Moreover, understanding the pathogenesis of irAEs, in this case, immune checkpoint-induced thyroiditis, may serendipitously provide data that can be used to design immune-based therapeutic strategies for select patients with advanced treatment refractory thyroid cancer (21, 22).
The purpose of this study was to comprehensively review and characterize anti-PD-1–induced thyroid irAEs in cancer patients within a single institution.
Our aims in this study were to (1) determine the incidence and clinical presentation of thyroid-related irAEs in cancer patients receiving pembrolizumab and (2) examine the potential mechanisms of anti-PD-1–induced thyroid irAEs by examining alterations in thyroid autoantibodies, thyroid uptake on 18fludeoxyglucose (FDG) positron emission tomography (PET)-computed tomography (CT) imaging, as well as comprehensively examining the circulating immune cell phenotype of patients with thyroid irAEs.
Materials and Methods
We performed an institutional review board–approved retrospective review of the electronic medical records of 93 cancer patients treated with pembrolizumab at Mayo Clinic Rochester between April 2014 and January 2015 to identify patients that developed thyroid abnormalities. When clinically indicated, pembrolizumab was administered with intravenous infusions of 2 mg/kg every 3 weeks. Screening thyroid function tests (TFTs), consisting of thyrotropin (TSH), thyroxine (FT4 or T4), and/or triiodothyronine, were performed in the majority of patients at baseline and in all patients every 3 weeks while on therapy. Prior treatment with other immunotherapies was recorded. Thyroiditis was defined by the presence of a suppressed TSH (<0.3 mIU/L) level with normal or elevated T4 or triiodothyronine that spontaneously resolved or progressed to overt hypothyroidism. Subclinical hypothyroidism was defined by an elevated TSH (>4.2 but ≤10 mIU/L) with normal serum FT4 and overt hypothyroidism was defined by elevated TSH (>10) with or without low FT4. Recurrent hypothyroidism was identified in select patients with a history of hypothyroidism and on stable thyroid hormone replacement who, following initiation of pembrolizumab, developed an acute rise in TSH requiring an increased in their levothyroxine dose by >50%.
In selected cases in which stored plasma was available, anti-thyroid peroxidase autoantibodies (TPO-abs) were measured at baseline and after development of thyroid dysfunction. When available, baseline and posttherapy 18FDG-PET/CT images and reports were reviewed for the presence or absence of increased 18FDG uptake within the thyroid gland. Thyroid uptake and scans were reviewed, as were TSH receptor antibodies (TRabs) and thyroid-stimulating immunoglobulin results were collected.
Peripheral blood immunophenotyping by flow cytometry
To characterize the circulating phenotype of patients with thyroiditis/hypothyroidism, we formed flow cytometry analyses on peripheral blood samples collected in K2EDTA tubes (Becton Dickinson, Franklin Lakes, NJ) from seven patients who developed anti-PD-1–induced acute thyroiditis and/or hypothyroidism. These profiles were compared with 45 healthy volunteers and nine patients with autoimmune thyroid disease (seven cases of Hashimoto disease and two cases of Graves disease) (Supplemental Table 1 (758.4KB, docx) ). Flow cytometry was performed enabling absolute cell number quantifications as well as relative percentages (23).
Unmanipulated whole blood was stained with antibodies directly. Flow cytometry was performed on the three-laser, 10-color Gallios Flow Cytometer (Beckman Coulter, Brea, CA). All procedures, antibodies, flow protocols, instrument settings, and gating strategies have been previously described by Gustafson et al. (23). Analysis of flow data was performed using Kaluza (Beckman Coulter) software. Descriptive statistics were used to determine mean and standard deviation or median and ranges depending on data distribution, whereas categorical data are shown as a percentage. Comparisons between different cohorts were tested for statistical significance via the Mann-Whitney nonparametric t test. All graphical representations and statistical analyses were performed in Prism 7 (GraphPad, San Diego, CA).
Results
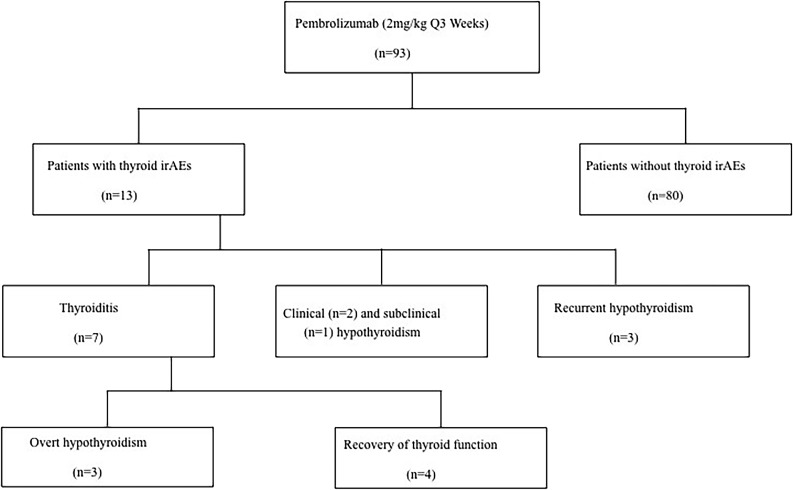
From our cohort of 93 cancer patients, with a median age of 59 (range, 24 to 82), 13 (14%) developed abnormal TFTs following initiation of pembrolizumab with a median follow-up of 8 months (range, 3 to 41). The clinical characteristics of these cases are presented in Table 1. Twelve of the 13 patients had metastatic malignant melanoma and one non–small cell lung carcinoma. The majority of patients (10 of 13) received ipilimumab (4 mg/kg every 4 weeks for a total of four cycles) before pembrolizumab. Of the 13 cases, seven (54%) developed acute thyroiditis, three of which progressed to hypothyroidism, whereas four patients resolved to euthyroid levels. Two patients (15%) developed new-onset hypothyroidism, one (0.7%) transient subclinical hypothyroidism, and three (23%) recurrent hypothyroidism (Fig. 1). The cause of hypothyroidism in patients 8, 9, and 10 (Table 2) before initiation of pembrolizumab were ipilimumab-induced, interferon-induced, and primary hypothyroidism, respectively. There was no sex predilection for thyroid-related irAEs (P = 0.56). The median time of onset of abnormal TFTs following pembrolizumab was 6 weeks (range, 4 to 28; i.e., within the first two cycles of pembrolizumab infusion). Cases of transient thyroiditis were mild and were managed conservatively without the need of initiation of beta-blocker therapy, steroid therapy, or any antithyroid medications. Patients returned to a euthyroid state in a median time of 6.5 weeks (range, 4 to 14 weeks).
Table 1.
Pembrolizumab-Induced Thyroid Dysfunction, Clinical Characteristics
| Characteristic | Result |
|---|---|
| No. with abnormal TFTs | 13 of 93 (14%) |
| Thyroiditis | 7 (4 recovered, 3 progressed to hypothyroidism) |
| New-onset hypothyroidism (clinical and subclinical) | 3 |
| Recurrent hypothyroidism | 3 |
| Hyperthyroidism not associated with thyroiditis | 0 |
| Sex | |
| Males | 7 of 57 (12%) |
| Females | 6 of 36 (17%) |
| Median age, y (range) | 59 (24– 82) |
| Cancer type | |
| Metastatic melanoma | 12 |
| Non–small cell lung carcinoma | 1 |
| Median follow-up, mo (range) | 8 (3– 41) |
| Median time to onset of thyroid dysfunction, wk | 6 |
| No. cases of thyroiditis/hypothyroidism with PET scans | 13 |
| FDG uptake in thyroid at baseline | 2 |
| New FDG uptake after pembrolizumab | 7 |
| Median time of increased FDG uptake, wk | 12 |
| No. cases with TPO-abs measured at time of abnormal TFTs | 12 |
| Elevated (mean titer) | 5 (85 IU/mL) |
| Received ipilimumab before pembrolizumab | 10 |
| Recovery of thyroid function | |
| No | 5 (50%) |
| Yes | 5 (50%) |
| Overall status at last follow-up | |
| Alive | 12 |
| Dead | 1 |
Figure 1.
Overview of the investigated cohort of pembrolizumab-treated cancer patients. Q, every.
Table 2.
Summary of the 13 Cases With Pembrolizumab-Induced Thyroid irAEs
| Case Summaries |
Baseline |
Following Pembrolizumab Therapy |
Overview of 18FDG-PET/CT Imaging |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual Uptake | |||||||||||
| Case | Thyroid-Related irAE | TSHa | TSHa | FT4b | T3 | FT3/FT4 | Anti-TPOc | TRab/TSI | Baseline | After Abnormal TFTs | Time From First Pembrolizumab Infusion, mo |
| 1 | Thyroiditis, transient | 1.4 | 0.02 | 1.3 | NA | — | 2.6 | Negative | NA | Increased | 3 |
| 2 | Thyroiditis, transient | 1.1 | 0.2 | 1.0 | 3.4d | 3.4 | n/a | NA | No increase | No increase | 7 |
| 3 | Thyroiditis, transient | 2.0 | 0.08 | 1.3 | 460c | — | 1.1 | Negative | Increased | No increase | 3 |
| 4 | Thyroiditis transient | 2.3 | 0.01 | 3.2 | NA | — | 0.3 | Negativef | No increase | Increased | 3 |
| 4.7 | 1.1 | ||||||||||
| 5 | Thyroiditis, progressing to hypothyroidism | 0.7 | 0.01 | 2.2 | NA | — | 0.7 | Negativef | No increase | Increased | 6 |
| 6 | Thyroiditis, progressing to hypothyroidism | 1.1 | <0.01 | 1.7 | 4.8d | 2.8 | 2.0 | Negative | No increase | Increased | 3 |
| 20 | 0.7 | 2.4 | |||||||||
| 7 | Thyroiditis, progressing to hypothyroidism | 2.8 | 0.01 | 3.4 | 125c | 16.1 | 0.7 | Negative | No increase | NA | NA |
| 37.3 | 0.4 | 55c | 5.5 | ||||||||
| 8 | Recurrent hypothyroidism | 5.1 | 95g | 0.7 | NA | — | >800 | NA | Increased | NA | NA |
| 9 | Recurrent hypothyroidism | 3 | 24g | 0.8 | NA | — | 31.7 | NA | No increase | Increased | 3 |
| 10 | Recurrent hypothyroidism | 0.47 | 83g | NA | NA | — | 27 | NA | No increase | No increase | 2 |
| 11 | New hypothyroidism | 2.3 | 43 | 0.7 | NA | — | 0.3 | NA | NA | No increase | 24 |
| 12 | New hypothyroidism | 3.4 | 20.2 | 0.9 | NA | — | 3.3 | NA | No increase | Increased | 5 |
| 13 | Subclinical hypothyroidism, transient | 1.9 | 6.1 | 1 | NA | — | 16.8 | NA | No increase | Increased | 3 |
Abbreviations: NA, not available; T3, triiodothyronine; TSI, thyroid-stimulating immunoglobulin.
TSH, 0.3 to 4.2 mIU/L.
FT4, 0.9 to 1.7 ng/dL.
Total T3, 80 to 200 ng/dL.
FT3, 2 to 3.5.
TPO, <9 IU/mL.
Thyroid uptake/scan negative.
Levothyroxine dose doubled.
In the majority of the cases (12 of 13), TPO-abs were available at the time of abnormal TFTs (Table 2.). In six of seven available cases of thyroiditis, TPO-abs were negative. TPO-abs were positive only in four of 13 cases (median 29 IU/mL), and the majority of these (3/4) were in patients with a preexisting diagnosis of hypothyroidism (Table 2). In four patients with pembrolizumab-induced thyroiditis, we evaluated TPO-abs levels at baseline using stored plasma samples (Table 3). Two patients had positive TPO-abs at baseline (median, 408 IU/mL), with one progressing to new-onset subclinical hypothyroidism, whereas the other developed recurrent hypothyroidism requiring a >50% increase in their levothyroxine dose. Two patients that developed thyroiditis with negative TPO-abs at the time of abnormal TFTs had negative TPO-abs at baseline. Finally, we measured TPO-abs from stored serum in 11 patients treated with pembrolizumab that did not develop thyroid abnormalities; none was found to have elevated TPO-abs at baseline.
Table 3.
Comparison of TPO-abs Levels at Baseline and Following Pembrolizumab Treatment
| Thyroid Antibody Status | Baseline | Following Pembrolizumab Treatment | |
|---|---|---|---|
| Case no. | Thyroid-related irAE | Anti-TPOa | Anti-TPOa |
| 1 | Thyroiditis, transient | 0.9 | 2.6 |
| 5 | Thyroiditis, progressing to hypothyroidism | 0.7 | 0.7 |
| 8 | Recurrent hypothyroidism | >800 | >800 |
| 13 | Subclinical hypothyroidism, transient | 17 | 16.8 |
Reference range, TPO <9 IU/mL.
In six of seven cases of thyroiditis, TRabs titers were tested and were negative, making Graves-like disease an unlikely etiology. In the patient without available TRabs, the FT3/FT4 ratio was 3.4, suggesting that a destructive thyroiditis was the most likely underlying process (24, 25). In addition, thyroid uptake and scans were performed in two of seven cases presenting with thyrotoxicosis and both scans demonstrated suppressed iodine uptake, consistent with an acute destructive thyroiditis (Table 2).
All 13 patients with thyroid irAEs underwent 18FDG PET/CT imaging at some point in their oncologic surveillance (Table 2). Increased thyroid 18FDG uptake at the time of abnormal TFTs was noted in 7/11 (64%) available cases. New diffuse 18FDG uptake in the thyroid gland after initiation of pembrolizumab therapy compared with baseline was identified in 6/9 patients (67%). The median time of onset of new FDG thyroid uptake was 12 weeks. We observed two patients with baseline diffuse increased 18FDG uptake in the thyroid; one developed transient thyroiditis and the other worsening hypothyroidism.
Although not the main focus of this study, from the total cohort of 93 patients, 14 (15%) developed ipilimumab-induced hypophysitis before the first infusion of pembrolizumab, consistent with published data (26). An additional two cases of hypophysitis were identified immediately after the first cycle of pembrolizumab, but each had received ipilimumab within 4 months, supporting an ipilimumab-induced effect (26).
Because the clinical features of pembrolizumab-induced thyroid irAEs seem to overlap with that of autoimmune thyroid disease, we examined whether there was similar immune cell involvement in these two settings. We performed prospective comprehensive immune phenotyping of the blood of seven patients that developed anti-PD-1–induced thyroiditis and compared these patterns with healthy volunteers and patients with autoimmune thyroid disease (Supplemental Table 1 (758.4KB, docx) ).
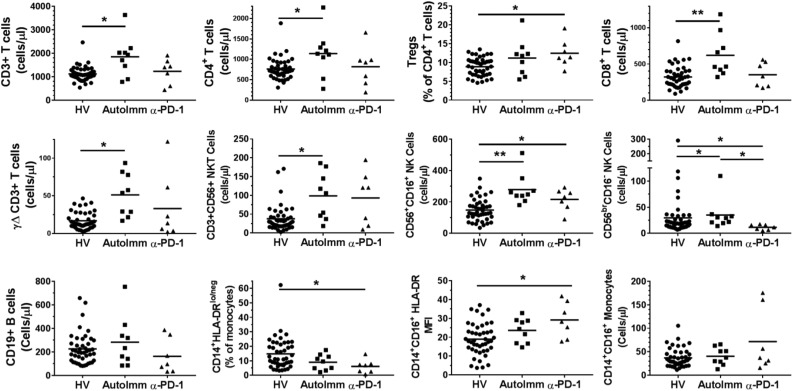
Autoimmune patients exhibited substantial increases in CD3+ T-cell counts (cells/µL) and both CD4+ and CD8+ T-cell subpopulations (Fig. 2). Additionally, other T-cell subsets such as γΔ T cells and CD56+CD3+ natural killer (NK) T-cell counts were elevated in autoimmune patients (Fig. 2). However, we saw no such increases in pembrolizumab-treated patients. On the other hand, CD56+CD16+ NK cells were elevated in both autoimmune and pembrolizumab-induced thyroiditis patients. Paradoxically, “immature” CD56brCD16− NK cells were decreased in pembrolizumab-induced thyroiditis patients, whereas they were elevated in autoimmune patients.
Figure 2.
Immunophenotypic differences in patients with autoimmune thyroiditis compared with patients with pembrolizumab-induced thyroiditis. Immune cell populations were measured by flow cytometry as cell counts (cells/µL), % of a parent population, or mean fluorescence intensity (MFI) where indicated. Healthy volunteers (HV; circles), patients with autoimmune thyroiditis (AutoImm; squares), and patients with pembrolizumab-induced thyroiditis (α-PD-1; triangles) are shown. The horizontal line represents the mean value for each cohort. *P < 0.05; **P < 0.001.
Loss of HLA-DR expression on CD14+ monocytes is a mechanism by which melanoma tumors cause systemic immunosuppression (27). We found that pembrolizumab-induced thyroiditis patients exhibited a substantial decrease in the number of immunosuppressive monocytes that have low HLA-DR expression (CD14+HLA-DRlo/neg monocytes). HLA-DR surface expression was specifically elevated in the inflammatory intermediate CD14+CD16+ monocytes, whereas the overall numbers did not change in peripheral blood. There were no major differences in the number of circulating immune cells representing circulating regulatory T cells, B cells, total monocytes, granulocytes, or dendritic cells among the three groups (Fig. 2 and data not shown). These data suggest that the absolute numbers of T-cell subsets may play a role in autoimmune disease that is not necessarily reflected in the pembrolizumab-treated patients. Monocyte activation, through upregulation of HLA-DR may be a potential mechanism of pembrolizumab-induced thyroiditis. Circulating T cells expressed very low levels of CTLA-4, and we saw no statistical differences in any of the comparisons (data not show).
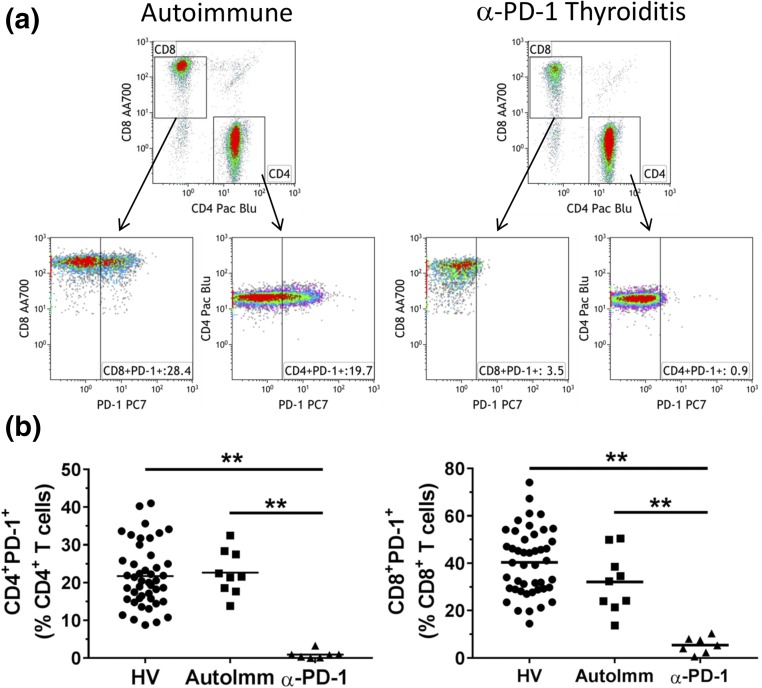
Because PD-1 blockade restores T-cell function, we hypothesized that perhaps the activation of T cells through a PD-1–dependent pathway, may also contribute to pembrolizumab-induced thyroiditis. Therefore, we compared PD-1 levels on T cells from these groups. Figure 3(a) shows the gating strategy for detecting PD-1 on both CD4+ and CD8+ T cells. As expected, there was no detectable surface expression of PD-1 on T cells from pembrolizumab-induced thyroiditis patients; on the contrary, PD-1 expression on T cells from autoimmune patients was not different than healthy volunteer controls [Fig. 3(b)]. As such, whereas the role of PD-1–dependent T-cell activation may contribute to T cell–mediated destruction of the thyroid in pembrolizumab-treated patients, the role of PD-1 in the autoimmune setting seem less likely, consistent with known antibody-mediated mechanism for the latter (28). These data provide additional support for a distinct pattern of immune-mediated thyroid destruction in autoimmune patients compared with pembrolizumab-induced thyroiditis patients.
Figure 3.
Comparisons of PD-1 expression on peripheral blood T cells from healthy volunteer controls, patients with autoimmune thyroiditis, and patients with pembrolizumab-induced thyroiditis. (a) Flow cytometric dot plots outlining the gating strategy for measuring PD-1 expression. CD4+ and CD8+ T cells were displayed from a CD3+ parent T-cell gate (not shown). PD-1 expression is displayed from both CD4+ and CD8+ T cells. Gates depicting PD-1–positive cells were set using fluorescence minus one staining protocols where PD-1 fluorescent antibody was removed. Representative dot plots from a patient with autoimmune thyroiditis and a patient with pembrolizumab-induced thyroiditis are shown. (b) PD-1 expression of the three cohorts: HV (circles), AutoImm (squares), and α-PD-1 (triangles). The horizontal line represents the mean value for each cohort. **P < 0.001.
Discussion
Immune checkpoint inhibitors, by blocking CTLA-4 and/or PD-1, result in reactivation of antitumor cytotoxic T cells and induce potent anti-tumor responses that are revolutionizing cancer therapies.
PD-1 is a type 1 transmembrane protein of the immunoglobulin superfamily (29) that is minimally expressed on resting immune cells. Upon activation through inflammatory signals, PD-1 receptor expression becomes broadly induced not only on T cells, but also on B cells, NK cells, NKT cells, dendritic cells, and macrophages (30). Binding of PD-1 to its ligands PD-L1 or PD-L2 induces negative regulatory signals resulting in inhibition of T-cell proliferation, cytokine production, and cytotoxic activity; a mechanism of controlling inflammation. PD-L1, the most abundant of the two ligands, is constitutively expressed on the surface of multiple tissue types, including hematopoietic cells and tumor cells. Expression of PD-L1 by the tumor cells results in “T-cell exhaustion,” allowing tumor cells to escape host immune surveillance (31).
CTLA-4, the first identified negative regulatory receptor on T cells, like PD-1, is expressed at the cell surface of activated cytotoxic T cells and may dampen anti-immune responses. In antitumor immunity, tumor-derived antigens are recognized and processed by antigen-presenting cells (APCs) and presented to naïve T cells via major histocompatibility complex class II molecules; costimulatory B7 ligands on APCs bind to T-cell receptor and C28 on T cells, respectively, resulting in a cytotoxic T-cell activation and antitumor immunity. Once activated, however, cytotoxic T cells upregulate CTLA-4 receptors on their cell surface, which have higher affinity for B7 than CD28. This results in a physiologic “break” on the T-cell activation leading to “T-cell exhaustion” (32). Monoclonal antibodies blocking negative regulatory pathways (i.e., against CTLA-4, PD-1 or PD-L1) may lead to a derepression and/or reactivation of cytotoxic T-cell activity that in cancer patients may translate into potent antitumor immune responses.
Despite their common negative regulatory effects on T-cell functions, PD-1 and CTLA-4 have distinct effects on the immune system. For example, CTLA-4–deficient mice exhibit rapidly progressive, fatal lymphoproliferative disease, characterized by multiorgan T-cell infiltration and death by 3 to 4 weeks of age (33, 34). PD-1–deficient mice, on the other hand, develop a delayed-onset, organ-specific autoimmunity (such as lupus-like arthritis, glomerulonephritis, and autoimmune-dilated cardiomyopathy) that appears in autoimmune-prone mice and in an age-dependent manner (35). These observations suggest that the CTLA-4 pathway modulates the early-phase activation followed by repression of naïve or memory T cells, and absence of CTLA-4 results in a generalized systemic autoimmune process (36). PD-1, on the other hand, appears to modulate the activity of T cells in the periphery during active inflammatory processes (such as chronic inflammation, viral infection, or cancer), thereby limiting autoimmunity in conditions of persistent antigen stimulation (37) and absence of which may result in tissue-specific autoimmunity.
Distinct irAE profiles are thus not surprisingly noted between anti-PD1 and anti-CTLA-4 therapies. In cancer patients treated with ipilimumab, comprehensive review of endocrine irAEs show that hypophysitis occurs in 0% to 17% (26, 38), whereas thyroiditis/hypothyroidism is less frequent, 1.5% to 9% (1, 39). In contrast, in our study and consistent with others, anti-PD-1 immunotherapies show a reversed immune toxicity profile with a <1% incidence of hypophysitis (38) and a much higher incidence (3% to 21%) of thyroid abnormalities (18–20), recognizing, however, that our study was not a comprehensive review of PD-1–induced hypophysitis.
Data examining the underlying pathogenesis of irAEs have been sparse; however, several studies on anti-CTLA-4–induced irAEs have identified tissue-specific mechanisms involving T cells, cytokines, and/or antibody-mediated destructive processes. Histological data derived from patients with anti-CTLA-4 induced enterocolitis have shown a neutrophilic and/or lymphocytic inflammation of the small bowel mucosa with an increased infiltration of CD8 and CD4 T cells (40). In mouse models with anti-CTLA-4–associated hypophysitis, an antibody-dependent toxicity was observed by circulatory anti-TSH and antiprolactin antibodies and deposition of complement in CTLA-4–expressing pituitary cells (41). Finally, a cytokine-mediated toxicity (interleukin-17) has been shown to contribute to anti-CTLA-4–induced enterocolitis (42).
Studies on the pathogenesis of anti-PD-1–induced irAEs are even more sparse, with some data indicating a depletion of both regulatory T cells and B cell–mediated mechanisms in patients with autoimmune bullous pemphigoid (43). In mice, data show that blocking PD-1 leads to activation of B cells and development of B-cell germinal centers, favoring a humoral rather than a cellular-mediated autoimmune response (44, 45).
In our study, we have identified thyroiditis, either acute and self-limiting or progressing to hypothyroidism, as the most common endocrine irAE following PD-1 blockade, which is perhaps not unexpected given that autoimmune thyroid disease represents the most common autoimmune disease. Limited data suggest that genetic susceptibilities linked to HLA phenotypes, as seen in autoimmune diabetes (46) and thyroid diseases (47), or polymorphisms in either CTLA-4 (48), PD-1, or cytokine-related genes, may alter immune responses and/or increase thyroid autoimmune susceptibility (49–51). Further studies are needed to determine whether immune checkpoint inhibitor-induced thyroiditis shares the same pathophysiological fingerprint with autoimmune thyroid disease.
To identify potential immune phenotypes that may mediate pembrolizumab-induced thyroiditis, we took a comprehensive system-based approach to measure peripheral blood leukocytes and compared these profiles with patients with autoimmune thyroid disease. Hashimoto thyroiditis, the most common autoimmune thyroid disease, involves complex immune responses, which include APCs and CD4+ T helper cells that activate CD8+ T cells, resulting in direct tissue injury and thyroid antigen exposure. The latter leads to B-cell activation and differentiation into plasma cells, resulting in expression of thyroid-restricted antibodies that in turn lead to an antibody or complement-dependent thyroid cell destruction. Secretion of inflammatory molecules (i.e., interferon-γ) by the thyroid itself may further enhance the autoimmune destructive process, ultimately resulting in a diffuse lymphocytic infiltration, generation of lymphoid germinal centers, and destruction of thyroid follicles (28, 52–54).
In our study, we illustrate by flow cytometry a complete loss of circulatory PD-1+ T cells (Fig. 3), consistent with pembrolizumab treatment that may either have interfered with fluorescence-activated cell sorter detection and/or may have resulted in loss of these circulating cell types. Autoimmune thyroid patients had PD-1 levels similar to healthy volunteer controls but had a broad increase of NK-cell and T-cell subpopulations, including CD4+ T helper cells, CD8+ cytotoxic T cells, γΔ T cells, and NKT cells. Patient with pembrolizumab-induced acute thyroiditis exhibited unique phenotypic differences from autoimmune disease patients, which included a decrease in immature NK cells (CD56brCD16−) as well as a decrease in CD14+HLA-DRlo/neg immunosuppressive monocytes. Our group has shown that the latter are elevated in a variety of cancer patients and associated with immunosuppression via several mechanisms (55); reductions in this cell type may increase risks for autoimmunity. Taken altogether, the data from this study suggest that there are phenotypic differences between autoimmune thyroid patients and pembrolizumab-induced thyroiditis patients. These potential biomarkers may provide avenues toward understanding pathogenesis of immune checkpoint inhibitor-induced irAEs as well as in identifying patients at risk for developing pembrolizumab-induced thyroiditis.
The role of thyroid autoantibodies in the pathogenesis of and/or in predicting an increased risk for anti-PD-1–induced thyroid abnormalities remains unclear. Acknowledging our small sample size and the absence of baseline TPO-abs in the entire cohort, our data, similar to a recent study (20), suggest that TPO-abs are uncommonly associated with immune checkpoint-induced thyroid dysfunction, given that the majority of patients with abnormal TFTs had negative TPO-abs, signifying an antibody-independent mechanism or the presence of other thyroid autoantibodies not presently measured. However, high TPO-abs titer at baseline or history of hypothyroidism predicts an increased risk of worsening/recurrent hypothyroidism following treatment with anti-PD-1, and close follow-up of this patient population is needed. In the study of Osorio et al. (18), antithyroid antibodies were present in the majority of patients that developed anti-PD-1 thyroid dysfunction (80% vs 8%, P < 0.0001). However, it is unclear whether the presence of thyroid autoantibodies is the cause of thyroid dysfunction or the result of a humoral immunological response to thyroid antigens released during a destructive thyroiditis process.
We now appreciate that the majority of patients with anti-PD-1–induced thyroiditis develop diffuse increased 18FDG uptake on PET/CT in the thyroid (Supplemental Fig. 1 (758.4KB, docx) ), similar to a recent study (20). In our cohort, 67% of patients with abnormal TFTs had diffuse increased 18FDG uptake of the thyroid following pembrolizumab treatment, with median time of onset of 12 weeks. A similar pattern of 18FDG uptake is commonly described in patients with chronic lymphocytic Hashimoto thyroiditis (56) and to a lesser extent in Graves disease (57); again, in line with an intense inflammatory process. Although focal uptake in the thyroid may represent thyroid cancer, this pattern of diffuse uptake is more consistent with inflammation rather than of a malignant process, either primary or metastatic. Future studies, including fluorescence-activated cell sorter and/or histologic characterizations of thyroid tissues following anti-PD-1–induced thyroiditis may significantly expand our understanding of the cell types mediating thyroid destruction.
Clinically, in our comprehensive retrospective study of pembrolizumab-treated cancer patients, the incidence of thyroid abnormalities was 14% higher than that reported in clinical trials (3, 4), but consistent with more recently published studies (18–20), likely because of rigorous diagnostic criteria applied. Thyroiditis (54%) was the most common thyroid abnormality, with spontaneous recovery in four of seven (57%) cases. Because no cases of Graves-like hyperthyroidism were noted, observation rather than additional diagnostic testing and/or antithyroid drugs is the recommended approach (19). In patients with preexisting thyroid disease, recurrent severe hypothyroidism (median TSH, 83) not attributable to colitis or other medications known to increase hepatic metabolism of levothyroxine (e.g., phenytoin, rifampin), developed in three patients (23%), necessitating a doubling in thyroid replacement. Thus, close monitoring of patients on thyroid hormone replacement when initiated on pembrolizumab therapy is required.
Strengths and limitations
Our study is a comprehensive review of pembrolizumab-induced thyroid irAEs, using well-established diagnostic criteria in a large number of cancer patients receiving pembrolizumab. This study describes the immunological features of cancer patients treated with anti-PD-1 using immune phenotyping data results. Up to now, studies have based their conclusions of the underlying mechanisms using solely serum thyroid antibody measurements (18) and/or results from 18FDG PET/CT imaging studies (20). Our analysis, although comprehensive and performed at a center with an extended experience in cancer patients treated with immune check point inhibitors, has limitations. Both the retrospective nature of the analysis and the limited comprehensive endocrine evaluations in many patients represent challenges in accurately diagnosing endocrine irAEs. The majority of the cancer patients were pretreated with ipilimumab, which may influence susceptibility for other irAEs following anti-PD-1 therapy. Although our data represent a comprehensive investigation of circulating immune phenotypes in thyroiditis patients, the relative small number of samples does limit definitive conclusions, but may be hypothesis-generating. Furthermore, we acknowledge that some immunophenotypes may be affected by the melanoma itself. We have previously reported that HLA-DR expression on intermediate monocytes is downregulated in stage 4 untreated melanoma patients (27), whereas in this study, intermediate monocyte HLA-DR was significantly increased in pembrolizumab-induced thyroiditis patients. As such, additional longitudinal studies are warranted to dissect the contributions of the cancer and the checkpoint inhibitors on the immune system. This study investigates the underlying involved mechanism of thyroid irAEs in patients receiving anti-PD-1 using this technique.
Conclusion
In summary, the incidence of anti-PD-1–induced thyroid irAEs is high. Results from our study, similar to others (18–20, 39), demonstrate a relatively uniform pattern of an acute but transient thyroiditis, biochemically accompanied by thyrotoxicosis, which may be striking, followed by resolution or progression to hypothyroidism. We observed an increase in peripheral CD56+CD16+ NK cells and HLA-DR surface expression of intermediate monocytes in patients with anti-PD-1–induced thyroid irAEs. Understanding the clinical presentation and management of these irAEs allows optimized patient care, ensures continuation of lifesaving therapies, and offers the opportunity to investigate common and otherwise rare autoimmune-mediated processes such as thyroiditis and hypophysitis. Further investigations into the immunologic mechanisms contributing to the development of these irAEs are needed. Given the propensity of anti-PD-1 alone, or in combination with anti-CTLA-4 antibodies, to target the thyroid, harnessing this effect may prove relevant for patients with advanced, therapeutically refractory thyroid cancers.
Acknowledgments
Acknowledgments
This project was supported by the Clinical and Translational Science Awards (Grant UL1 TR000135) from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosure Summary: L.K. has previously consulted for Novartis Pharmaceuticals (honorarium) and Bristol-Myers Squibb (monies paid to institution). The remaining authors have nothing to disclose.
Footnotes
- APC
- antigen-presenting cell
- CT
- computed tomography
- CTLA-4
- cytotoxic T-lymphocyte associated protein 4
- FDG
- fludeoxyglucose
- FT4
- thyroxine
- irAE
- immune-related adverse event
- NK
- natural killer
- PD-1
- programmed death receptor-1
- PET
- positron emission tomography
- T4
- thyroxine
- TFT
- thyroid function test
- TPO-abs
- thyroid peroxidase autoantibodies
- TRabs
- TSH receptor antibodies
- TSH
- thyrotropin.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schadendorf DHF, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen T-T, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced unresectable melanoma. J Clin Oncol. 2015;33(17):1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, Patnaik A, Dronca R, Zarour H, Joseph RW, Boasberg P, Chmielowski B, Mateus C, Postow MA, Gergich K, Elassaiss-Schaap J, Li XN, Iannone R, Ebbinghaus SW, Kang SP, Daud A. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A; KEYNOTE-006 investigators . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 8.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 10.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. [DOI] [PubMed] [Google Scholar]
- 12.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, Cohen JB, Collins G, Savage KJ, Trneny M, Kato K, Farsaci B, Parker SM, Rodig S, Roemer MG, Ligon AH, Engert A. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361–1375. [DOI] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, Merghoub T, Rudin CM, Fish S, Hellmann MD. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small cell lung cancer. Ann Oncol. 2016;28(3):583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, Gore M, Larkin J. 2016 Thyroid abnormalities following the use of CTLA-4 and PD-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017;86(4):614–620. [DOI] [PubMed] [Google Scholar]
- 20.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101(11):4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastman JJ, Serracino HS, Zhu Y, Koenig MR, Mateescu V, Sams SB, Davies KD, Raeburn CD, McIntyre RC Jr, Haugen BR, French JD. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2016;101(7):2863–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, Gastineau DA, Dietz AB. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PLoS One. 2015;10(3):e0121546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amino N, Yabu Y, Miki T, Morimoto S, Kumahara Y, Mori H, Iwatani Y, Nishi K, Nakatani K, Miyai K. Serum ratio of triiodothyronine to thyroxine, and thyroxine-binding globulin and calcitonin concentrations in Graves’ disease and destruction-induced thyrotoxicosis. J Clin Endocrinol Metab. 1981;53(1):113–116. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura Noh J, Momotani N, Fukada S, Ito K, Miyauchi A, Amino N. Ratio of serum free triiodothyronine to free thyroxine in Graves’ hyperthyroidism and thyrotoxicosis caused by painless thyroiditis. Endocr J. 2005;52(5):537–542. [DOI] [PubMed] [Google Scholar]
- 26.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(2):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavan R, Salvador D, Gustafson MP, Dietz AB, Nevala W, Markovic SN. Untreated stage IV melanoma patients exhibit abnormal monocyte phenotypes and decreased functional capacity. Cancer Immunol Res. 2014;2(3):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLachlan SM, Rapoport B. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev. 2014;35(1):59–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. [DOI] [PubMed] [Google Scholar]
- 33.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. [DOI] [PubMed] [Google Scholar]
- 34.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. [DOI] [PubMed] [Google Scholar]
- 36.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19(1):82–92. [DOI] [PubMed] [Google Scholar]
- 39.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;100(5):1738–1741. [DOI] [PubMed] [Google Scholar]
- 40.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. [DOI] [PubMed] [Google Scholar]
- 42.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, Kirkwood JM. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naidoo J, Schindler K, Querfeld C, Busam K, Cunningham J, Page DB, Postow MA, Weinstein A, Lucas AS, Ciccolini KT, Quigley EA, Lesokhin AM, Paik PK, Chaft JE, Segal NH, D’Angelo SP, Dickson MA, Wolchok JD, Lacouture ME. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. OncoImmunology. 2012;1(8):1223–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11(6):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cervin C, Lyssenko V, Bakhtadze E, Lindholm E, Nilsson P, Tuomi T, Cilio CM, Groop L. Genetic similarities between latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Diabetes. 2008;57(5):1433–1437. [DOI] [PubMed] [Google Scholar]
- 47.Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, Ban Y, Jacobson EM, Concepcion ES, Li CW, Tomer Y. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA. 2008;105(37):14034–14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ting WH, Chien MN, Lo FS, Wang CH, Huang CY, Lin CL, Lin WS, Chang TY, Yang HW, Chen WF, Lien YP, Cheng BW, Lin CH, Chen CC, Wu YL, Hung CM, Li HJ, Chan CI, Lee YJ. Association of cytotoxic T-lymphocyte-associated protein 4 (CTLA4) Gene polymorphisms with autoimmune thyroid disease in children and adults: case-control study. PLoS One. 2016;11(4):e0154394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun. 2008;30(1-2):58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid. 2010;20(7):715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nada AM, Hammouda M. Immunoregulatory T cells, LFA-3 and HLA-DR in autoimmune thyroid diseases. Indian J Endocrinol Metab. 2014;18(4):574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116(10):2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehlers M, Schott M. Hashimoto’s thyroiditis and papillary thyroid cancer: are they immunologically linked? Trends Endocrinol Metab. 2014;25(12):656–664. [DOI] [PubMed] [Google Scholar]
- 54.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28(5):492–520. [DOI] [PubMed] [Google Scholar]
- 55.Laborde RR, Lin Y, Gustafson MP, Bulur PA, Dietz AB. Cancer vaccines in the world of immune suppressive monocytes (CD14(+)HLA-DR(lo/neg) cells): the gateway to improved responses. Front Immunol. 2014;5:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, Peller PJ, Bahn RS, Lowe VJ. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48(6):896–901. [DOI] [PubMed] [Google Scholar]
- 57.Chen YK, Chen YL, Liao AC, Shen YY, Kao CH. Elevated 18F-FDG uptake in skeletal muscles and thymus: a clue for the diagnosis of Graves’ disease. Nucl Med Commun. 2004;25(2):115–121. [DOI] [PubMed] [Google Scholar]