Abstract
Context:
Preeclampsia (PE) can be classified into early-onset (<34 weeks of gestation) and late-onset (>34 weeks of gestation) subtypes. Soluble endoglin, an auxiliary receptor for transforming growth factor (TGF)-β ligands, is increased in PE circulation and believed to inhibit TGF-β action by sequestering the ligands. However, soluble endoglin, with a low affinity to TGF-β ligands, has been demonstrated to have little effect by itself on TGF-β action.
Objectives:
We examined whether multiple soluble TGF-β receptors are elevated in PE circulation and whether they synergistically block TGF-β signaling.
Design:
TGF-β receptors were measured using enzyme-linked immunosorbent assay in sera collected from preeclamptic pregnancies and gestation-age-matched controls. TGF-β signaling was assessed using an in vitro bioassay and a tube formation assay.
Results:
TGF-β type I, II, and III receptors were all identified in pregnant serum; all were substantially elevated in early-onset but not late-onset PE. Endoglin was increased in both subtypes. At the greatest concentrations detected in PE, none of these soluble TGF-β receptors alone, including endoglin, inhibited TGF-β signaling. However, when all four soluble receptors were present, signaling of both TGF-β1 and TGF-β2 was substantially reduced. Removal of any one of these soluble receptors alleviated TGF-β1 inhibition; however, removal of soluble TGFβRIII was necessary to relieve TGF-β2 inhibition.
Conclusions:
Multiple soluble TGF-β receptors are present in pregnant circulation and elevated in early-onset PE; they synergistically inhibit TGF-β signaling, which might be more likely to occur in early-onset than late-onset PE. Reducing soluble TGFβRIII, rather than endoglin, would be more effective in alleviating the inhibition of both TGF-β1 and TGF-β2 signaling in PE.
Multiple soluble TGF-β receptors synergistically inhibit TGF-β signaling, which can occur in early-onset PE. Removal of TGFβRIII alleviated the inhibition of both TGF-β1 and TGF-β2 signaling.
Preeclampsia (PE) is a serious pregnancy disorder affecting 2% to 8% of pregnancies worldwide (1). PE is characterized by new-onset hypertension and proteinuria after 20 weeks of gestation (2). The condition can progress rapidly, leading to multiple organ failure, swelling, headaches, convulsions, and stroke (3). Currently, the only effective treatment of PE is delivery of the placenta. However, preeclamptic pregnancy and premature birth itself predispose neonates to developing chronic diseases later in life (4). Women who have had PE also have a greater risk of developing cardiovascular diseases many years postpartum (5).
PE can be classified into two distinct subtypes, early-onset PE, occurring before 34 weeks of gestation, and late-onset PE, occurring after 34 weeks (6). Compelling evidence suggests that the two PE subtypes have vastly different etiologies (7–9), and early-onset PE poses far more substantial maternal risks, with a 20-fold greater mortality rate relative to late-onset PE (10). Early-onset PE is often associated with inadequate trophoblast invasion into the uterine spiral arteries and increased uteroplacental vascular resistance (11), resulting in altered blood flow in the umbilical arteries and a reduced blood supply to the fetus (11). In contrast, most evidence suggests a lower association between abnormal trophoblast invasion and late-onset PE (12, 13).
In PE patients, the placenta releases abnormal amounts and/or types of factors into the maternal circulation, either as a response to reduced placental perfusion or because of genetic and/or environmental predisposition. These placental factors are thought to cause endothelial dysfunction and the maternal syndrome of PE (14). The factors substantially elevated in PE circulation include cytokines, antiangiogenic factors, syncytiotrophoblast microparticles, and activated leukocytes (15–18). Excessive levels of antiangiogenic factors in the circulation are believed to cause an angiogenic imbalance, disruptions of the angiogenic processes, and endothelial dysfunction (12, 19). One prominent soluble factor that is substantially increased in the PE circulation is soluble endoglin, and its levels are reported to correlate with disease severity (20).
Endoglin is an auxiliary receptor for transforming growth factor (TGF)-β signaling. A soluble form of endoglin will be substantially elevated in women who develop PE 2 to 3 months before the onset of the disease (21, 22). An in vitro study reported that soluble endoglin blocks TGF-β1–induced vasodilation in rat vessels by inhibiting binding of TGF-β1 to its receptors (20). It has, therefore, been suggested that elevated soluble endoglin sequesters TGF-β ligands to inhibit TGF-β signaling, causing endothelial dysfunction (20). However, it has been demonstrated that the affinity of soluble endoglin to the TGF-β ligands is too low to scavenge the ligands and inhibit their signaling (23). Consistent with this view, a recent study showed that high levels of soluble endoglin alone in the circulation do not induce endothelial dysfunction in a mouse model (24).
TGF-β ligands exist in three isoforms, TGF-β1, -β2, and -β3 (25). Each ligand initiates activities by forming a receptor complex with type I (TGFβRI) and type II (TGFβRII) serine/threonine kinase receptors on the cell surface. Initially, TGF-β binds to TGFβRII, promoting recruitment and phosphorylation of TGFβRI (26). The activated TGFβRI then triggers an intracellular phosphorylation cascade involving SMAD2/3 transcription factors. TGF-β receptor III (TGFβRIII or betaglycan), a membrane-bound proteoglycan, facilitates the binding of TGF-β ligands to TGFβRI/TGFβRII, leading to a heightened cellular response (27). This is particularly important for TGF-β2, because it has a weak affinity for its type II receptor (28). It has been reported that TGFβRIII can be shed from the cell surface after a proteolytic attack and that soluble TGFβRIII is able to block TGF-β–mediated receptor activation (29). Endoglin shares similar sequences in some regions with TGFβRIII (30). However, unlike TGFβRIII, endoglin only binds to TGF-β1 and TGF-β3 with high affinity and does not interact with TGF-β2 (30). Mice null for either endoglin or TGFβRII die in utero with defects in hematopoiesis and vasculogenesis (31, 32), suggesting that these receptors are critical for TGF-β signaling in endothelial cells and angiogenesis. It has thus been suggested that perhaps soluble endoglin in the PE circulation is present in complex with other soluble TGF-β receptors to inhibit TGF-β signaling (23).
In the present study, we examined whether soluble TGFβRI, TGFβRII, and TGFβRIII are present in the serum of normal pregnancy, whether their circulating levels are altered in PE similarly or differently from soluble endoglin, and whether these changes are associated with certain subtypes of PE. We also investigated whether any of these soluble TGF-β receptors, at the concentrations detected in the serum of preeclamptic pregnancy, alone or in combination, could inhibit TGF-β1 and TGF-β2 signaling in an in vitro model.
Materials and Methods
Serum samples
Maternal sera were obtained at the PE diagnosis, consisting of early-onset (28 to 34 weeks of gestation; n = 14) and late-onset (>34 weeks of gestation; n = 17) subtypes, together with their gestational age-matched normotensive controls (28 to 34 weeks of gestation, n = 19; >34 weeks of gestation, n = 31), at Wuxi Maternity and Children’s Health Hospital of Nanjing Medical University (Wuxi, China). The Human Research Ethic Committee of Wuxi Maternity and Children’s Hospital, Nanjing Medical University (Wuxi, China) approved the study, and all the participants provided written consent. The clinical characteristics of the participants are listed in Table 1. Maternal sera were also obtained from a separate cohort of healthy pregnant women during gestational weeks 11 to 13 (n = 14), 23 to 29 (n = 15), and 32 to 40 (n = 15), representing the first, second, and third trimester, respectively.
Table 1.
Clinical Characteristics of Study Subjects
| Characteristic | Early-Onset (28–34 wk) |
Late-Onset (>34 wk) |
||
|---|---|---|---|---|
| Control (n = 19) | PE (n = 14) | Control (n = 31) | PE (n = 17) | |
| Maternal age, y | 26.6 ± 3.6 | 28.9 ± 5.4 | 26 ± 3.9 | 27.5 ± 5.3 |
| GA when PE diagnosed, wk | NA | 30 ± 3 | NA | 36 ± 1 |
| GA at delivery, wk | 39 ± 1 | 34 ± 1 | 39 ± 1.6 | 37 ± 1 |
| Systolic blood pressure, mm Hg | 117 ± 12 | 159 ± 12 | 123 ± 9 | 156 ± 15 |
| Diastolic blood pressure, mm Hg | 72 ± 8 | 112 ± 14 | 78 ± 7 | 102 ± 12 |
| Fetal birth weight, g | 3312 ± 383 | 1658 ± 716 | 3166 ± 504 | 2668 ± 659 |
| Proteinuria | Negative | >1+ | Negative | >1+ |
Abbreviations: GA, gestational age; NA, not applicable.
Data presented as mean ± standard deviation.
Detection of soluble TGF-β receptors in serum using enzyme-linked immunosorbent assay
An enzyme-linked immunosorbent assay was used to measure the serum concentrations of endoglin (R&D Systems, Minneapolis, MN), TGFβRI (Cloud-Clone Corp., Houston, TX), and TGFβRII and TGFβRIII (Abnova, Taipei, Taiwan). The samples were assayed in duplicates according to the manufacturers’ instructions. The detection limit and inter- and intra-assay variability for these enzyme-linked immunosorbent assays were as follows: endoglin, 0.007 ng/mL, 6.5%, and 3%; TGFβRI, 0.121 ng/mL, 12%, and 8%; TGFβRII, 0.016 ng/mL, 15%, and 6%; and TGFβRIII, 0.157 ng/mL, 9%, and 8%, respectively.
Assessment of TGF-β signaling by an in vitro TGF-β bioassay
Human embryonic kidney (HEK) 293 cells were maintained at 37°C in a humidified atmosphere of 5% carbon dioxide in air and cultured in Dulbecco’s modified Eagle medium (Thermo Fisher Scientific, Scoresby, VIC, Australia) supplemented with 1% antibiotics (Thermo Fisher Scientific), 2 mM l-glutamine (Sigma-Aldrich, St. Louis, MO), and 10% fetal bovine serum (FBS; Thermo Fisher Scientific). For the TGF-β bioassay, the cells were plated in 48-well plates (Thermo Fisher Scientific) at 75 × 103/well density in antibiotic-free media for 24 hours. Each well was then transfected with 25 ng of A3-luc (33), a TGF-β–responsive luciferase reporter, and 50 ng of FAST2 transcription factor (34), with 1.25 μL Lipofectamine 2000 (Invitrogen Australia PTY, Waverely, VIC, Australia) in Opti-MEM media (Thermo Fisher Scientific) (35). The cells were stimulated for 24 hours after transfection, with TGF-β1 or TGF-β2 ligands (both from R&D Systems) in Dulbecco’s modified Eagle medium containing 0.2% FBS and 50 mM HEPES (Thermo Fisher Scientific), in the presence or absence of soluble endoglin, TGFβRI, TGFβRII, and TGFβRIII (all from R&D Systems). The inhibitory capacity of these receptors was examined independently and in various combinations. After treatment, the cells were lysed, and luciferase activity was measured using d-luciferin as a substrate (Sigma-Aldrich) on a Spectramax plate reader (Molecular Devices, Sunnyvale, CA). All treatments were prepared in duplicate or triplicate, and all experiments were repeated three times.
Endothelial tube formation assay
Human umbilical vein endothelia cells (HUVECs; ATCC, Manassas, VA) were maintained at 37°C in a humidified atmosphere of 5% carbon dioxide in air and cultured in EGMTM-2-MV Bullet Kit media (Lonza, Basel, Switzerland) supplemented with 1% antibiotics (Thermo Fisher Scientific) and 10% FBS (Thermo Fisher Scientific). To determine the effect of soluble TGF-β receptors on endothelial tube formation, HUVECs were first grown in 12-well plates (Thermo Fisher Scientific) at 2 × 105/well density for 24 hours and treated for 24 hours with TGF-β1 or TGF-β2 ligand without or with individual or combined soluble TGF-β receptors in basic endothelium media, supplemented with 1% antibiotics and 10% FBS. They were then reseeded at 1.5 × 105/well density in a 48-well plate precoated with growth factor-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ) and treated with the same conditions as before transfer for another 16 hours. The cells were washed three times with Hanks’ balanced salt solution (Thermo Fisher Scientific) and labeled with 4 μg/mL calcein AM fluorescent dye (BD Biosciences) for 30 minutes at 37°C in 5% carbon dioxide. The tubes were assessed through an inverted fluorescent microscope at ×4 magnification (Olympus Australia PTY, Notting Hill, VIC, Australia), and the total tube length per area was quantified using FIJI software (National Institutes of Health, Bethesda, MD). Each treatment was repeated independently three times.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism, version 6 (GraphPad Software Inc., La Jolla, CA). The serum concentrations of each receptor across gestation were normally distributed and analyzed using one-way analysis of variance, followed by Tukey’s post hoc test. Comparisons of the receptor concentrations in PE subtypes with their gestational age-matched controls were conducted using an unpaired t test on log transformed data (normally distributed after transformation). The inhibitory activities of each receptor, alone and in various combinations, on TGF-β–induced luciferase activities and HUVEC tube formation were analyzed using one-way analysis of variance, followed by Dunnett’s post hoc tests. Differences were considered statistically significant at P < 0.05.
Results
Detection of TGFβRI, TGFβRII, and TGFβRIII in serum of normal pregnancy
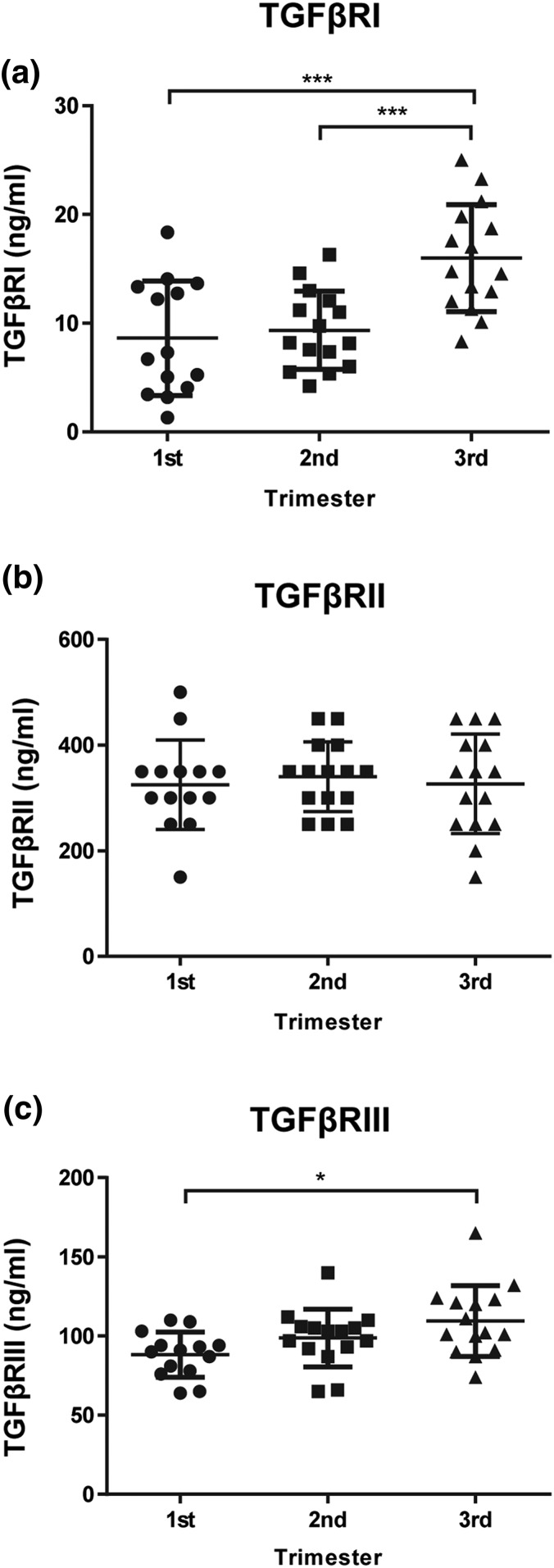
All three TGF-β receptors, TGFβRI, TGFβRII, and TGFβRIII, were detected in the serum of normal pregnancy (Fig. 1). The serum concentration of TGFβRI remained stable in the first and second trimester and then substantially increased in the third trimester [Fig. 1(a)]. In contrast, the serum levels of TGFβRII remained constant across the gestational period [Fig. 1(b)]. The levels of TGFRIII increased throughout gestation, with the highest concentration detected in the third trimester [Fig. 1(c)].
Figure 1.
Serum levels of TGF-β receptors across gestation in normal pregnancy. Serum levels of (a) TGFβRI, (b) TGFβRII, and (c) TGFβRIII were determined using an enzyme-linked immunosorbent assay. Samples are presented in trimesters (first trimester, n = 14; second trimester, n = 15; and third trimester, n = 15). Data are expressed as mean ± standard deviation. *P < 0.05 and ***P < 0.001.
Differential elevation of serum TGF-β receptors in early-onset vs late-onset PE
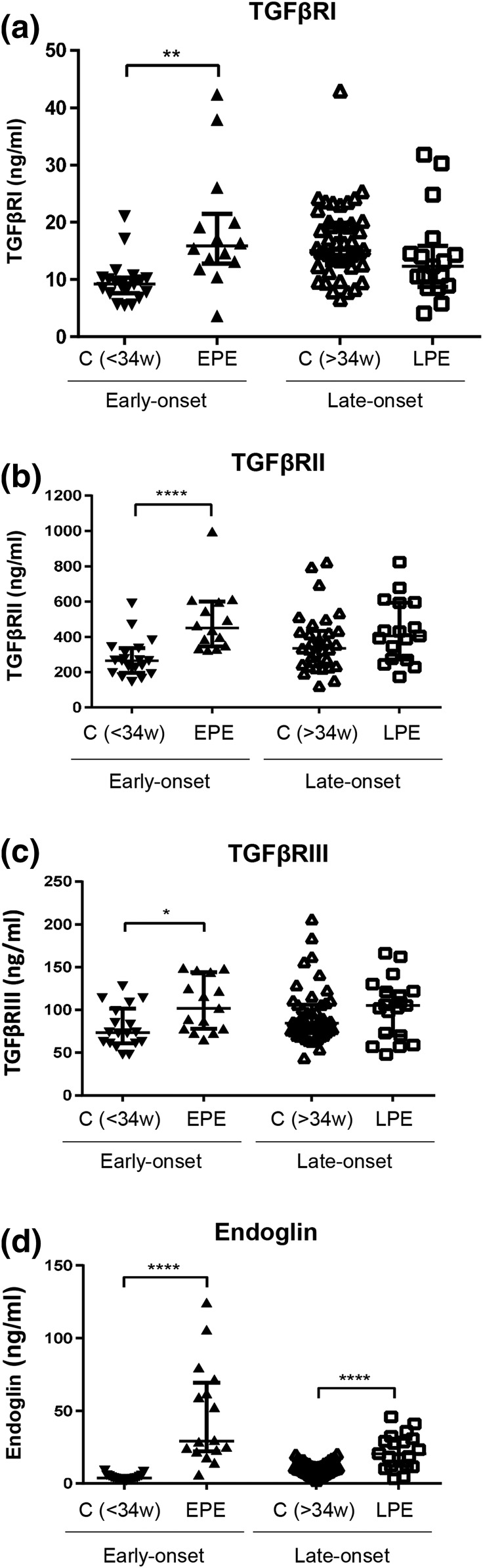
We next determined the serum levels of the three TGF-β receptors in pregnant women who either had early-onset or late-onset PE compared with their gestational age-matched controls (Fig. 2). All three receptors, TGFβRI, TGFβRII, and TGFβRIII, were substantially elevated in early-onset PE compared with their gestational age-matched controls; however, this difference was not seen in late-onset PE compared with their respective controls [Fig. 2(a–c)]. We also measured endoglin, which was substantially greater in both early-onset and late-onset PE compared with their respective controls [Fig. 2(d)].
Figure 2.
Serum levels of TGF-β receptors in preeclamptic pregnancy at disease presentation. Enzyme-linked immunosorbent assay was used to determine the levels of TGF-β receptors in the sera of early-onset PE (EPE; 28 to 34 weeks of gestation, n = 14) vs gestational age-matched controls (C<34w; n = 19), and late-onset PE (LPE; >34 weeks of gestation, n = 17) vs gestational age-matched controls (C>34w; n = 31). These samples were distinct from those shown in Fig. 1. (a) TGFβRI, (b) TGFβRII, (c) TGFβRIII, and (d) Endoglin. Data are expressed as median ± interquartile range. *P < 0.05, ** P < 0.01, and ****P < 0.0001.
Establishment of an in vitro bioassay to assess TGF-β signaling
To determine whether all three soluble TGF-β receptors are capable of inhibiting TGF-β signaling, we next established an in vitro bioassay to assess TGF-β signaling. Because TGF-β1 and TGF-β3 isoforms exert a similar mode of action, but TGF-β2 is distinct (36), we chose TGF-β1 and TGF-β2 to study their signaling using a TGF-β–responsive luciferase bioassay. HEK293 cells were transfected with a TGF-β–responsive luciferase reporter (A3-luc) and transcription factor (FAST2) and treated with increasing doses of TGF-β1 (Supplemental Fig. 1A (344.8KB, pdf) ) or TGF-β2 (Supplemental Fig. 1B (344.8KB, pdf) ). Both TGF-β ligands stimulated luciferase activity in a dose-dependent manner (Supplemental Fig. 1 (344.8KB, pdf) ). We selected a 10-pM concentration for both TGF-β1 and TGF-β2 ligands to further examine the inhibitory effect of soluble receptors on TGF-β action, because this concentration was in the linear range of substantial stimulation.
Soluble TGFβRI, TGFβRII, TGFβRIII, and endoglin are required to inhibit TGF-β1 signaling
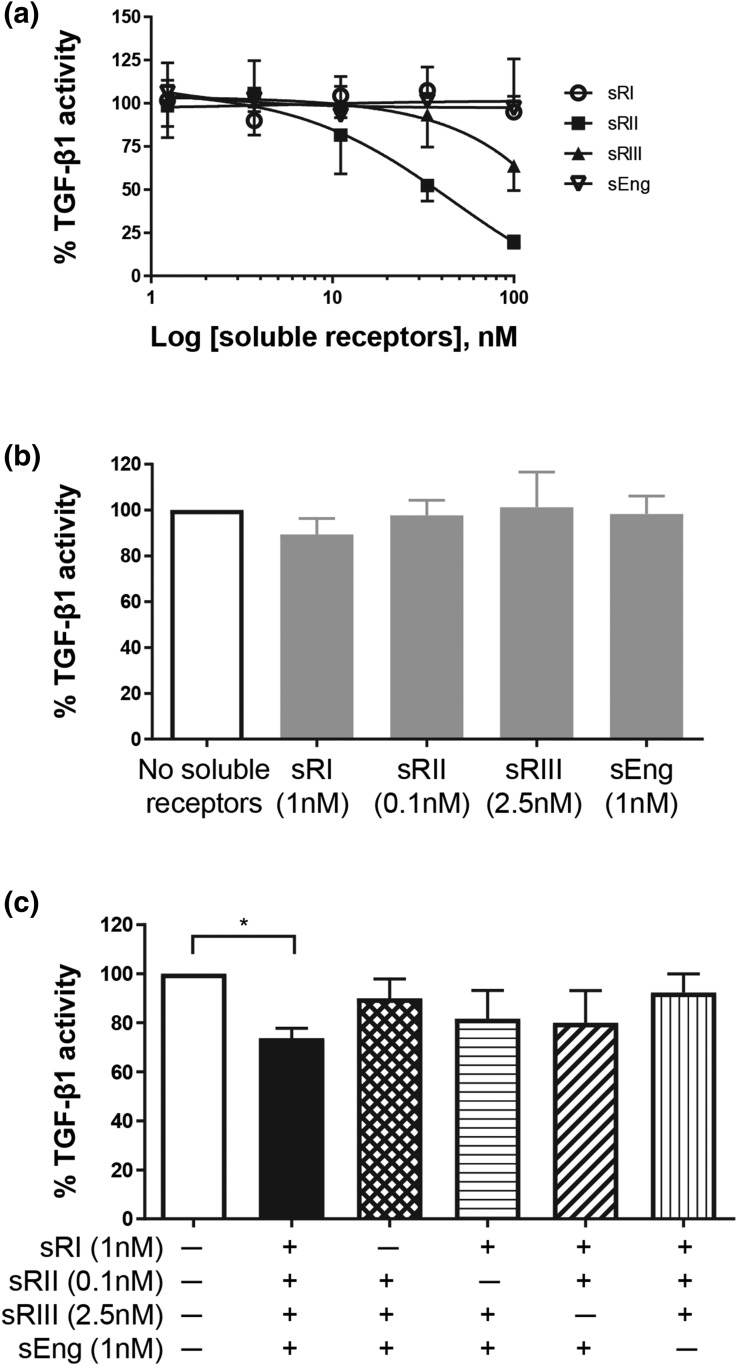
We first examined the inhibitory activities of each individual soluble TGF-β receptor (dose range, 1.23 to 100 nM) against TGF-β1 ligand [Fig. 3(a)]. TGFβRII was the only soluble receptor that inhibited TGF-β1 action in a dose-dependent manner. TGFβRIII showed some inhibition only at the highest concentration (100 nM); however, neither soluble endoglin nor TGFβRI affected TGF-β1 activity, even at the highest concentration. Next, we tested each soluble receptor at the highest concentration seen in early-onset PE serum [Fig. 3(b)]. At these physiological PE concentrations, none of the soluble TGF-β receptors inhibited TGF-β1 action [Fig. 3(b)]. These soluble receptors were then tested again at the physiological PE concentrations, but in various combinations, to determine whether they would synergistically exert an antagonistic activity against TGF-β1 [Fig. 3(c)]. When all four soluble TGF-β receptors were present, TGF-β1 signaling was reduced by >25% [Fig. 3(c)]. However, on removal of any one of these soluble receptors, the inhibition was alleviated [Fig. 3(c)], indicating that all four soluble receptors are required to synergistically inhibit TGF-β1 action. As elevation of all four soluble receptors was seen only in early-onset PE (Fig. 2), inhibition of TGF-β1 action might be more likely to be associated with early-onset PE only.
Figure 3.
Effects of soluble TGF-β receptors on TGF-β1 action. HEK293 cells were transfected with TGF-β–responsive luciferase reporter and treated with 10 pM TGF-β1 ligand in the presence or absence of soluble TGF-β receptors. TGF-β1–induced luciferase reading without any soluble receptor was set as 100%. The effect of soluble receptors was expressed as a percentage (mean ± standard deviation) of TGF-β1–induced luciferase activity from three independent experiments. (a) Dose responses of individual soluble TGF-β receptors on TGF-β1 action. (b) Effect of each soluble receptor alone at the highest concentration seen in early-onset PE serum on TGF-β1 action. (c) Effects of soluble TGF-β receptor combinations on TGF-β1 action. *P < 0.05. sEng, soluble endoglin; sRI, soluble TGFβRI; sRII, soluble TGFβRII; sRIII, soluble TGFβRIII.
Inhibition of TGF-β2 action requires soluble TGFβRIII
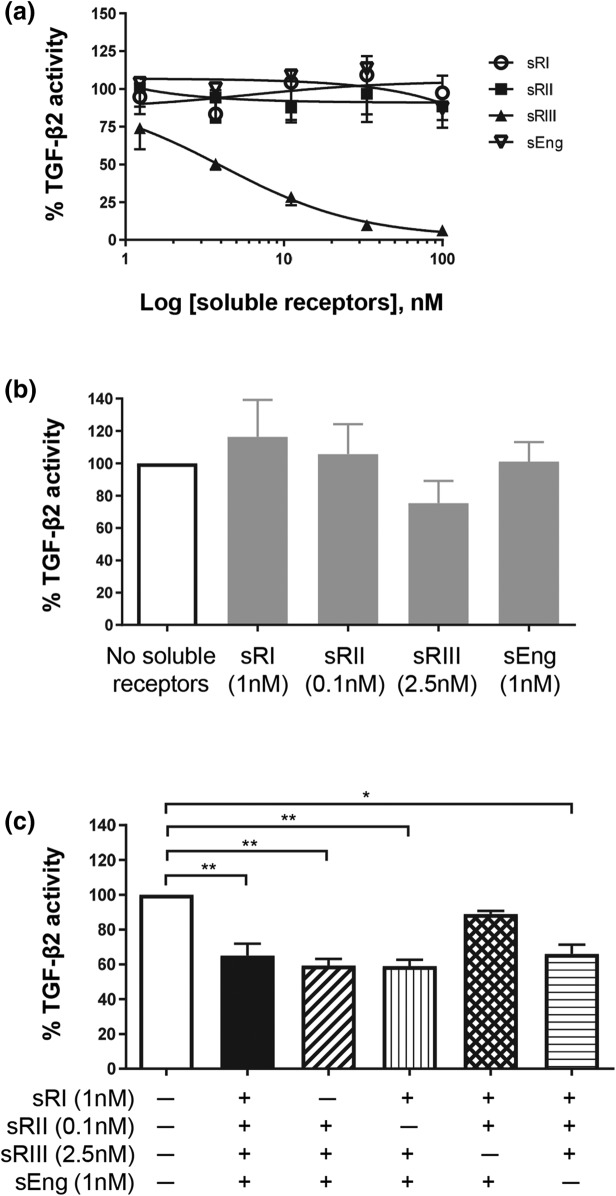
The ability of these soluble TGF-β receptors to inhibit TGF-β2–induced luciferase activity was also examined (Fig. 4). When each of the four soluble TGF-β receptors was examined individually with increasing doses, only TGFβRIII potently inhibited TGF-β2 action [Fig. 4(a)]. None of the other three soluble receptors, even at the highest concentration examined (100 nM), showed inhibitory effects on TGF-β2 action [Fig. 4(a)]. When each soluble receptor at the highest concentration seen in early-onset PE was examined individually against TGF-β2 stimulation, none of the receptors substantially affected TGF-β2 action [Fig. 4(b)]. Soluble TGFβRIII appeared to inhibit TGF-β2 activity; however, the difference was not statistically significant [Fig. 4(b)]. When all four soluble receptors were present, TGF-β2 action was reduced by 35%, a relevant decrease [Fig. 4(c)]. Furthermore, the co-addition of soluble TGFβRIII with other soluble receptors also substantially reduced TGF-β2 action [Fig. 4(c)]. When TGFβRIII was absent, the TGF-β2–stimulated luciferase activity remained unchanged, even when all three of the other soluble receptors were present [Fig. 4(c)].
Figure 4.
Effect of soluble TGF-β receptors on TGF-β2 action. HEK293 cells were transfected with TGF-β–responsive luciferase reporter and treated with 10 pM TGF-β2 ligand in the presence or absence of soluble TGF-β receptors. TGF-β2–induced luciferase reading without any soluble receptor was set as 100%. The effect of soluble receptors was expressed as a percentage (mean ± standard deviation) of TGF-β2–induced luciferase activity from three independent experiments. (a) Dose responses of individual soluble TGF-β receptors on TGF-β2 action. (b) Effect of each soluble receptor alone at the highest concentration seen in early-onset PE serum on TGF-β2 action. (c) Effects of soluble TGF-β receptor combinations on TGF-β2 action. *P < 0.05 and **P < 0.01. sEng, soluble endoglin; sRI, soluble TGFβRI; sRII, soluble TGFβRII; sRIII, soluble TGFβRIII.
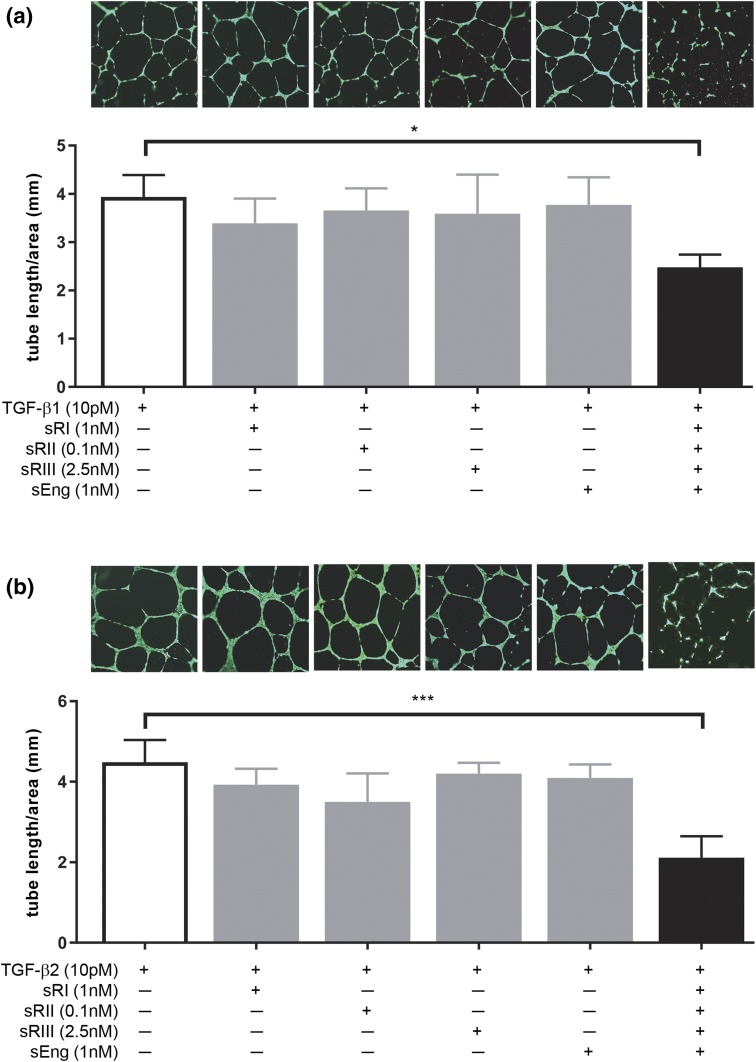
Soluble TGF-β receptors in combination inhibit ligand-induced angiogenesis in HUVECs
To further determine the effect of soluble receptors on TGF-β signaling and angiogenesis, endothelial cell tube formation was assessed using HUVEVs as a model (Fig. 5). HUVECs formed nice tube-like structures after 16 hours of culture with the TGF-β1 ligand alone [Fig. 5(a), image 1]. The addition of individual soluble receptors at the highest concentration seen in PE serum did not affect tube formation [Fig. 5(a), images 2 to 5]. However, when all four soluble receptors were added, tube formation was severely disrupted [Fig. 5(a), image 6]. This disruption was quantified by measuring the total length of the HUVEC tubes formed, which was substantially reduced in the cells treated with combined soluble receptors compared with those treated in any other conditions [Fig. 5(a)]. Similarly, HUVECs cultured with TGF-β2 ligand alone formed regular tubes [Fig. 5(b), image 1], and none of the soluble receptors individually had any effect on tube formation when co-treated with TGF-β2 [Fig. 5(b), images 2 to 5]. However, when all four soluble receptors were added, tube formation was severely affected, and the total tube length was substantially reduced [Fig. 5(b), image 6]. These data are consistent with the TGF-β bioassay results of HEK293 cells (Figs. 3 and 4).
Figure 5.
Effect of soluble TGF-β receptors on TGF-β–mediated angiogenesis. HUVECs were cultured in Matrigel and treated with 10 pM TGF-β ligands without or with individual or combined soluble receptors at the highest concentration observed in PE serum. (a) Effect of soluble TGF-β receptors on TGF-β1–mediated tube formation. The bar graph shows the total tube length per square millimeter area for each treatment condition; each bar represents the average tube length calculated from three independent experiments (n = 3). The photograph above each bar is a representative image of that condition. (b) Effect of soluble TGF-β receptors on TGF-β2–mediated tube formation. Data are presented as mean ± standard deviation. *P < 0.05 and ***P < 0.001. sEng, soluble endoglin; sRI, soluble TGFβRI; sRII, soluble TGFβRII; sRIII, soluble TGFβRIII.
Discussion
To the best of our knowledge, the results of the present study have demonstrated for the first time that multiple soluble TGF-β receptors, in addition to soluble endoglin, are present in pregnant serum and that all are substantially elevated in early-onset PE. None of these soluble TGF-β receptors alone, including soluble endoglin, at the concentrations detected in the serum of early-onset PE, inhibited TGF-β signaling. However, in combination, they synergistically inhibited both TGF-β1 and TGF-β2 signaling.
Elevated soluble endoglin in the maternal circulation has been linked to PE; it is believed to sequester TGF-β ligands to inhibit TGF-β signaling. However, soluble endoglin only binds to TGF-β1 and TGF-β3 and not to TGF-β2, and the affinity of soluble endoglin to TGF-β1 and TGF-β3 has been shown to be too weak to affect TGF-β signaling (23). Thus, it is unclear whether the high levels of soluble endoglin found in PE circulation actually inhibit the signaling of any of the TGF-β ligands. In the present study, we have confirmed previous reports that soluble endoglin is substantially increased in the serum of PE women (21, 22). However, our data show that soluble endoglin is not the only soluble circulating TGF-β receptor; soluble TGFβRI, TGFβRII, and TGFβRIII are also present, together with soluble endoglin, in the circulation of pregnant women. Furthermore, the serum profile of these factors is distinct between the early-onset and late-onset PE subtypes. Although serum endoglin was elevated in both early-onset and late-onset PE, the other three soluble TGF-β receptors were substantially increased only in early-onset, but not late-onset, PE.
We further demonstrated that none of these soluble TGF-β receptors alone, including the much studied soluble endoglin, at the concentrations seen in PE serum, inhibited the luciferase activities or angiogenesis mediated by either TGF-β1 or TGF-β2. Only when all four soluble TGF-β receptors were present, was a relevant inhibition observed for both TGF-β1 and TGF-β2 ligands. Because only early-onset, but not late-onset, PE exhibited elevation of all four soluble TGF-β receptors, it is logical to suggest that inhibition of TGF-β signaling might be more likely to be associated with early-onset than with late-onset PE. Our findings further indicated that removal of any one of the four soluble TGF-β receptors, including soluble endoglin, would alleviate the inhibition of TGF-β1 signaling. However, removal of soluble TGFβRIII was essential to relieve the inhibition of TGF-β2 signaling. Thus, these data suggest that reducing the circulating levels of soluble TGFβRIII, rather than soluble endoglin (either by removal or inhibition of its production), would be more effective in alleviating the inhibition of both TGF-β1 and TGF-β2 signaling that likely occurs in early-onset PE.
Previous studies have reported that serum endoglin levels increase with gestation in normal pregnancy (37, 38). In the present study, we found that although serum levels of TGFβRI and TGFβRIII correlated positively with gestational age, the levels of TGFβRII remained constant across gestation. In addition, the absolute levels of TGFβRII detected in the maternal serum were much lower than all other three TGF-β receptors, indicating that the production of TGFβRII is tightly controlled. Our data have also confirmed that soluble endoglin is increased in both PE subtypes compared with their respective controls (22). However, unlike soluble endoglin, soluble TGFβRI, TGFβRII, and TGFβRIII were all elevated only in early-onset PE, but not late-onset PE, consistent with the emerging view that the two PE subtypes differ considerably in etiology (7–9).
Much of the research to date has focused on elevated soluble endoglin as a key causal factor of dysregulation of TGF-β signaling in PE. Our data extend this view and suggest that soluble endoglin is accompanied by other soluble TGF-β receptors, especially in early-onset PE. Remarkably, despite the speculation that soluble endoglin is the major inhibitor of TGF-β function in PE, our in vitro cell model demonstrated that none of these soluble TGF-β receptors alone, including soluble endoglin, at the concentrations seen in PE circulation, exert any inhibition on TGF-β signaling or TGF-β–mediated angiogenesis. In addition, our study showed no inhibition of TGF-β1 action by soluble endoglin alone, even at the highest concentration tested (100 nM). These data are in strong agreement with a previous report showing that soluble endoglin alone does not inhibit TGF-β signaling (23). We have further demonstrated that at the concentrations observed in PE serum, all four soluble TGF-β receptors are required to inhibit TGF-β1 action and that removal of any one of these soluble receptors relieved the inhibitory activity. These data strongly suggest that these four soluble receptors act synergistically to inhibit TGF-β1 signaling.
TGF-β2 behaved very differently from TGF-β1 in the presence of the soluble receptors. In our system, soluble TGFβRIII functioned as the major inhibitor of TGF-β2 signaling. Although soluble TGFβRIII alone did not substantially inhibit TGF-β2 action, the combination of TGFβRIII with any other soluble TGF-β receptor substantially inhibited TGF-β2–induced luciferase activity. However, in the absence of soluble TGFβRIII, even combining all three of the other soluble receptors failed to block TGF-β2 action. This finding is consistent with those from other studies showing that TGFβRIII is essential for the binding of TGF-β2 to its receptors and that the loss of TGFβRIII on the cell surface suppresses TGF-β2–mediated cellular functions (28, 39, 40). Our data thus suggest that elevated TGFβRIII in the PE circulation can effectively increase the formation of the TGF-β2–soluble receptor complex, thus reducing the availability of TGF-β2 ligand to bind to its cell surface receptors to activate downstream signaling.
In conclusion, the present study has demonstrated that all four soluble TGF-β receptors are present in pregnant serum and are elevated differently in early-onset PE compared with late-onset PE. Although soluble endoglin is increased in both PE subtypes, the other three TGF-β receptors are substantially elevated only in early-onset PE. We have also demonstrated that all four soluble receptors are required to act synergistically to inhibit TGF-β signaling. Removal of any one of them will alleviate the inhibition of TGF-β1 action; however, removal of TGFβRIII is necessary to relieve the inhibition of TGF-β2 actions. Our findings thus suggest that inhibition of TGF-β signaling by soluble TGF-β receptors is more likely to be associated with early-onset PE, because this subtype exhibits elevation of all four TGF-β receptors. Our data also suggest that strategies to reduce the circulating levels of soluble TGFβRIII would be more effective to alleviate the inhibition of both TGF-β1 and TGF-β2 signaling.
One limitation of the present study was that we used an in vitro model to investigate TGF-β signaling, which might not reflect the in vivo biological events. However, we used TGF-β receptors at the highest concentrations observed in early-onset PE and found that none of the four receptors alone affected TGF-β action, in contrast to the view that soluble endoglin is the major contributor of TGF-β dysregulation in PE. Furthermore, we demonstrated that the presence of all four soluble receptors is required to disrupt TGF-β–induced tube formation in endothelial cells, suggesting that the elevation of all four soluble receptors might be a contributing factor to the endothelial dysfunction in early-onset PE.
Acknowledgments
Acknowledgments
This work was supported by National Health and Medical Research Council of Australia Fellowship Grant 1041835 and Project Grant 1108365 (to G.N.), the Gates Foundation, and the Victorian State Government Operational Infrastructure Scheme.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FBS
- fetal bovine serum
- HEK
- human embryonic kidney
- HUVEC
- human umbilical vein endothelia cell
- PE
- preeclampsia
- TGF
- transforming growth factor
- TGFβRI
- TGF-β receptor type I
- TGFβRII
- TGF-β receptor type II
- TGFβRIII
- TGF-β receptor type III.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. [DOI] [PubMed] [Google Scholar]
- 2.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61:932–942. [DOI] [PubMed] [Google Scholar]
- 3.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Diseases in children born to mothers with preeclampsia: a population-based sibling cohort study. Am J Obstet Gynecol. 2011;204(2):157.e1–157.e5. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tranquilli AL. Early and late-onset pre-eclampsia. Pregnancy Hypertens. 2014;4(3):241. [DOI] [PubMed] [Google Scholar]
- 7.Singh H, Zhao M, Chen Q, Wang Y, Li Y, Kaitu’u-Lino TJ, Tong S, Nie G. Human HtrA4 expression is restricted to the placenta, is significantly up-regulated in early-onset preeclampsia, and high levels of HtrA4 cause endothelial dysfunction. J Clin Endocrinol Metab. 2015;100(7):E936–E945. [DOI] [PubMed] [Google Scholar]
- 8.Teoh SS, Zhao M, Wang Y, Chen Q, Nie G. Serum HtrA1 is differentially regulated between early-onset and late-onset preeclampsia. Placenta. 2015;36(9):990–995. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Wang Y, Zhao M, Hyett J, da Silva Costa F, Nie G. Serum levels of GDF15 are reduced in preeclampsia and the reduction is more profound in late-onset than early-onset cases. Cytokine. 2016;83:226–230. [DOI] [PubMed] [Google Scholar]
- 10.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–538. [DOI] [PubMed] [Google Scholar]
- 11.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension 2008;51:970–975. [DOI] [PubMed] [Google Scholar]
- 12.Myatt L, Roberts JM. Preeclampsia: syndrome or disease? Curr Hypertens Rep. 2015;17(11):83. [DOI] [PubMed] [Google Scholar]
- 13.Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111(4):298–302. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57(5 Pt 2):1R–7R. [DOI] [PubMed] [Google Scholar]
- 16.Schipper EJ, Bolte AC, Schalkwijk CG, Van Geijn HP, Dekker GA. TNF-receptor levels in preeclampsia–results of a longitudinal study in high-risk women. J Maternal Fetal Neonatal Med. 2005;18:283–287. [DOI] [PubMed] [Google Scholar]
- 17.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von Dadelszen P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27(1):56–61. [DOI] [PubMed] [Google Scholar]
- 18.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002;39:155–160. [DOI] [PubMed] [Google Scholar]
- 19.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. [DOI] [PubMed] [Google Scholar]
- 21.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA; CPEP Study Group . Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. [DOI] [PubMed] [Google Scholar]
- 23.Gregory AL, Xu G, Sotov V, Letarte M. Review: the enigmatic role of endoglin in the placenta. Placenta. 2014;35(Suppl):S93–S99. [DOI] [PubMed] [Google Scholar]
- 24.Nemeckova I, Serwadczak A, Oujo B, Jezkova K, Rathouska J, Fikrova P, Varejckova M, Bernabeu C, Lopez-Novoa JM, Chlopicki S, Nachtigal P. High soluble endoglin levels do not induce endothelial dysfunction in mouse aorta. PLoS One. 2015;10(3):e0119665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Chen L, Liu B, Vialli C, Stone P, Ching LM, Chamley L. The role of autocrine TGFbeta1 in endothelial cell activation induced by phagocytosis of necrotic trophoblasts: a possible role in the pathogenesis of pre-eclampsia. J Pathol. 2010;221(1):87–95. [DOI] [PubMed] [Google Scholar]
- 26.Farnworth PG, Wang Y, Escalona R, Leembruggen P, Ooi GT, Findlay JK. Transforming growth factor-beta blocks inhibin binding to different target cell types in a context-dependent manner through dual mechanisms involving betaglycan. Endocrinology. 2007;148(11):5355–5368. [DOI] [PubMed] [Google Scholar]
- 27.Bilandzic M, Chu S, Wang Y, Tan HL, Fuller PJ, Findlay JK, Stenvers KL. Betaglycan alters NFκB-TGFβ2 cross talk to reduce survival of human granulosa tumor cells. Mol Endocrinol. 2013;27(3):466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Re E, Babitt JL, Pirani A, Schneyer AL, Lin HY. In the absence of type III receptor, the transforming growth factor (TGF)-beta type II-B receptor requires the type I receptor to bind TGF-beta2. J Biol Chem. 2004;279(21):22765–22772. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyay A, Wang L, López-Casillas F, Mendoza V, Yeh IT, Sun L. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. Prostate. 2005;63(1):81–90. [DOI] [PubMed] [Google Scholar]
- 30.Cheifetz S, Bellón T, Calés C, Vera S, Bernabeu C, Massagué J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267(27):19027–19030. [PubMed] [Google Scholar]
- 31.Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179(1):297–302. [DOI] [PubMed] [Google Scholar]
- 32.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104(10):1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13(7):804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F, Pouponnot C, Massagué J. Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes. Genes Dev. 1997;11(23):3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Y-T, Liu J-J, Luo Y, e C, Haltiwanger RS, Abate-Shen C, Shen MM. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol. 2002;22(13):4439–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheifetz S, Hernandez H, Laiho M, ten Dijke P, Iwata KK, Massagué J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990;265(33):20533–20538. [PubMed] [Google Scholar]
- 37.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Maternal Fetal Neonatal Med. 2008; 21:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in preeclampsia. J Clin Endocrinol Metab. 2007;92(7):2672–2679. [DOI] [PubMed] [Google Scholar]
- 39.Bilandzic M, Wang Y, Ahmed N, Luwor RB, Zhu HJ, Findlay JK, Stenvers KL. Betaglycan blocks metastatic behaviors in human granulosa cell tumors by suppressing NFκB-mediated induction of MMP2. Cancer Lett. 2014;354(1):107–114. [DOI] [PubMed] [Google Scholar]
- 40.Looyenga BD, Wiater E, Vale W, Hammer GD. Inhibin-A antagonizes TGFbeta2 signaling by down-regulating cell surface expression of the TGFbeta coreceptor betaglycan. Mol Endocrinol. 2010;24(3):608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]