Abstract
Context:
Low 25-hydroxyvitamin D [25(OH)D] is associated with coronary heart disease (CHD) in people who are white and Chinese but not black or Hispanic. Vitamin D binding globulin (VDBG) avidly binds 25(OH)D, reducing its bioavailability, and differs in isoform and concentration by race.
Objective:
Evaluate associations of VDBG with CHD and whether accounting for VDBG or estimating bioavailable 25(OH)D explains the heterogeneity of the association of 25(OH)D with CHD.
Design and Setting:
We conducted a case–cohort study within the Multi-Ethnic Study of Atherosclerosis. Participants with an incident CHD event over 12 years of follow-up (n = 538) and a randomly assigned subcohort (n = 999) were included. We measured baseline 25(OH)D, VDBG, and isoforms using mass spectrometry and estimated bioavailable 25(OH)D from published equations.
Results:
VDBG was associated with an increased risk of CHD [hazard ratio, 1.77 (95% confidence interval, 1.46 to 2.14) per standard deviation increment, P < 0.0001], without evidence of heterogeneity by race or isoform (each P for interaction > 0.1). Low total 25(OH)D was differentially associated with CHD events, by race, with or without adjustment for VDBG (P for interaction = 0.04 or 0.05, respectively). Associations of 25(OH)D with CHD were strengthened with adjustment for VDBG among participants who were white or Chinese, and bioavailable 25(OH)D was associated with CHD events only among white participants.
Conclusions:
High VDBG concentration was associated with CHD events in all racial and ethnic groups. Incorporation of VDBG strengthened existing associations of 25(OH)D with CHD but did not explain racial heterogeneity in associations of 25(OH)D with CHD.
Higher circulating VDBG concentrations are strongly associated with CHD events, and more studies are warranted to determine the nature of this relationship.
Low circulating 25-hydroxyvitamin D [25(OH)D] concentrations are associated with increased risk of coronary heart disease (CHD) across observational studies of predominantly white participants (1–6). 25(OH)D, which is the precursor of calcitriol, is the primary circulating form of vitamin D and the biomarker that is traditionally used to determine vitamin D deficiency or sufficiency states. Circulating 25(OH)D concentrations are substantially lower among black and Hispanic people but are not associated with CHD among these race and ethnicity groups (7–13).
One possible explanation for the observed racial heterogeneity is differences in the amount of 25(OH)D that is available to bind to target tissues. The majority of circulating 25(OH)D is tightly bound to vitamin D binding globulin (VDBG), with a smaller amount (10% to 15%) loosely bound to albumin, and <1% circulating in a free, unbound form (14, 15). VDBG is a highly polymorphic gene; isoforms differ strongly by race and by affinity for 25(OH)D (16). A recent study reported similar bioavailable 25(OH)D concentrations among whites and blacks, despite marked differences in total 25(OH)D (17). However, subsequent studies have failed to replicate this finding when VDBG concentrations were measured using gold-standard liquid chromatography–tandem mass spectrometry (LC-MS/MS) methods (18–21). Differences in VDBG and vitamin D availability may be critical to interpreting 25(OH)D concentrations and associated health outcomes, including differences by race and ethnicity.
We tested the hypothesis that VDBG concentration and isotype alter the association of 25(OH)D with CHD events in a community-based, multiracial and multiethnic study population. We measured serum VDBG concentrations, VDBG isotype, and 25(OH)D by using highly specific LC-MS/MS assays and evaluated associations with incident CHD events by race over long-term follow-up.
Methods
Study population
In the Multi-Ethnic Study of Atherosclerosis (MESA), 6814 community-dwelling adults between the ages of 45 and 84 years were recruited between July 2000 and August 2002 from six US communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan and the Bronx, New York; and St. Paul, Minnesota (22). MESA excluded patients who had prevalent clinical cardiovascular disease at baseline, defined as myocardial infarction, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, use of nitroglycerin, prior angioplasty, coronary artery bypass graft surgery, valve replacement, pacemaker or defibrillator implant, or any surgery on the heart or arteries. The cohort of participants is 38% non-Hispanic white, 28% African American, 22% Hispanic, and 12% Chinese American. The institutional review boards at all participating centers approved the study, and all participants gave written informed consent.
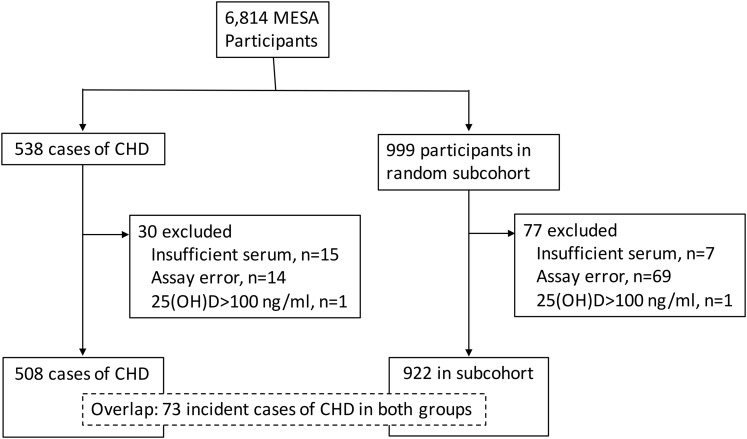
We selected participants for measurement of VDBG and albumin in 2015 by using a case–cohort design. Included were a random sample of MESA participants (subcohort, n = 999) and all those who had an incident CHD event by 31 December 2013 (cases, n = 538) (Fig. 1). Incident CHD was defined as the first occurrence of any one of the following events during follow-up: acute myocardial infarction, death from CHD, resuscitated cardiac arrest, definite angina, or probable angina with coronary revascularization (23). Participants with 25(OH)D concentrations >100 ng/mL (suggestive of high-dose supplementation) and participants without CHD follow-up data were excluded from analyses.
Figure 1.
Case–cohort design and flow of participants included in this analysis.
Measurement of exposures
VDBG concentration and isoform were measured simultaneously via an LC-MS/MS assay (18, 20). This assay quantifies VDBG concentration by measuring the abundance of two VDBG peptide sequences that are conserved across VDBG isoforms, generating results that are not biased by VDBG genotype (a concern with commercially available antibody-based assays) (21, 24). The assay uses internal standards to increase precision, with interassay coefficients of variation of 3.1% to 9.1% across a range of concentrations. At the same time, the assay detects the presence or absence of peptide sequences that define common VDBG isoforms, allowing direct determination of expressed isoforms (including multiple isoforms for heterozygotes). Isoform identification has previously been validated with genetic data (18). Total 25(OH)D (sum of 25-hydroxyvitamins D2 and D3) was measured via high-performance LC-MS/MS (25). Calibration was confirmed with National Institute of Standards and Technology standard reference material 972a (26). Albumin was measured via the modified Doumas and Rodkey procedures on the Beckman-Coulter DxC. Bioavailable 25(OH)D concentrations were calculated via published equations allowing for six affinity coefficients based on VDBG isoforms (17, 27, 28). Analyses were repeated with bioavailable 25(OH)D calculated with a single binding coefficient and yielded similar results.
Measurement of covariates
Covariates were ascertained at the baseline MESA examination, concurrent with VDBG, albumin, and 25(OH)D. Participants completed self-administered questionnaires, interviewer-administered standardized interviews, and extensive in-person examinations, yielding demographic and lifestyle characteristics, medical history, anthropometric measurements, and laboratory data. Race and ethnicity were characterized based on participants’ responses to questions modeled from the year 2000 US Census. General health was self-reported on a questionnaire as excellent, very good, good, fair, or poor. Leisure-time physical activity was estimated as the total amount of intentional exercise performed in a usual week and measured in metabolic equivalent task-minutes. Diabetes status was defined by the use of an oral hypoglycemic medication or insulin, fasting blood glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, or hemoglobin A1c ≥6.5% (29). Body mass index was calculated as weight in kilograms divided by height in meters squared. Blood pressure was ascertained as the mean of the last two of three seated measurements. Total and high-density lipoprotein cholesterol were measured via the cholesterol oxidase method. Low-density lipoprotein cholesterol levels were calculated via the Friedewald equation. Intact serum parathyroid hormone (PTH) was quantified by a DxI automated two-site immunoassay on a clinical analyzer (Beckman-Coulter Inc, Brea, CA) (30). Circulating fibroblast growth factor-23 (FGF-23) was measured in previously unthawed serum via the Kainos immunoassay, which detects the full-length, biologically intact FGF-23 molecule via midmolecule and distal epitopes. Urine albumin excretion was quantified as the ratio of albumin to creatinine in a single-voided urine sample. Serum calcium and phosphate were measured by indirect potentiometry on a DxC Synchron analyzer (Beckman-Coulter Inc) and timed-rate colorimetric reaction method, respectively (31). Glomerular filtration rate was estimated from the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation (32). Data on vitamin D supplement use were not available.
Statistical analysis
We examined baseline characteristics of the subcohort participants across tertiles of VDBG and bioavailable 25(OH)D concentrations; these were summarized with means and standard deviations, or medians and interquartile ranges for highly skewed variables. For the entire analysis population, we tabulated the number of CHD events overall and by race and ethnicity. We accounted for the small (<5%) amount of missing data by using multiple imputation with chained equations in all regression analyses (33); resulting estimates were combined according to Rubin’s rules to account for the variability in the imputation procedure (34).
In the primary analysis, we examined the associations of VDBG, total 25(OH)D, and bioavailable 25(OH)D concentrations with incident CHD in a series of Cox regression models with robust variance estimation that accounted for the case–cohort study design, using the Prentice method (35–37). Participants were considered at risk from the date of their baseline MESA examination until the first occurrence of the composite outcome or until they were censored due to death from non-CHD cause (n = 160), loss to follow-up, or the end of available follow-up. The first model included adjustment for age, sex, race or ethnicity, MESA study site, income, and season. The second model further adjusted for additional potential confounders: diabetes, body mass index, smoking status, self-reported general health status, log-transformed intentional physical activity, estimated glomerular filtration rate, systolic blood pressure, use of antihypertensive medications, use of lipid-lowering medications, low-density lipoprotein cholesterol, C-reactive protein (CRP) (log-transformed) concentration, and serum albumin. When we examined the association of VDBG with incident CHD, a third model of potential mediators further adjusted for calcium, phosphorus, log-transformed PTH, log-transformed FGF-23, total 25(OH)D, and a total 25(OH)D by race or ethnicity interaction term.
When we examined the associations of total 25(OH)D with incident CHD, the third model further adjusted for VDBG concentration alone. To formally test differences in effect estimates from associations of total 25(OH)D with incident CHD before and after adjustment for VDBG, we calculated a confidence interval for the difference in β coefficients, by means of bootstrap methods (38) (2000 bootstraps for each of the 20 imputation datasets). Estimates were combined across imputations according to Rubin’s rules.
Categorical covariates were modeled via indicator variables, except for self-reported health, which was modeled as a continuous term; continuous covariates were modeled linearly unless noted. Subgroup-specific hazard ratios (HRs) were calculated via linear combination of regression coefficients for main effect and cross-product terms. Interactions were tested by the Wald test of a product term for the exposure of interest and race and ethnicity or isoform categories. Sensitivity analyses completely stratified by race and ethnicity or VDBG isoform yielded similar results. All analyses were conducted with R 3.3.0 (39); a two-sided P of <0.05 was considered statistically significant for all analyses.
Results
VDBG concentrations and isoforms
At baseline, the mean [standard deviation (SD)] age in the subcohort was 59.4 (9.8) years, and 57% of participants were women. The measured VDBG isoform varied by race and ethnicity. The most common haplotype among white and Hispanic participants was Gc1s/Gc1s (41.8% and 31.7%, respectively) and among black and Chinese participants was Gc1f/Gc1f (58.5% and 35.1%, respectively). However, VDBG concentration did not vary substantially by race or ethnicity [mean (SD) in micrograms per milliliter: white, 250.9 (39.5); Chinese, 236.9 (29.2); black, 249.6 (37.5); and Hispanic, 251.4 (37.1)]. Gc2 and Gc1f haplotype carriage were associated with lower and higher concentrations of VDBG, respectively (Supplemental Table 1 (39.2KB, docx) ). Participants with higher VDBG concentrations were more likely to be female and to smoke and less likely to have diabetes, and they were characterized by higher serum concentrations of 25(OH)D, 1,25(OH)D, high-density lipoprotein, and CRP and lower physical activity levels (Table 1). VDBG concentrations varied seasonally and were highest in the summer [mean (SD), 254.4 (41.9) µg/mL] and lowest in the winter [238.9 (33.6) µg/mL]. VDBG concentrations were weakly positively correlated with 25(OH)D (r = 0.20), weakly inversely correlated with bioavailable 25(OH)D (r = −0.17), and moderately correlated with log-transformed CRP (r = 0.30) (Supplemental Table 2 (39.2KB, docx) ).
Table 1.
Baseline Characteristics of a Random Subcohort of Participants in the MESA
| Vitamin D Binding Protein Tertile, Concentration (No. of Participants) |
|||||
|---|---|---|---|---|---|
| All Subcohort Participants (N = 922) | 149–232 µg/mL (N = 319) | 232–264 µg/mL (N = 318) | 265–418 µg/mL (N = 285) | P | |
| Age (y) | 59.4 (9.8) | 60.1 (10.0) | 59.4 (10.0) | 58.5 (9.2) | 0.042 |
| Female sex | 525 (57) | 138 (43) | 169 (53) | 218 (76) | <0.001 |
| Race or ethnicity | 0.012 | ||||
| White | 431 (47) | 155 (49) | 134 (42) | 142 (50) | |
| Chinese | 93 (10) | 40 (13) | 39 (12) | 14 (5) | |
| Black | 181 (20) | 57 (18) | 68 (21) | 56 (20) | |
| Hispanic | 217 (24) | 67 (21) | 77 (24) | 73 (26) | |
| Study site | <0.001 | ||||
| Forsyth County, North Carolina | 151 (16) | 61 (19) | 46 (14) | 44 (15) | |
| New York and Bronx counties, New York | 168 (18) | 44 (14) | 56 (18) | 68 (24) | |
| Baltimore and Baltimore County, Maryland | 109 (12) | 38 (12) | 44 (14) | 27 (9) | |
| St. Paul, Minnesota | 159 (17) | 48 (15) | 49 (15) | 62 (22) | |
| Chicago, Illinois | 142 (15) | 43 (13) | 58 (18) | 41 (14) | |
| Los Angeles, California | 193 (21) | 85 (27) | 65 (20) | 43 (15) | |
| Season | 0.006 | ||||
| Winter | 269 (29) | 124 (39) | 85 (27) | 60 (21) | |
| Spring | 214 (23) | 52 (16) | 87 (27) | 75 (26) | |
| Summer | 265 (29) | 81 (25) | 85 (27) | 99 (35) | |
| Fall | 174 (19) | 62 (19) | 61 (19) | 51 (18) | |
| Total gross family income ($) | 0.116 | ||||
| <20,000 | 167 (18) | 60 (19) | 62 (19) | 45 (16) | |
| 20,000 to <50,000 | 331 (36) | 101 (32) | 119 (37) | 111 (39) | |
| ≥50,000 | 398 (43) | 146 (46) | 132 (42) | 120 (42) | |
| General self-reported health | 0.052 | ||||
| Poor | 5 (1) | 2 (1) | 3 (1) | 0 (0) | |
| Fair | 77 (8) | 22 (7) | 22 (7) | 33 (12) | |
| Good | 355 (39) | 130 (41) | 119 (37) | 106 (37) | |
| Very good | 353 (38) | 112 (35) | 128 (40) | 113 (40) | |
| Excellent | 131 (14) | 53 (17) | 46 (14) | 32 (11) | |
| Physical examination, mean (SD) | |||||
| Body mass index (kg/m2) | 28.4 (5.7) | 28.2 (5.3) | 28.4 (6.1) | 28.6 (5.6) | 0.427 |
| Systolic blood pressure (mm Hg) | 123.8 (21.1) | 124.8 (20.7) | 123.2 (21.7) | 123.3 (20.9) | 0.390 |
| Medical history | |||||
| Diabetes mellitus | 100 (11) | 40 (13) | 42 (13) | 18 (6) | 0.018 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 79.3 (15.6) | 79.1 (14.3) | 81.0 (16.7) | 77.8 (15.7) | 0.607 |
| Urine ratio of albumin to creatinine, median (IQR) | 5.0 (3.2–8.7) | 5.0 (3.4–8.4) | 4.9 (3.2–9.2) | 5.1 (3.2–8.5) | 0.564 |
| Antihypertensive medication use | 307 (33) | 100 (31) | 94 (30) | 113 (40) | 0.031 |
| Lipid-lowering medication use | 117 (13) | 37 (12) | 37 (12) | 43 (15) | 0.172 |
| Smoking | 0.008 | ||||
| Never | 450 (49) | 164 (51) | 149 (47) | 137 (48) | |
| Former | 323 (35) | 120 (38) | 112 (35) | 91 (32) | |
| Current | 147 (16) | 34 (11) | 57 (18) | 56 (20) | |
| Total intentional exercise (metabolic equivalent tasks, min/wk), median (IQR) | 825.0 (105.0–1980.0) | 1035.0 (87.5–2205.0) | 735.0 (151.9–1876.9) | 735.0 (105.0–1683.8) | <0.001 |
| LDL-C (mg/dL) | 117.1 (31.0) | 115.3 (31.2) | 119.9 (29.3) | 116.0 (32.4) | 0.675 |
| CRP (mg/L), median (IQR) | 2.0 (0.8–4.5) | 1.3 (0.6–2.9) | 2.0 (0.7–4.4) | 3.3 (1.4–6.9) | <0.001 |
| Calcium (mg/dL) | 9.7 (0.4) | 9.7 (0.4) | 9.7 (0.4) | 9.7 (0.4) | 0.306 |
| Phosphorus (mg/dL) | 3.7 (0.5) | 3.7 (0.5) | 3.7 (0.5) | 3.8 (0.5) | 0.005 |
| PTH (pg/mL), median (IQR) | 39.0 (29.6–50.3) | 41.7 (32.2–53.2) | 38.1 (29.4–48.7) | 38.1 (28.0–49.5) | 0.004 |
| FGF-23 (pg/mL), median (IQR) | 36.5 (29.3–46.0) | 38.2 (31.0–47.7) | 36.2 (28.7–46.1) | 35.3 (28.9–44.2) | 0.088 |
| Total 25(OH)D (ng/mL) | 26.2 (11.2) | 24.3 (10.8) | 26.4 (11.3) | 28.0 (11.3) | <0.001 |
| Albumin (g/dL) | 4.1 (0.3) | 4.1 (0.3) | 4.2 (0.3) | 4.2 (0.3) | 0.028 |
| Gc2 haplotype carriage | <0.001 | ||||
| 0 | 176 (54) | 229 (71) | 249 (87) | ||
| 1 | 106 (33) | 76 (24) | 31 (11) | ||
| 2 | 37 (12) | 13 (4) | 5 (2) | ||
| Gc1s haplotype carriage | <0.001 | ||||
| 0 | 136 (43) | 110 (34) | 77 (27) | ||
| 1 | 121 (38) | 115 (36) | 97 (34) | ||
| 2 | 62 (19) | 93 (29) | 111 (39) | ||
Abbreviations: IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol.
VDBG and CHD events
Participants were followed for a median (interquartile range) of 12.4 (5.6–12.8) years. For incident CHD, the qualifying event was myocardial infarction for 204 participants, angina for 200 participants, resuscitated cardiac arrest for 16 participants, and CHD death for 88 participants. (Supplemental Table 3 (39.2KB, docx) ).
Participants with higher circulating concentrations of VDBG were more likely to experience a CHD event (fully adjusted HR, 1.78; 95% confidence interval, 1.46 to 2.14, per SD increment in VDBG concentration, P < 0.0001) (Table 2). We did not observe HR heterogeneity in the association of VDBG and CHD by race or ethnicity (P for interaction = 0.59) (Table 2) or by VDBG isoform (P for interaction = 0.86) (Supplemental Table 4 (39.2KB, docx) ). Secondary analyses that repeated the primary analysis with a more restrictive definition of incident CHD that included only incident myocardial infarction, CHD death, and resuscitated cardiac arrest yielded stronger results (Supplemental Table 5 (39.2KB, docx) ). Haplotype carriage was also associated with CHD events, but magnitudes of association appeared weaker than those of VDBG concentration (Supplemental Table 6 (39.2KB, docx) ).
Table 2.
Association of VDBG Concentrations With Incident CHD Events
| VDBG Levels | N Participants (N Events) | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| All participants | ||||
| <232 µg/mL | 458 (170) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 232–262 µg/mL | 449 (153) | 0.99 (0.70, 1.38) | 0.97 (0.68, 1.40) | 1.02 (0.70, 1.50) |
| >263 µg/mL | 450 (185) | 2.32 (1.61, 3.35) | 2.52 (1.67, 3.79) | 2.80 (1.83, 4.29) |
| Per SD increment | 1.54 (1.32, 1.80) | 1.62 (1.35, 1.94) | 1.78 (1.47, 2.16) | |
| P | <0.0001 | <0.0001 | <0.0001 | |
| Global P for interaction by race or ethnicity | 0.34 | 0.58 | 0.59 | |
| White participants | ||||
| <232 µg/mL | 221 (86) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 232–262 µg/mL | 186 (63) | 1.07 (0.66, 1.74) | 1.13 (0.66, 1.94) | 1.32 (0.73, 2.37) |
| >263 µg/mL | 209 (78) | 2.09 (1.24, 3.51) | 2.33 (1.31, 4.12) | 2.98 (1.63, 5.44) |
| Per SD increment | 1.45 (1.17, 1.80) | 1.55 (1.21, 1.98) | 1.79 (1.38, 2.32) | |
| P | 0.0006 | 0.0005 | < 0.0001 | |
| Chinese participants | ||||
| <232 µg/mL | 56 (16) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 232–262 µg/mL | 49 (14) | 0.64 (0.21, 1.90) | 0.46 (0.16, 1.38) | 0.50 (0.17, 1.48) |
| >263 µg/mL | 30 (16) | 4.11 (1.34, 12.56) | 3.91 (1.22, 12.54) | 4.67 (1.36, 16.1) |
| Per SD increment | 2.59 (1.41, 4.75) | 2.52 (1.31, 4.87) | 2.91 (1.39, 6.08) | |
| P | 0.002 | 0.006 | 0.004 | |
| P for interaction vs. white participants | 0.07 | 0.16 | 0.21 | |
| Black participants | ||||
| <232 µg/mL | 91 (39) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 232–262 µg/mL | 109 (44) | 0.93 (0.46, 1.88) | 0.81 (0.40, 1.64) | 0.74 (0.35, 1.54) |
| >263 µg/mL | 99 (47) | 2.08 (1.05, 4.13) | 1.79 (0.88, 3.65) | 1.88 (0.91, 3.90) |
| Per SD increment | 1.63 (1.21, 2.19) | 1.62 (1.18, 2.22) | 1.73 (1.24, 2.42) | |
| P | 0.001 | 0.003 | 0.001 | |
| P for interaction vs. white participants | 0.53 | 0.82 | 0.87 | |
| Hispanic participants | ||||
| <232 µg/mL | 90 (29) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 232–262 µg/mL | 105 (32) | 1.17 (0.59, 2.32) | 1.26 (0.60, 2.66) | 1.28 (0.59, 2.77) |
| >263 µg/mL | 112 (44) | 2.88 (1.45, 5.74) | 3.88 (1.81, 8.30) | 3.72 (1.66, 8.32) |
| Per SD increment | 1.50 (1.12, 2.01) | 1.62 (1.17, 2.23) | 1.66 (1.17, 2.36) | |
| P | 0.007 | 0.003 | 0.004 | |
| P for interaction vs. white participants | 0.87 | 0.82 | 0.72 |
Model 1 adjusts for age, sex, race and ethnicity (analyses of all participants), study site, income, and season. Model 2 additionally adjusts for diabetes mellitus, body mass index, smoking status, self-reported general health status, log-transformed intentional physical activity, estimated glomerular filtration rate, systolic blood pressure, antihypertensive medications, lipid-lowering medications, low-density lipoprotein cholesterol, log-transformed CRP concentration, and serum albumin. Model 3 additionally adjusts for calcium, phosphorus, log-transformed PTH, log-transformed FGF-23, total 25(OH)D, and a total 25(OH)D by race and ethnicity interaction. P values from analysis of continuous exposure.
25(OH)D and CHD events
Mean (SD) bioavailable 25(OH)D concentration was 4.6 (2.4) ng/mL and varied by race and ethnicity: 5.7 (2.5) ng/mL for white participants, 4.4 (1.9) ng/mL for Chinese participants, 2.5 (1.3) ng/mL for black participants, and 4.3 (1.9) ng/mL for Hispanic participants (P < 0.0001). Participants with high circulating bioavailable 25(OH)D were less likely to be female or diabetic, were more likely to have high income, better self-reported health, and high physical activity levels, and were characterized by low CRP and interleukin-6 (Supplemental Table 7 (39.2KB, docx) ).
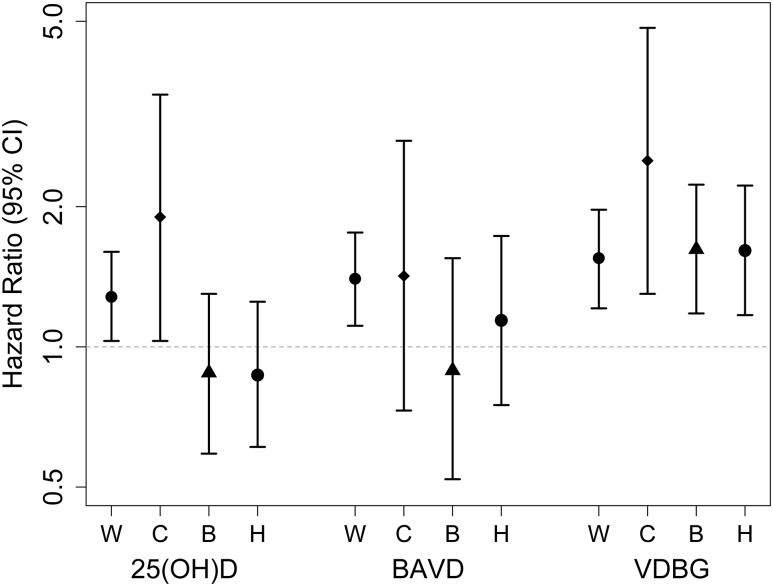
Low circulating bioavailable 25(OH)D was associated with incident CHD events among white participants but not among black or Hispanic participants (Fig. 2 and Table 3). Results were not materially changed when bioavailable 25(OH)D was calculated assuming a single binding coefficient across all six isoforms.
Figure 2.
Associations of total 25(OH)D, bioavailable 25(OH)D (6 coefficients), and VDBG with incident CHD, by race. B, black participants; C, Chinese-American participants; H, Hispanic participants; W, white participants. HRs are per SD decrement for 25(OH)D (SD = 11.0 ng/mL) and bioavailable 25(OH)D (BAVD) (SD = 2.4 ng/mL), and per SD increment for VDBG (SD = 38.8 µg/mL), from a race interaction model that adjusts for age, sex, race and ethnicity, study site, income, season, diabetes mellitus, body mass index, smoking status, self-reported general health status, log-transformed intentional physical activity, estimated glomerular filtration rate, systolic blood pressure, antihypertensive medications, lipid-lowering medications, low-density lipoprotein cholesterol, log-transformed CRP concentration, and serum albumin. Global P for interaction by race for each metabolite was 0.05 for total 25(OH)D, 0.45 for BAVD, and 0.58 for VDBG.
Table 3.
Association of Bioavailable 25(OH)D With Incident CHD Events
| N Participants (N Events) | Model 1 | Model 2 | |
|---|---|---|---|
| All participants | |||
| ≥ 3.4 ng/mL | 457 (190) | 1.0 (reference) | 1.0 (reference) |
| 2.29–3.4 ng/mL | 455 (173) | 1.48 (1.05, 2.09) | 1.50 (1.04, 2.16) |
| <2.29 ng/mL | 445 (145) | 2.20 (1.48, 3.26) | 2.34 (1.53, 3.59) |
| Per SD decrement | 1.24 (1.04, 1.48) | 1.27 (1.06, 1.54) | |
| P | 0.01 | 0.01 | |
| Global P for interaction by race or ethnicity | 0.59 | 0.45 | |
| White participants | |||
| ≥3.4 ng/mL | 95 (36) | 1.0 (reference) | 1.0 (reference) |
| 2.29–3.4 ng/mL | 209 (91) | 2.15 (1.39, 3.32) | 2.41 (1.51, 3.85) |
| <2.29 ng/mL | 312 (100) | 2.26 (1.26, 4.03) | 2.71 (1.48, 4.95) |
| Per SD decrement | 1.33 (1.07, 1.65) | 1.40 (1.11, 1.75) | |
| P | 0.01 | 0.004 | |
| Chinese participants | |||
| ≥3.4 ng/mL | 41 (18) | 1.0 (reference) | 1.0 (reference) |
| 2.29–3.4 ng/mL | 62 (17) | 0.66 (0.21, 2.01) | 0.49 (0.15, 1.59) |
| <2.29 ng/mL | 32 (11) | 2.05 (0.66, 6.43) | 2.17 (0.66, 7.15) |
| Per SD decrement | 1.48 (0.79, 2.79) | 1.43 (0.73, 2.78) | |
| P | 0.22 | 0.29 | |
| P for interaction vs. white participants | 0.75 | 0.95 | |
| Black participants | |||
| ≥3.4 ng/mL | 222 (97) | 1.0 (reference) | 1.0 (reference) |
| 2.29–3.4 ng/mL | 65 (27) | 0.23 (0.06, 0.88) | 0.15 (0.04, 0.62) |
| <2.29 ng/mL | 12 (6) | 0.34 (0.10, 1.14) | 0.21 (0.06, 0.73) |
| Per SD decrement | 0.94 (0.55, 1.58) | 0.90 (0.52, 1.55) | |
| P | 0.81 | 0.69 | |
| P for interaction vs. white participants | 0.22 | 0.14 | |
| Hispanic participants | |||
| ≥3.4 ng/mL | 99 (39) | 1.0 (reference) | 1.0 (reference) |
| 2.29–3.4 ng/mL | 119 (38) | 0.97 (0.50, 1.86) | 0.82 (0.40, 1.67) |
| <2.29 ng/mL | 89 (28) | 2.01 (1.00, 4.04) | 1.97 (0.92, 4.21) |
| Per SD decrement | 1.16 (0.79, 1.71) | 1.14 (0.75, 1.73) | |
| P | 0.45 | 0.53 | |
| P for interaction vs. white participants | 0.54 | 0.40 |
Model 1 adjusts for age, sex, race and ethnicity, study site, income, and season. Model 2 additionally adjusts for diabetes mellitus, body mass index, smoking status, self-reported general health status, log-transformed intentional physical activity, estimated glomerular filtration rate, systolic blood pressure, antihypertensive medications, lipid-lowering medications, low-density lipoprotein cholesterol, log-transformed CRP concentration, and serum albumin. P values from analysis of continuous exposure.
Like bioavailable 25(OH)D, low circulating total 25(OH)D was associated with CHD events among white and Chinese participants. Associations of 25(OH)D with CHD were strengthened by additional adjustment for VDBG (Supplemental Table 8 (39.2KB, docx) ). Among white participants, adjustment for VDBG significantly increased the magnitude of associations of 25(OH)D with CHD events by 15% [ratio of HR from Model 3 to Model 2, per 10-ng/mL decrement of 25(OH)D, 1.15 (1.05, 1.26), P = 0.002]. Adjustment for VDBG did not account for the heterogeneity by race and ethnicity (global P for interaction by race or ethnicity = 0.04).
Discussion
In this multiethnic community-based cohort of adults without clinical cardiovascular disease at baseline, high circulating serum VDBG was strongly associated with increased risk of adjudicated incident CHD events. This association was strong, independent of known CHD risk factors, and consistent across race and ethnicity. Adjustment for VDBG strengthened associations of total 25(OH)D concentration with CHD events in white participants, and estimated bioavailable 25(OH)D was significantly associated with CHD events in whites. However, accounting for VDBG through adjustment or estimation of bioavailable 25(OH)D did not eliminate the racial and ethnic heterogeneity observed in 25(OH)D associations with CHD events, with null associations still present for black and Hispanic participants.
With the polyclonal enzyme-linked immunosorbent assay for VDBG, free and bioavailable 25(OH)D were more strongly associated with bone mineral density than total 25(OH)D (28), but VDBG was not associated with heart failure (40). One recent case–control study, which measured VDBG via the isoform-independent LC-MS/MS assay, found that higher VDBG concentrations were strongly associated with incident end-stage renal disease (41). These associations are consistent in direction with our observed associations with incident CHD events. In contrast, among patients with liver failure, lower VDBG concentration is associated with in-hospital mortality, perhaps because it is a sign of severely impaired liver synthetic function in this setting (42–45).
The associations between VDBG and CHD may or may not be causal. VDBG is an acute phase reactant, and our noted associations could therefore reflect confounding by inflammation. However, adjustment for CRP, another acute phase reactant modestly correlated with VDBG, did not attenuate the associations. VDBG could promote CHD by sequestering circulating 25(OH)D and 1,25(OH)2D and reducing transport to target tissues. However, animal studies have explored the influence of VDBG on the biological activity of 1,25(OH)2D3 and question this hypothesis: VDBG-null mice have severely depleted circulating 1,25(OH)2D3 in the blood, but they have preserved 1,25(OH)2D3 levels in tissue and are normocalcemic (46). VDBG is the major protein responsible for the sequestration of G-actin monomers from plasma after tissue injury. Low concentrations of VDBG have been associated with worse survival among trauma patients (47, 48). Circulating VDBG could also plausibly be related to CHD via other mechanisms, through modulation of inflammatory processes and innate immunity, and through binding of fatty acids as well as directly helping to regulate neutrophil, macrophage, fibroblast, and osteoclast activity (49).
Previously, VDBG isoforms and concentrations measured by monoclonal immunoassay were reported to vary by race (17). An important implication of this observation was that differences in VDBG may help explain racial variation in associations of total 25(OH)D concentration with health outcomes. However, subsequent studies demonstrated that the monoclonal immunoassay is biased by isoform and that VDBG concentrations measured by other methods did not differ significantly by race (18, 20, 21). Our data, generated via a mass spectrometry assay that is thought to quantify VDBG concentration without bias by race, ethnicity, or isoform, suggest that differences in VDBG do not explain racial or ethnic heterogeneity in associations of 25(OH)D with CHD and that alternative explanations should be sought.
If the free and weakly bound portions of circulating 25(OH)D are most accessible to target tissues, accounting for VDBG could improve the precision of classifying 25(OH)D status and strengthen relationships with health outcomes. Indeed, free and bioavailable 25(OH)D were found to be more strongly associated with BMD than total 25(OH)D (50) among healthy young adults and more strongly associated with PTH and calcium among incident hemodialysis patients (27). Consistent with these observations, we observed that adjustment for VDBG concentration substantially increased the magnitude of association of total 25(OH)D with CHD events among white and Chinese participants, and bioavailable 25(OH)D was strongly associated with CHD events among white participants. However, associations of bioavailable 25(OH)D with health outcomes should be interpreted with caution because they may be confounded by VDBG concentration, which we found to be strongly associated with both lower bioavailable 25(OH)D concentration and higher CHD risk. The finding of associations between the Gc2 and Gc1 haplotypes and incident CHD warrants investigation and replication.
Strengths of this study its racially and ethnically diverse, community-based population; the evaluation of incident events relevant to 25(OH)D and VDBG over a long period of longitudinal follow-up; the large number of observed events sampled in an efficient study design; and the use of state-of-the-art assays to measure 25(OH)D, VDBG, and isoform. As in any observational study, potential associations of VDBG and 25(OH)D with CHD events may be confounded by unmeasured factors that are associated with both mineral metabolism markers and CHD outcomes. However, confounding is minimized in MESA because, by design, participants were free of self-reported clinical cardiovascular disease at baseline and because potential confounding variables were quantified in a high-quality manner. An additional limitation is that our study may have limited power to detect associations of small magnitude within individual racial and ethnic groups and within individual VDBG isoforms.
Conclusions
Higher circulating VDBG concentrations are strongly associated with CHD events, and additional studies are warranted to determine the nature of this relationship. Incorporation of VDBG assessment strengthened existing associations of 25(OH)D with CHD events among white and Chinese participants, suggesting that accounting for VDBG may help classify health risks associated with low circulating 25(OH)D in these populations. However, associations of bioavailable 25(OH)D with health outcomes must be interpreted cautiously because they may be confounded by VDBG concentration. Moreover, neither VDBG concentrations nor genotype mitigated racial variation in the association of 25(OH)D with CHD, suggesting that explanations other than differences in VDBG should be sought for race and ethnicity heterogeneity in associations of 25(OH)D and CHD.
Acknowledgments
The authors thank the MESA investigators, staff, and participants for their valuable contributions.
This study was supported by National Heart, Lung, and Blood Institute Grant R01HL096875 and Grant N01-HC-95159 through N01-HC-95169. This study was also supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK088762, Grant R01DK099199, and Grant K01DK109019.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 25(OH)D
- 25-hydroxyvitamin D
- CHD
- coronary heart disease
- CRP
- C-reactive protein
- FGF-23
- fibroblast growth factor-23
- HR
- hazard ratio
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- MESA
- Multi-Ethnic Study of Atherosclerosis
- PTH
- parathyroid hormone
- SD
- standard deviation
- VDBG
- vitamin D binding globulin.
References
- 1.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. [DOI] [PubMed] [Google Scholar]
- 2.de Boer IH, Levin G, Robinson-Cohen C, Biggs ML, Hoofnagle AN, Siscovick DS, Kestenbaum B. Serum 25-hydroxyvitamin D concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med. 2012;156(9):627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromsø study. Eur J Endocrinol. 2010;162(5):935–942. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Chen M, Hankins SR, Nùñez AE, Watson RA, Weinstock PJ, Newschaffer CJ, Eisen HJ; Drexel Cardiovascular Health Collaborative Education, Research, and Evaluation Group . Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am J Cardiol. 2012;110(6):834–839. [DOI] [PubMed] [Google Scholar]
- 7.Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michos ED, Reis JP, Post WS, Lutsey PL, Gottesman RF, Mosley TH, Sharrett AR, Melamed ML. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012;28(4):367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr L, Selvin E, Reis JP, Gross M, Eckfeldt JH, Folsom AR. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail. 2015;3(5):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scragg R, Sowers M, Bell C; Third National Health and Nutrition Examination Survey . Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. [DOI] [PubMed] [Google Scholar]
- 11.Reis JP, Michos ED, von Mühlen D, Miller ER III. Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88(6):1469–1477. [DOI] [PubMed] [Google Scholar]
- 12.van Ballegooijen AJ, Robinson-Cohen C, Katz R, Criqui M, Budoff M, Li D, Siscovick D, Hoofnagle A, Shea SJ, Burke G, de Boer IH, Kestenbaum B. Vitamin D metabolites and bone mineral density: the Multi-Ethnic Study of Atherosclerosis. Bone. 2015;78:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michos ED, Misialek JR, Selvin E, Folsom AR, Pankow JS, Post WS, Lutsey PL. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: the ARIC study. Atherosclerosis. 2015;241(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61(5):969–975. [DOI] [PubMed] [Google Scholar]
- 15.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D–binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. [DOI] [PubMed] [Google Scholar]
- 16.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D–binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986;72(4):281–293. [DOI] [PubMed] [Google Scholar]
- 17.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D–binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson CM, Lutsey PL, Misialek JR, Laha TJ, Selvin E, Eckfeldt JH, Hoofnagle AN. Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D–binding protein in blacks and whites. Clin Chem. 2016;62(1):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB; Chronic Renal Insufficiency Cohort study investigators . Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D–binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res. 2016;31(6):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D–binding protein concentrations quantified by mass spectrometry. N Engl J Med. 2015;373(15):1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, Pauwels S, Prentice A, Smith RD, Shi T, Gao Y, Schepmoes AA, Zmuda JM, Lapidus J, Cauley JA, Bouillon R, Schoenmakers I, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Research Group . Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101(5):2226–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 23.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R. Thoracic aortic calcification and coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2011;215(1):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutsey PL, Parrinello CM, Misialek JR, Hoofnagle AN, Henderson CM, Laha TJ, Michos ED, Eckfeldt JH, Selvin E. Short-term variability of vitamin D-related biomarkers. Clin Chem. 2016;62(12):1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, Ix JH, Budoff M, Lutsey PL, Siscovick DS, Kestenbaum B, de Boer IH. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97(6):1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88(2):511S–512S. [DOI] [PubMed] [Google Scholar]
- 27.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almås B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D [published correction appears in Scand J Clin Lab Invest. 2014;74(5):464] Scand J Clin Lab Invest. 2014;74(3):177–183. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association Standards of medical care in diabetes: 2007. Diabetes Care. 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 30.Bosworth C, Sachs MC, Duprez D, Hoofnagle AN, Ix JH, Jacobs DR Jr, Peralta CA, Siscovick DS, Kestenbaum B, de Boer IH. Parathyroid hormone and arterial dysfunction in the Multi-Ethnic Study of Atherosclerosis. Clin Endocrinol (Oxf). 2013;79(3):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linefsky JP, O’Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS, Siscovick DS, Kestenbaum B. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the Cardiovascular Health Study. J Am Coll Cardiol. 2011;58(3):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227–241. [Google Scholar]
- 34.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 35.Therneau TM, Li H. Computing the Cox model for case cohort designs. Lifetime Data Anal. 1999;5(2):99–112. [DOI] [PubMed] [Google Scholar]
- 36.Barlow WE. Robust variance estimation for the case–cohort design. Biometrics. 1994;50(4):1064–1072. [PubMed] [Google Scholar]
- 37.Prentice R. A case–cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 38.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- 39.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 40.Petrone AB, Weir NL, Steffen BT, Tsai MY, Gaziano JM, Djoussé L. Plasma vitamin D–binding protein and risk of heart failure in male physicians. Am J Cardiol. 2013;112(6):827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rebholz CM, Grams ME, Lutsey PL, Hoofnagle AN, Misialek JR, Inker LA, Levey AS, Selvin E, Hsu CY, Kimmel PL, Vasan RS, Eckfeldt JH, Coresh J; Chronic Kidney Disease Biomarkers Consortium . Biomarkers of vitamin D status and risk of ESRD. Am J Kidney Dis. 2016;67(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WM, Galbraith RM, Watt GH, Hughes RD, McIntire DD, Hoffman BJ, Williams R. Predicting survival in fulminant hepatic failure using serum Gc protein concentrations. Hepatology. 1995;21(1):101–105. [PubMed] [Google Scholar]
- 43.Antoniades CG, Berry PA, Bruce M, Cross TJ, Portal AJ, Hussain MJ, Bernal W, Wendon JA, Vergani D. Actin-free Gc globulin: a rapidly assessed biomarker of organ dysfunction in acute liver failure and cirrhosis. Liver Transpl. 2007;13(9):1254–1261. [DOI] [PubMed] [Google Scholar]
- 44.Bagchi A, Kumar S, Ray PC, Das BC, Gumma PK, Kar P. Predictive value of serum actin-free Gc-globulin for complications and outcome in acute liver failure. J Viral Hepat. 2015;22(2):192–200. [DOI] [PubMed] [Google Scholar]
- 45.Bagchi S, Oniku AE, Topping K, Mamhoud ZN, Paget TA. Programmed cell death in Giardia. Parasitology. 2012;139(7):894–903. [DOI] [PubMed] [Google Scholar]
- 46.Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D–binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149(7):3656–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahl OE, Gudmundsen TE, Bjørnarå BT, Solheim DM. Risk of clinical pulmonary embolism after joint surgery in patients receiving low-molecular-weight heparin prophylaxis in hospital: a 10-year prospective register of 3,954 patients. Acta Orthop Scand. 2003;74(3):299–304. [DOI] [PubMed] [Google Scholar]
- 48.Dahl B, Schiødt FV, Rudolph S, Ott P, Kiaer T, Heslet L. Trauma stimulates the synthesis of Gc-globulin. Intensive Care Med. 2001;27(2):394–399. [DOI] [PubMed] [Google Scholar]
- 49.Gomme PT, Bertolini J. Therapeutic potential of vitamin D–binding protein. Trends Biotechnol. 2004;22(7):340–345. [DOI] [PubMed] [Google Scholar]
- 50.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D–binding protein modifies the vitamin D–bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]