Abstract
Context:
Polycystic ovary syndrome (PCOS), a common endocrine condition, is the leading cause of anovulatory infertility.
Objective:
Given that common disease-susceptibility variants account for only a small percentage of the estimated PCOS heritability, we tested the hypothesis that rare variants contribute to this deficit in heritability.
Design, Setting, and Participants:
Unbiased whole-genome sequencing (WGS) of 80 patients with PCOS and 24 reproductively normal control subjects identified potentially deleterious variants in AMH, the gene encoding anti-Müllerian hormone (AMH). Targeted sequencing of AMH of 643 patients with PCOS and 153 control patients was used to replicate WGS findings.
Main Outcome Measures:
Dual luciferase reporter assays measured the impact of the variants on downstream AMH signaling.
Results:
We found 24 rare (minor allele frequency < 0.01) AMH variants in patients with PCOS and control subjects; 18 variants were specific to women with PCOS. Seventeen of 18 (94%) PCOS-specific variants had significantly reduced AMH signaling, whereas none of 6 variants observed in control subjects showed significant defects in signaling. Thus, we identified rare AMH coding variants that reduced AMH-mediated signaling in a subset of patients with PCOS.
Conclusion:
To our knowledge, this study is the first to identify rare genetic variants associated with a common PCOS phenotype. Our findings suggest decreased AMH signaling as a mechanism for the pathogenesis of PCOS. AMH decreases androgen biosynthesis by inhibiting CYP17 activity; a potential mechanism of action for AMH variants in PCOS, therefore, is to increase androgen biosynthesis due to decreased AMH-mediated inhibition of CYP17 activity.
Using next-generation sequencing, we identified in patients with PCOS 17 rare AMH variants that reduced signaling in vitro. These results suggest a previously unrecognized mechanism for AMH action in PCOS.
Polycystic ovary syndrome (PCOS) is a complex genetic disease affecting 5% to 15% of premenopausal women worldwide (1). It is associated with substantially increased risk for infertility, prediabetes, and type 2 diabetes (T2D) (1). PCOS is a highly heritable disorder with disease correlation of 71% in monozygotic twins, almost twice as large as that in dizygotic twins (38%) (2), suggesting a genetic susceptibility to the disorder. Furthermore, male and female first-degree relatives have metabolic and reproductive features of the syndrome, including increased T2D risk, consistent with a genetic contribution to these phenotypes (1).
The etiology of PCOS remains unknown. It is diagnosed by the presence of two or more reproductive phenotypes: hyperandrogenism, chronic oligoanovulation, and polycystic ovarian morphology (POM) (1). Genome-wide association studies (GWAS) in Han Chinese and European PCOS have implicated gonadotropin secretion and action, ovarian androgen biosynthesis, insulin resistance, body weight, and sex hormone–binding globulin in the development of PCOS (3–5). However, as with other complex diseases (6), the 16 susceptibility loci identified have modest effect sizes and thus account for only a small fraction of the estimated heritability of PCOS.
One hypothesis for this deficit in heritability is that low-frequency or rare genetic variants with larger biologic effects play a more important role in complex disease pathogenesis than do variants that can be detected by GWAS (6). GWAS are designed to detect common genetic variation [minor allele frequencies (MAFs) ≥ 0.05] (6). These variants are expected to have modest phenotypic impact because they have not been subjected to strong selective pressure (7). Rare variants that cause larger biologic effects than common susceptibility variants have been found in other common, complex diseases. In T2D, rare variants likely to be deleterious were found in MTNR1B, which encodes melatonin receptor 1B (8). Only the rare MTNR1B variants that resulted in total or partial loss of melatonin receptor 1B function increased T2D risk (8). Rare coding variants have also been found in GCKR, another T2D GWAS susceptibility gene encoding glucokinase regulatory protein, in subjects with higher circulating triglyceride levels (9). Collectively, these findings support the hypothesis that rare, functional coding variants can produce common, complex disease/trait phenotypes (6).
Using an unbiased whole-genome sequencing (WGS) approach followed by targeted resequencing of AMH in a replication cohort, we identified significant evidence for association between functional mutations in the anti-Müllerian hormone gene (AMH) and PCOS. In females, anti-Müllerian hormone (AMH; or Müllerian inhibiting substance) is a regulator of folliculogenesis (10). AMH has also been shown to modulate steroidogenesis through its inhibition of CYP17 (11). AMH is secreted by the granulosa cells of small, growing ovarian follicles (12), and its expression is inversely correlated with follicle size (12). Polycystic ovaries are characterized by an increase in ovarian follicles from the primary stages onward (1). Levels of AMH are typically elevated in PCOS and correlate with antral follicle counts, although the mechanism for elevated AMH levels in PCOS remains unknown (12). Elevated AMH levels are proposed to be a marker for the distinctive alteration in folliculogenesis that is a cardinal feature of polycystic ovarian morphology (12). Furthermore, it has recently been demonstrated that AMH can regulate gonadotropin-releasing hormone release (13), a mechanism that is perturbed in PCOS (14). Accordingly, AMH is a highly plausible candidate gene for PCOS.
Materials and Methods
Subjects
The subjects were self-reported white people of European ancestry and had participated in our previous studies of PCOS (4, 15) (Table 1). The details of the assessment of study subjects and their clinical and biochemical features at enrollment were reported previously (4, 16, 17). Patients with PCOS had the “classic” or National Institutes of Health (NIH) phenotype of PCOS with elevated levels of total testosterone (T) or non–sex hormone–binding globulin-bound T (uT) levels, and chronic oligomenorrhea (eight or fewer menses per year) (18). Control subjects had normal androgen levels, regular 27- to 35-day menstrual cycles, and no history of reproductive disorders. (See Supplemental Data for additional details.) Ovarian morphology was not assessed, because this finding is not a criterion for the diagnosis of the NIH PCOS phenotype.
Table 1.
Clinical Features and Reproductive Hormone Levels of Study Participants
|
Patients With PCOS (n = 700) |
Control Subjects (n = 165) |
P Valuea | |||
|---|---|---|---|---|---|
| No. | Median (First Through Third Quartiles) | No. | Median (First Through Third Quartiles) | ||
| Age, y | 700 | 28 (24–32) | 165 | 29 (24–34) | 0.016 |
| BMI, kg/m2 | 700 | 35.4 (28.7–41.5) | 165 | 27.6 (22.4–34.1) | <0.0001 |
| Tb, ng/dL | 669 | 71 (59–90) | 165 | 26 (19–35) | <0.0001 |
| uT, ng/dL | 497 | 23 (17–29) | 65 | 4 (3–7) | <0.0001 |
| SHBG, nM | 498 | 53 (34–78) | 65 | 107 (72–156) | <0.0001 |
| DHEAS, ng/mL | 595 | 2065 (1446–2886) | 90 | 1349 (1059–1729) | <0.0001 |
| LH, mIU/mL | 518 | 11 (7–17) | 59 | 3 (3–7) | <0.0001 |
| FSH, mIU/mL | 518 | 9 (8–11) | 59 | 10 (7–12) | 0.74* |
| AMH, ng/mL | 259 | 9.3 (5.3–17.5) | 126 | 2.3 (1.2–4.0) | <0.0001* |
All analyses were with Mann-Whitney U test except for those denoted by an asterisk, which used analysis of covariance adjusted for age and BMI.
To convert values for T from nanograms per deciliter to nanomoles per liter, multiply by 0.03467; to convert the values for uT from nanograms per deciliter to nanomoles per liter, multiply by 0.03467; to convert the values for DHEAS from nanograms per milliliter to micromolar per liter, multiply by 0.00271; to convert values for AMH from nanograms per milliliter to picomoles per liter, multiply by 7.1429.
Next-generation sequencing
WGS was performed on DNA from 80 patients with PCOS and 24 control subjects by Complete Genomics (Mountain View, CA). Targeted resequencing of AMH was performed in DNA from 643 patients with PCOS and 153 control subjects at the Center for Inherited Disease Research (Johns Hopkins University, Baltimore, MD), using the Illumina HiSeq2000 (San Diego, CA) platform. Samples from 23 patients with PCOS and 12 control subjects were sequenced on both platforms. Genomic sequence data were thus available for analysis for a total of 700 patients with PCOS and 165 control subjects.
Bioinformatic pipeline
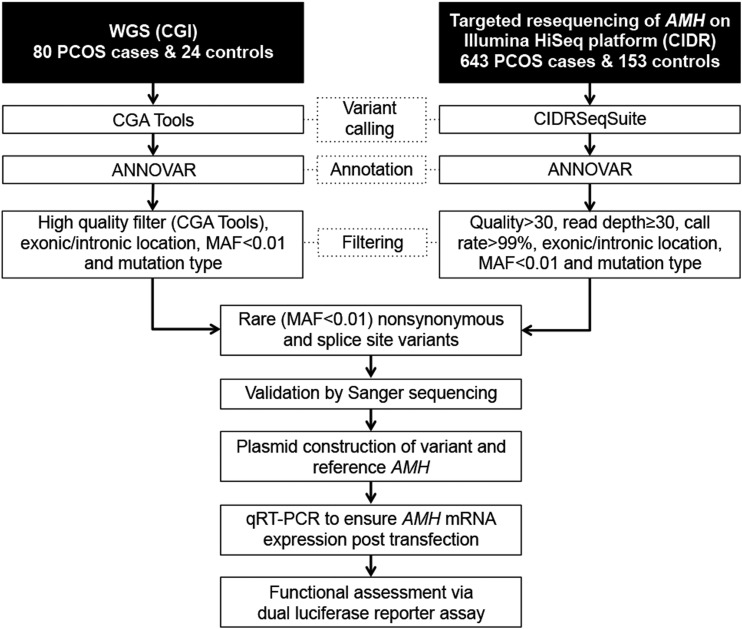
Analyses for WGS and targeted sequencing results are outlined in Fig. 1. From WGS analyses, genes having PCOS-specific, rare (MAF < 0.01), likely deleterious (i.e., missense, nonsense, or splice site) variants, as well as a biological rationale for being a PCOS susceptibility locus were carried on to targeted resequencing.
Figure 1.
Pipeline of sequencing data analyses and functional assessment of variants. Shown are the analytical steps applied to identify high-quality, rare (MAF < 0.01) AMH variants from WGS and targeted resequencing data, as well as the molecular approach used to determine effects of AMH variants on downstream signaling activity. The ANNOVAR tool (for annotation of genetic variants) is detailed at http://annovar.openbioinformatics.org/en/latest/. CGA Tools are from CGI. CGI, Complete Genomics Inc.; CIDR, Center for Inherited Disease Research; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
Variant validation
All annotated variants surviving the filters described earlier were verified using polymerase chain reaction (PCR) amplification of genomic DNA and Sanger sequencing.
Cell culture
COS7 cells (African green monkey kidney fibroblastlike cell line; American Type Culture Collection, Manassas, VA) were maintained in Dulbecco’s modified Eagle medium, high glucose + GlutaMAX (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (Hyclone, Logan, UT) at 37°C with 5% CO2.
AMH plasmid constructs
The AMH coding region was PCR amplified from carriers of AMH variants. PCR products were subcloned into pcDNA 3.1. The reference AMH (build GRCh37/hg19) construct was generated in parallel to variant constructs.
Transfection and dual reporter assay
To quantify the signaling potential of AMH variants in their homozygous variant and heterozygous states relative to the homozygous reference, we used a dual luciferase reporter assay in which binding to MISRII activates a firefly luciferase reporter (19). Applied Biosystem TaqMan primers were used to quantify mRNA expression of AMH (Hs01006984_g1) relative to the housekeeping gene GAPDH (Hs02758991_g1).
Hormone assays
T, uT, dehydroepiandrosterone sulfate (DHEAS), sex hormone–binding globulin (SHBG), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) levels were determined as previously reported (4, 16, 17) (Supplemental Table 1 (96.9KB, docx) ). AMH was measured by enzyme-linked immunosorbent assay [Ansh Laboratories, Webster, TX (17)] in 259 patients with PCOS and 126 control subjects for whom serum samples were available.
Statistical analysis
Statistical tests were implemented using SPSS statistics (IBM, Armonk, NY). Normality of hormonal and demographic end points were evaluated by the Kolmogorov-Smirnov test. All tests were two-tailed and α levels <0.05 were considered statistically significant.
Association testing
We tested for association between functional AMH variants and PCOS using a gene-based burden test approach (20) and combined all functional AMH variants into one statistical test. We tested for association between functional AMH variants and PCOS using two independent control groups: 165 reproductively normal women from this study and population-based control subjects (n > 100,000; Table 2).
Table 2.
Rare AMH Variants Identified in Patients With PCOS and Control Subjects, and Impact on Signaling Activity
| Base Change (mRNA) (No._000479.3) | AA Change | SNP ID | Population-Based MAFa | Cases, n | Controls, n | % Decrease Activity | P Valueb |
|---|---|---|---|---|---|---|---|
| T254G | V12G | rs149082963 | 7.4E-04 | 6 | 0 | 46 | 3.0E-04 |
| G289A | A24T | rs775579158 | 6.6E-05 | 1 | 0 | 65 | 1.8E-05 |
| C355G | P46A | rs148294311 | 8.3E-05 | 1 | 0 | 43 | 5.7E-05 |
| G491A | R91H | rs534377664 | 1.7E-05 | 1 | 0 | 45 | 6.0E-04 |
| A514T | T99S | rs200226465 | 8.5E-05 | 2 | 0 | 56 | 1.5E-05 |
| C647T | T143I | rs139265145 | 4.1E-03 | 11 | 4 | 12 | 1.0E-01 |
| C670T | P151S | rs370532523 | 3.3E-05 | 1 | 0 | 85 | 5.7E-09 |
| G685A | A156T | rs374588581 | 2.3E-04 | 1 | 0 | 76 | 1.4E-07 |
| C772G | Q185E | rs200523942 | 7.9E-05 | 1 | 0 | 86 | 3.0E-08 |
| GT | splicing (ex2/3) | rs759859203 | 1.0E-05 | 1 | 0 | 83 | 1.8E-07 |
| G800A | R194H | rs376035065 | 2.1E-05 | 1 | 0 | 66 | 3.6E-06 |
| C1027T | P270S | rs757506343 | 1.4E-04 | 1 | 0 | 80 | 3.5E-06 |
| C1069T | P284S | rs769350289 | 9.0E-06 | 0 | 1 | −8 | 8.0E-01 |
| C1083G | D288E | rs199831511 | 1.7E-04 | 3 | 1 | 15 | 4.0E-01 |
| G1124A | R302Q | rs536688211 | 9.9E-04c | 1 | 0 | 46 | 2.4E-05 |
| A1193G | Q325R | rs140765565 | 4.4E-03 | 3 | 4 | 14 | 7.0E-02 |
| C1273T | P352S | rs764049634 | 1.1E-04 | 3 | 0 | 82 | 2.0E-06 |
| C1304T | P362S | rs765380360 | 6.5E-05 | 1 | 0 | 65 | 3.8E-06 |
| C1317T | P366L | chr19:2251370-2251370d | — | 1 | 0 | 69 | 4.6E-06 |
| C1334T | A372V | rs541377806 | 3.5E-04 | 1 | 0 | 1 | 8.0E-01 |
| C1373T | A385V | chr19:2251427-2251427d | — | 1 | 0 | 66 | 3.6E-06 |
| C1737G | H506Q | rs138571039 | 4.2E-05 | 1 | 0 | 68 | 2.1E-06 |
| C1775T | A519V | rs200031151 | 1.4E-03 | 0 | 1 | 12. | 1.0E-01 |
| G1876T | V553L | rs770189890 | 9.0E-05 | 1 | 1 | 8 | 5.0E-01 |
Abbreviations: AA, amino acid; mRNA, messenger RNA; SNP ID, single nucleotide polymorphism identification.
MAF based on ExAc Aggregated Populations (http://exac.broadinstitute.org).
Two-tailed t test.
MAF based on 1000Genomes European ancestry (http://www.1000genomes.org).
Build GRCh37/hg1.
Results
Study participant characteristics
Patients with PCOS were significantly younger and heavier than control subjects (Table 1). T, uT, DHEAS, LH, and AMH levels were significantly elevated in patients with PCOS. SHBG levels were significantly decreased in patients with PCOS compared with control subjects. These observations are consistent with the biochemical profile of PCOS (16). The results of these comparisons using other assays methodologies were similar to those reported in Table 1. There were no significant differences in the clinical or biochemical features of the patients with PCOS who underwent WGS compared with those who underwent targeted resequencing.
PCOS-associated AMH rare variants identified by next-generation sequencing
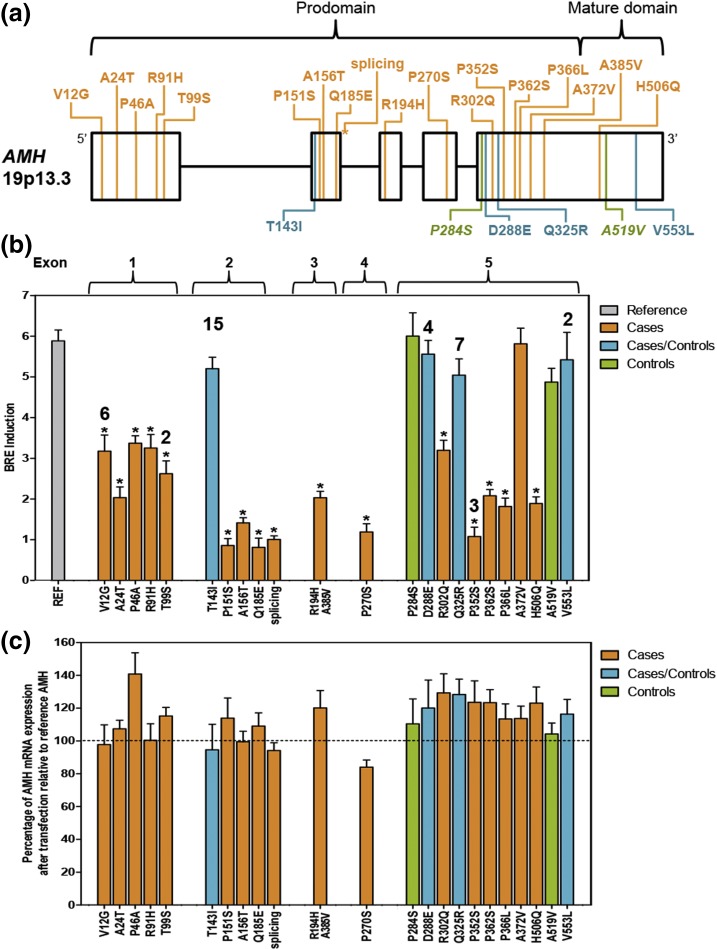
Analysis of WGS data in 80 patients with PCOS and 24 control subjects (Fig. 1) identified three rare, putative functional coding variants in AMH, the gene encoding AMH. These variants were found in five women with PCOS [T143I, n = 3; P270S, n = 1; exon2/3 splice site, n = 1; Fig. 2(a)] and were confirmed by Sanger sequencing. No AMH coding variants were found in control subjects. No other genes were identified in the WGS cohort with rare, likely-to-be-detrimental variants limited to patients with PCOS that were logical PCOS susceptibility loci; thus, AMH was carried forward for further analysis.
Figure 2.
Genetic variation within AMH and effects on signaling. (a) Rare (MAF < 0.01) variants identified in AMH. PCOS-specific variants are indicated in orange (n = 18). Non–PCOS-specific variants are shown in blue (n = 4), and variants present in control subjects only are shown in green (n = 2). (b) Average induction of BRE-firefly luciferase normalized to Renilla reporter and empty vector control in COS7 cells transfected with reference or variant AMH constructs. Results represent three independent experiments done in triplicate with standard error of the mean (SEM). Asterisks indicate significantly reduced BRE induction. The number of subjects (more than one) is indicated above the BRE induction bar. (c) Percentage of AMH mRNA expression for each variant after transfection relative to reference AMH. Results represent three independent experiments with qRT-PCR run in duplicate with SEM. mRNA, messenger RNA.
We used a targeted resequencing approach to further evaluate genetic variation in AMH in a larger PCOS cohort (Fig. 1). Resequencing patients with PCOS (n = 643) and control subjects (n = 153), including 22 women with PCOS and 12 control subjects from the WGS cohort, identified 21 additional rare (MAF < 0.01) coding variants (Table 2). All subjects were heterozygous for the variants and, where parental DNA was available for sequencing, variants were inherited rather than generated de novo. The variants were dispersed across all five exons of AMH, including one splice-site variant following the second exon [Fig. 2(a)]. Twenty-one variants were in the prodomain region and three variants were in the mature domain of the AMH protein. Eighteen of the variants were found only in patients with PCOS (PCOS-specific variants). Fifteen of the 18 variants were singletons (found only in one subject), and three variants occurred in multiple patients with PCOS (V12G occurred in six patients; T99S in two; P352S in three). One patient with PCOS had two missense variants, R194H and A385V, mapping to the same copy of the gene [Supplemental Fig. 1(A) (96.9KB, docx) ]. Four variants were found in patients with PCOS and in control subjects; two variants were found only in control subjects. One woman in the control group was compound heterozygous for variants D288E and V553L [Supplemental Fig. 1(B) (96.9KB, docx) ]. Five AMH variants [i.e., V12G, P151S, splicing (ex2/3), R302Q, and H506Q] found in 10 patients with PCOS have also been identified in persistent Müllerian duct syndrome (PMDS) (21). None of the PMDS variants were found in control subjects.
Variant deleteriousness was evaluated using combined annotation-dependent depletion (22). The combined annotation-dependent depletion scores were broadly distributed and did not correspond well with known loss of function in PMDS variants. Analogous analyses with the programs Polymorphism Phenotyping (Polyphen; http://genetics.bwh.harvard.edu/pph2/) (23), Sorting Intolerant From Tolerant (SIFT; http://blocks.fhcrc.org/sift/SIFT.html) (24), and Genomic Evolutionary Rate Profiling (GERP; http://genome.ucsc.edu) (25) were also inconsistent.
PCOS-specific AMH variants significantly reduce signaling activity
Dual luciferase assays demonstrated a significant reduction of AMH-mediated BRE induction in 17 of the 18 PCOS-specific variants [Fig. 2(b); Table 2] in COS7 cells. In contrast, none of the variants that were found in both patients with PCOS and in control subjects (n = 4) or in control subjects only (n = 2) showed a significant reduction in signaling compared with reference AMH [Fig. 2(b); Table 2]. Quantitative real-time reverse transcription polymerase chain reaction of RNA isolated from COS7 cells transfected with AMH variants found in PCOS patients and control subjects demonstrated that all variant AMH transcripts were similarly expressed to reference AMH transcripts 48 hours after transfection [Fig. 2(c)]; thus, the signaling impairment observed in the functional variants was not simply due to a defect in variant expression but is due to a reduction in signaling capacity.
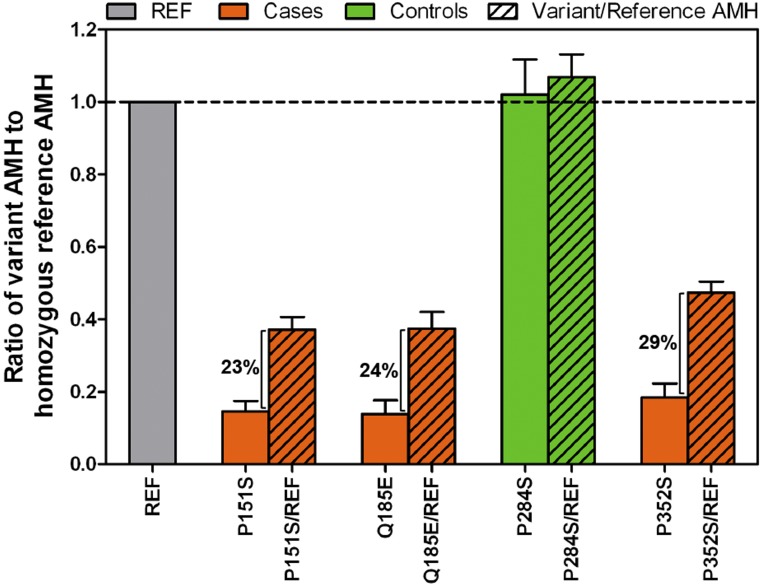
PCOS-specific AMH variants show dominant-negative effect
Dual luciferase assays of COS7 cells cotransfected with equal amounts of PCOS-specific variant and reference constructs (i.e., heterozygous state) displayed 25.05% (±3.45% standard deviation) greater signaling capacity than PCOS-specific variant constructs alone (i.e., homozygous variant state), averaged across the three PCOS-specific variants tested (Fig. 3), rather than the expected 50% to75% signaling increase if no impairment of activity was due to a dominant-negative interaction between wild-type and variant peptides. Variant P284S, which was identified only in control subjects, consistently showed wild-type signaling in homozygous and heterozygous states (Fig. 3).
Figure 3.
Dominant-negative effect of PCOS-specific AMH variants on signaling. Average BRE-firefly luciferase induction normalized to Renilla reporter and empty vector control in COS7 cells transfected with REF and/or variant AMH constructs. PCOS-specific AMH variants are shown in orange, and AMH variant present only in control subjects is shown in green. Solid colors indicate homozygous state, and the diagonal line pattern indicates the heterozygous state. Results represent three independent experiments done in triplicate with standard error of the mean. REF, reference.
Significant association of functional AMH variants with PCOS
We tested for association between functional AMH variants and PCOS using two independent control groups: the 165 reproductively normal women whose genomes were sequenced in this study and population-based control subjects (men and women) from the ExAc aggregated populations cohort (n > 100,000; http://exac.broadinstitute.org) or 1000Genomes European ancestry (n = 1006; http://www.1000genomes.org) cohort when no data were available in ExAc aggregated populations. The functional AMH variants were significantly associated with PCOS in our cohort of 700 women with PCOS and 165 control subjects (χ2 = 10.55; P = 0.0012; Table 2). Furthermore, evidence for association between functional AMH variants and PCOS was highly significant with the population-based control cohort (χ2 = 154; P < 10−8; Table 2). Age, body mass index, T, uT, DHEAS, SHBG, FSH, and LH levels did not differ significantly between patients with PCOS with functional AMH variants compared with patients with PCOS without AMH coding variants (Supplemental Table 2 (96.9KB, docx) ).
Discussion
PCOS is a highly heritable, complex genetic disease (2). Several common genetic PCOS susceptibility loci have been reproducibly mapped using family-based association tests (3, 26) or GWAS (3, 27). Taken together, these loci account for only a fraction of the heritability of PCOS, analogous to findings in other complex traits or diseases (6). One hypothesis for the observed deficit in heritability is that uncommon or rare genetic variants with greater phenotypic effects contribute to disease pathogenesis (6). We tested this hypothesis using an unbiased WGS approach followed by targeted resequencing of AMH in a replication cohort and validation of variants in a functional assay.
We identified 24 rare coding and splice-site variants in AMH. Eighteen of these variants were PCOS-specific. The remaining variants were found in patients with PCOS and control subjects or solely in control subjects. Seventeen of the 18 PCOS-specific variants decreased AMH-mediated signaling in COS7 cells. The one PCOS-specific variant with wild-type signaling activity encoded an alanine to valine substitution (A372V), a relatively conservative amino acid change (28). In contrast to the PCOS-specific variants, none of the six variants present in control subjects (i.e., T143I, D288E, Q325R, V553L, P284S, and A519V) reduced AMH signaling in COS7 cells. The functional AMH variants were significantly associated with PCOS relative to reproductively normal women (i.e., our phenotyped control subjects; n = 165) and highly significant relative to a much larger population-based control cohort (in ExAc Aggregated Populations; n ∼ 120,000).
Sixteen of the 17 functional PCOS-specific variants were in the prodomain region of AMH. Upon proteolytic cleavage, the prodomain remains noncovalently associated with the mature domain and has been shown to influence mature domain activity (29); thus, variants in the prodomain are likely to have an effect on mature protein processing and/or bioactivity. All the subjects were heterozygous for the variants. Given that AMH functions as a homodimer (29), AMH variants in the heterozygous state were hypothesized to have a phenotypic impact via a dominant-negative interaction between a wild-type and variant AMH peptide (29). Specifically, wild-type AMH dimers would only account for a quarter of total AMH in individuals heterozygous for AMH variants. In support of a dominant-negative interaction model, COS7 cells expressing AMH variants in the heterozygous state generated approximately a 25% greater signaling capacity than AMH variants in the homozygous variant state, exactly as predicted under a completely dominant-negative model. These results suggest that only 25% of wild-type AMH dimers were able to achieve signaling capability in vitro.
Approximately 3% (24 of 700) of our PCOS cohort of European ancestry had functional AMH variants. The women with PCOS were younger and heavier than the control subjects, a common finding in studies of PCOS (4). Accordingly, the analyses were adjusted for age and body mass index. There were no significant phenotypic differences between patients with PCOS with functional AMH variants and those with wild-type AMH genotypes. The AMH levels in patients with mutations were within the range of AMH levels observed in PCOS (1) and significantly higher than those observed in control subjects (Table 1). There were no significant differences in clinical and biochemical features between patients with PCOS with AMH functional variants and those with PCOS without coding variants.
In men, homozygous or compound heterozygous mutations in AMH and its receptor, AMHR2, are the most common causes of PMDS (21), a rare, autosomal recessive intersex disorder characterized by the presence of Müllerian duct structures in genotypic males (21). Five of the AMH variants identified in our cohort of patients with PCOS [i.e., V12G, P151S, splicing (ex2/3), R302Q, and H506Q] have also been identified in PMDS males (21). In the women harboring PCOS-associated AMH variants that reduced signaling, 42% (10 of 24) are PMDS-associated AMH variants with documented loss of AMH activity in PMDS (21). The phenotypic features of females in PMDS families with AMH variants have not been reported but, based on our findings, one would predict that female carriers of AMH variants have PCOS. Males with PMDS and AMH variants have circulating AMH levels ranging from undetectable to normal (21). These findings are consistent with our observation that circulating AMH levels are not correlated with AMH signaling capacity and suggest that AMH levels, per se, are not an accurate surrogate of AMH signaling activity in PCOS.
AMH has been proposed as a plausible PCOS candidate gene, given its role in folliculogenesis. Polycystic ovarian morphology (PCOM) is a key reproductive feature of PCOS (12, 30). PCOM is characterized by a distinctive twofold to fourfold increase in small preantral follicles, suggesting an alteration in gonadotropin-independent folliculogenesis (30). AMH is secreted by the granulosa cells of these early developing follicles (12, 30). Serum AMH levels correlate with antral follicle counts in both reproductively normal women as well as in those with PCOS (12). Accordingly, elevated AMH levels in women with PCOS have been considered a consequence of the increased number of these early-stage follicles (12).
There have been conflicting reports on the role of AMH in primordial follicular recruitment (10, 31, 32). In female mice, targeted disruption of AMH inhibited primordial follicle recruitment and FSH-induced follicular growth resulted in premature ovarian failure (33), the opposite phenotype of PCOM (12). Furthermore, the loss of one AMH allele in mice caused a significant enhancement of follicle recruitment followed by premature ovarian failure compared with control littermates, suggesting a gene-dosage effect for AMH in the rodent ovary (34). However, in contrast to findings in mice, knockdown of AMH bioactivity by active immunization with keyhole limpet hemocyanin-conjugated AMH peptides in female sheep did not affect the rate of primordial follicle recruitment (32). These findings suggest that there are species differences in the ovarian actions of AMH.
In women, AMH variation has been implicated in the pathology of premature ovarian insufficiency (POI). Mercadal et al. (35) found three heterozygous AMH variants with reduced AMH-mediated BMP Responsive Element induction (G264R, D288E, and R444H) in women with POI (35). Two variants (D288E and Q325R) were also found in our patients with PCOS and in our reproductively normal women in the control group. However, both D288E and Q325R demonstrated wild-type AMH bioactivity [Fig. 2(b)] in our assay. In addition, none of the 24 patients with PCOS with AMH variants that reduced AMH-mediated BRE induction had evidence for POI as defined by FSH levels (i.e., FSH level >40 µIU/mL) (36). Differences in the experimental approach to evaluate signaling potential of the AMH variants may explain the differences in signaling potential observed between our study and that of Mercadal et al. (35). Mercadal et al. (35) first purified and quantitated variant AMH before the luciferase assay, whereas in our assay, AMH bioactivity was measured directly in the cells expressing AMH variants. Given that we have evaluated a broad spectrum of AMH variants in women with PCOS and control subjects, and these women have no clinical evidence of POI, we conclude that AMH functional variation is generally not associated with POI.
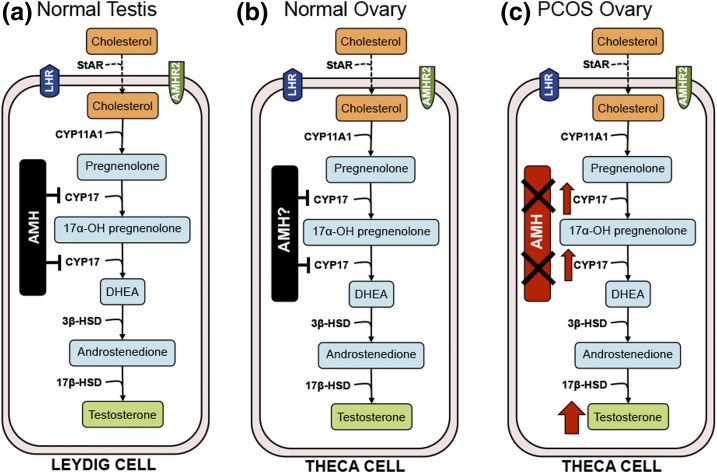
A plausible mechanism by which reduced AMH signaling could contribute to the pathogenesis of PCOS would be by decreased inhibition of ovarian testosterone production. In boys, AMH levels decrease just before puberty and are inversely correlated with testosterone postnatally (37). In transgenic male mice overexpressing AMH (37), as well as in isolated mouse Leydig cell cultures (11, 37), AMH inhibits testosterone production by downregulating transcription of CYP17, reducing both its 17α-hydroxylase and 17,20-lyase activities, which are rate limiting for androgen biosynthesis. In female mice, intraperitoneal administration of recombinant AMH significantly lowers testosterone levels (38). Wild-type AMH is thus predicted to inhibit theca cell androgen production, analogous to its action in Leydig cells (11). Accordingly, AMH mutations with reduced bioactivity are predicted to increase theca cell androgen production due to loss of CYP17 inhibition by AMH (Fig. 4). In support of this hypothesis, Nelson et al. (39) observed an increase in CYP17 mRNA in theca interna cells of women with PCOS compared with that in reproductively normal women in the control group. Alternatively, AMH inhibits FSH-induced aromatase activity (40) and reduced aromatase activity results in increased testosterone levels due to reduced aromatization of androgens to estrogen. Interestingly, Kevenaar et al. (41) demonstrated that women with AMH and AMHR2 variants had higher serum estradiol levels. It remains to be determined which of these mechanisms contributes to hyperandrogenemia in the subset of patients with PCOS with AMH mutations.
Figure 4.
Model of AMH role in gonadal steroidogenesis. (a) AMH inhibition of T synthesis in normal male testis via downregulation of CYP17 transcription observed in rodent Leydig cells [adapted from Teixeira et al. (11)]. (b) Model of AMH inhibition of testosterone production in normal female ovarian theca cells via inhibition of CYP17 transcription. (c) Impact of AMH inactivating mutation on T production in PCOS theca cells.
As expected, women with PCOS with functional AMH variants had markedly elevated T levels compared with the control group, and whereas median T levels in women with AMH mutations are nominally higher than what is observed in women without AMH variants, this observation is not statistically significant. Given that PCOS is a complex, multifactorial trait, it is our hypothesis that the underlying cause of hyperandrogenemia in PCOS is due to multiple distinct mechanisms. In a subset of women with PCOS, hyperandrogenemia is due to mutations in AMH or other members of the AMH signaling pathway, whereas in other women with PCOS, as predicted by the GWAS findings, hyperandrogenemia is due to alternative pathways (3–5). Further evaluation of these pathways will elucidate the relative impact of a given PCOS subphenotype in individual patients with PCOS.
We identified rare AMH variants with decreased bioactivity in 3.4% of our patients with PCOS. Rare variants identified in the insulin receptor gene and in genes regulating adipogenesis, such as those encoding lamin A/C and peroxisome proliferating factor-γ, cause extreme phenotypes of PCOS: type A syndrome and familial partial lipodystrophies, respectively (42). However, to our knowledge, this is the first report of rare variants associated with the common, nonsyndromic form of PCOS (3, 26). For complex traits like PCOS, disease burden attributable to a single gene is expected to be modest. A large-scale analysis of rare PPARG variants with reduced function in an adipocyte differentiation assay that substantially increased T2D risk identified one such variant per 1000 individuals screened (0.1% carrier rate) (43). Our findings suggest that mutations in other members of the AMH signaling pathway could also contribute to PCOS. Genetic variation in AMH regulatory elements that decrease gene transcription or in AMHR2 and other members of the AMH signaling cascade are predicted to have a similar phenotypic effect as AMH coding variants that reduce AMH bioactivity. Although common variants in AMHR2 were not associated with PCOS per se in patients with PCOS who were of Dutch ancestry, they were associated with AMH levels in women with PCOS (44, 45). In a Greek cohort, the common AMHR2 variant rs2002555 was associated with PCOS directly (46). Although these studies were limited to common variation, they provide further evidence for a role of AMH signaling in PCOS. The current study was not designed to detect regulatory variants. Moreover, because our discovery WGS cohort was small, we cannot exclude mutations in other members of the AMH signaling cascade. Similarly, we cannot exclude rare genetic variants in other genes or pathways accounting for some cases of PCOS. In fact, given the complex, multifactorial nature of PCOS, we expect that other pathways to also contribute to its etiology.
Our study subjects were of European ancestry and fulfilled the NIH criteria for PCOS of hyperandrogenism and chronic anovulation; ovarian morphology was not assessed (4, 16). Further studies are needed to assess the contribution of AMH mutations to the other Rotterdam PCOS phenotypes, hyperandrogenism and PCOM without anovulation, and chronic anovulation and PCOM without hyperandrogenism (47), as well as to PCOS in other racial/ethnic groups. Elevated AMH levels have been found in the sons (48), brothers, and fathers of affected women. It will now be possible to assess the contribution of AMH mutations to the male PCOS phenotype.
In conclusion, we identified 17 variants in AMH with reduced signaling potential in 24 women with PCOS. No such variants were observed in unaffected women in the control group. Thus, rare genetic variants do contribute to the pathogenesis of PCOS and account for some of the heritability not explained by the common PCOS susceptibility variants identified in GWAS. Furthermore, no evidence for association with PCOS in the AMH genomic region was detected in GWAS studies of PCOS (3, 4), underscoring the need for rare variant screens in parallel with GWAS to detect the full spectrum of PCOS-associated genetic variation. It is our hypothesis that these AMH mutations lead to the PCOS phenotype by abrogating AMH’s transcriptional inhibition of CYP17 role in androgen biosynthesis, resulting in hyperandrogenemia. Our findings provide a mechanism for the characteristic increase in circulating AMH levels in PCOS—decreased bioactivity of the molecule. Furthermore, they implicate the AMH signaling pathway in the pathogenesis of PCOS.
Acknowledgments
Current Affiliation: Gulum Kosova’s current affiliation is Genetics and Pharmacogenomics, Merck & Co., Inc. West Point, PA 19486.
Acknowledgments
This study was supported by US National Institutes of Health (NIH) Grants R01 HD057450 (to M.U.), R01 HD057223 (to A.D.), P50 HD044405 (to A.D. and M.U), U54 HD34449 (to A.D.), R01 HD056510 (to R.S.L.), and R01 HD072489 (to J.M.T.). Partial funding for the clinical studies was provided by Grants UL1 TR000150, UL1 RR033184, UL1 TR000430, and UL1 RR025758 from the National Center for Advancing Translational Sciences. Some hormone assays were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by Grant U54 HD28934 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- anti-Müllerian hormone
- DHEAS
- dehydroepiandrosterone sulfate
- FSH
- follicle-stimulating hormone
- GWAS
- genome-wide association studies
- LH
- luteinizing hormone
- MAF
- minor allele frequency
- NIH
- National Institutes of Health
- PCOM
- polycystic ovarian morphology
- PCOS
- polycystic ovary syndrome
- PCR
- polymerase chain reaction
- PMDS
- persistent Müllerian duct syndrome
- POI
- premature ovarian insufficiency
- SHBG
- sex hormone–binding globulin
- T
- testosterone
- T2D
- type 2 diabetes
- uT
- non–sex hormone–binding globulin-bound testosterone
- WGS
- whole-genome sequencing.
References
- 1.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H, Lv Y, Li L, Chen ZJ. Genetic studies on polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:56–65. [DOI] [PubMed] [Google Scholar]
- 4.Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A; Reproductive Medicine Network . Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015;6:7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV, Lawlor DA, Laval G, Mathieson I, McCardle WL, Louwers Y, Meun C, Ring S, Scott RA, Sulem P, Uitterlinden AG, Wareham NJ, Thorsteinsdottir U, Welt C, Stefansson K, Laven JS, Ong KK, Perry JR. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florez JC, Sjögren M, Burtt N, Orho-Melander M, Schayer S, Sun M, Almgren P, Lindblad U, Tuomi T, Gaudet D, Hudson TJ, Daly MJ, Ardlie KG, Hirschhorn JN, Altshuler D, Groop L. Association testing in 9,000 people fails to confirm the association of the insulin receptor substrate-1 G972R polymorphism with type 2 diabetes. Diabetes. 2004;53(12):3313–3318. [DOI] [PubMed] [Google Scholar]
- 8.Bonnefond A, Clément N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, Dechaume A, Payne F, Roussel R, Czernichow S, Hercberg S, Hadjadj S, Balkau B, Marre M, Lantieri O, Langenberg C, Bouatia-Naji N, Charpentier G, Vaxillaire M, Rocheleau G, Wareham NJ, Sladek R, McCarthy MI, Dina C, Barroso I, Jockers R, Froguel P; Meta-Analysis of Glucose and Insulin-Related Traits Consortium (MAGIC) . Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44(3):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees MG, Raimondo A, Wang J, Ban MR, Davis MI, Barrett A, Ranft J, Jagdhuhn D, Waterstradt R, Baltrusch S, Simeonov A, Collins FS, Hegele RA, Gloyn AL. Inheritance of rare functional GCKR variants and their contribution to triglyceride levels in families. Hum Mol Genet. 2014;23(20):5570–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira J, Fynn-Thompson E, Payne AH, Donahoe PK. Müllerian-inhibiting substance regulates androgen synthesis at the transcriptional level. Endocrinology. 1999;140(10):4732–4738. [DOI] [PubMed] [Google Scholar]
- 12.Dumont A, Robin G, Catteau-Jonard S, Dewailly D. Role of anti-Müllerian hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol. 2015;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, Pigny P, Prescott M, Campbell R, Herbison AE, Prevot V, Giacobini P. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels TL, Berga SL. Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab. 1997;82(12):4179–4183. [DOI] [PubMed] [Google Scholar]
- 15.Mutharasan P, Galdones E, Peñalver Bernabé B, Garcia OA, Jafari N, Shea LD, Woodruff TK, Legro RS, Dunaif A, Urbanek M. Evidence for chromosome 2p16.3 polycystic ovary syndrome susceptibility locus in affected women of European ancestry. J Clin Endocrinol Metab. 2013;98(1):E185–E190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legro RS, Spielman R, Urbanek M, Driscoll D, Strauss JF III, Dunaif A. Phenotype and genotype in polycystic ovary syndrome. Recent Prog Horm Res. 1998;53:217–256. [PubMed] [Google Scholar]
- 17.Torchen LC, Kumar A, Kalra B, Savjani G, Sisk R, Legro RS, Dunaif A. Increased antimüllerian hormone levels and other reproductive endocrine changes in adult male relatives of women with polycystic ovary syndrome. Fertil Steril. 2016;106(1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zawadzki J, Dunaif A eds. Diagnostic criteria for polycystic ovary syndrome; towards a rational apporach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific; 1991.
- 19.Renlund N, Pieretti-Vanmarcke R, O’Neill FH, Zhang L, Donahoe PK, Teixeira J. c-Jun N-terminal kinase inhibitor II (SP600125) activates Mullerian inhibiting substance type II receptor-mediated signal transduction. Endocrinology. 2008;149(1):108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgenthaler S, Thilly WG. A strategy to discover genes that carry multi-allelic or mono-allelic risk for common diseases: a cohort allelic sums test (CAST). Mutat Res. 2007;615(1-2):28–56. [DOI] [PubMed] [Google Scholar]
- 21.Josso N, Rey RA, Picard JY. Anti-müllerian hormone: a valuable addition to the toolbox of the pediatric endocrinologist. Int J Endocrinol. 2013;2013:674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLOS Comput Biol. 2010;6(12):e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1-2):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes MG, Urbanek M, Hivert MF, Armstrong LL, Morrison J, Guo C, Lowe LP, Scheftner DA, Pluzhnikov A, Levine DM, McHugh CP, Ackerman CM, Bouchard L, Brisson D, Layden BT, Mirel D, Doheny KF, Leya MV, Lown-Hecht RN, Dyer AR, Metzger BE, Reddy TE, Cox NJ, Lowe WL Jr; HAPO Study Cooperative Research Group . Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes. 2013;62(9):3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawn JD. Biochemistry. In: Daisy LP, Hodgin KC, O’Quin TL, Olsen SF, and Swan JA, eds. Biochemistry. Burlington, NC: Neil Patterson Publisher; 1989:52–58.
- 29.di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, Picard JY, Whitty A, Josso N, Pepinsky RB, Cate RL. Processing of anti-mullerian hormone regulates receptor activation by a mechanism distinct from TGF-beta. Mol Endocrinol. 2010;24(11):2193–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240–245. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt KL, Kryger-Baggesen N, Byskov AG, Andersen CY. Anti-Müllerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol. 2005;234(1-2):87–93. [DOI] [PubMed] [Google Scholar]
- 32.Campbell BK, Clinton M, Webb R. The role of anti-Müllerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology. 2012;153(9):4533–4543. [DOI] [PubMed] [Google Scholar]
- 33.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124(5):601–609. [DOI] [PubMed] [Google Scholar]
- 34.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–5796. [DOI] [PubMed] [Google Scholar]
- 35.Alvaro Mercadal B, Imbert R, Demeestere I, Gervy C, De Leener A, Englert Y, Costagliola S, Delbaere A. AMH mutations with reduced in vitro bioactivity are related to premature ovarian insufficiency. Hum Reprod. 2015;30(5):1196–1202. [DOI] [PubMed] [Google Scholar]
- 36.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22(5):657–674. [DOI] [PubMed] [Google Scholar]
- 38.Kano M, Sosulski AE, Zhang L, Saatcioglu HD, Wang D, Nagykery N, Sabatini ME, Gao G, Donahoe PK, Pépin D. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci USA. 2017;114(9):E1688–E1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson VL, Legro RS, Strauss JF III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–957. [DOI] [PubMed] [Google Scholar]
- 40.Grossman MP, Nakajima ST, Fallat ME, Siow Y. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008; 89(5, Suppl)1364–1370. [DOI] [PubMed] [Google Scholar]
- 41.Kevenaar ME, Themmen AP, Laven JS, Sonntag B, Fong SL, Uitterlinden AG, de Jong FH, Pols HA, Simoni M, Visser JA. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo-ovulatory women. Hum Reprod. 2007;22(6):1547–1554. [DOI] [PubMed] [Google Scholar]
- 42.Semple RK, Savage DB, Cochran EK, Gorden P, O’Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. 2011;32(4):498–514. [DOI] [PubMed] [Google Scholar]
- 43.Majithia AR, Flannick J, Shahinian P, Guo M, Bray MA, Fontanillas P, Gabriel SB, Rosen ED, Altshuler D; GoT2D Consortium; NHGRI JHS/FHS Allelic Spectrum Project; SIGMA T2D Consortium; T2D-GENES Consortium . Rare variants in PPARG with decreased activity in adipocyte differentiation are associated with increased risk of type 2 diabetes [published correction appears in Proc Natl Acad Sci U S A. 2014;11;111(45):16225] Proc Natl Acad Sci USA. 2014;111(36):13127–13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kevenaar ME, Laven JS, Fong SL, Uitterlinden AG, de Jong FH, Themmen AP, Visser JA. A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2008;93(4):1310–1316. [DOI] [PubMed] [Google Scholar]
- 45.Kevenaar ME, Themmen AP, van Kerkwijk AJ, Valkenburg O, Uitterlinden AG, de Jong FH, Laven JS, Visser JA. Variants in the ACVR1 gene are associated with AMH levels in women with polycystic ovary syndrome. Hum Reprod. 2009;24(1):241–249. [DOI] [PubMed] [Google Scholar]
- 46.Georgopoulos NA, Karagiannidou E, Koika V, Roupas ND, Armeni A, Marioli D, Papadakis E, Welt CK, Panidis D. Increased frequency of the anti-mullerian-inhibiting hormone receptor 2 (AMHR2) 482 A>G polymorphism in women with polycystic ovary syndrome: relationship to luteinizing hormone levels. J Clin Endocrinol Metab. 2013;98(11):E1866–E1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 48.Recabarren SE, Sir-Petermann T, Rios R, Maliqueo M, Echiburú B, Smith R, Rojas-García P, Recabarren M, Rey RA. Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J Clin Endocrinol Metab. 2008;93(9):3318–3324. [DOI] [PubMed] [Google Scholar]