Abstract
Background
Hyperkalemia is observed in chronic kidney disease patients and may be a risk factor for life-threatening arrhythmias and death. Race/ethnicity may be important modifiers of the potassium-mortality relationship in maintenance hemodialysis (MHD) patients given that potassium intake and excretion vary among minorities.
Methods
We examined racial/ethnic differences in baseline serum potassium levels and allcause and cardiovascular mortality using Cox proportional hazard models and restricted cubic splines in a cohort of 102,241 incident MHD patients. Serum potassium was categorized into 6 groups: ≤3.6, >3.6 to ≤4.0, >4.0 to ≤4.5 (reference), >4.5 to ≤5.0, >5.0 to ≤5.5, and >5.5 mEq/L. Models were adjusted for case-mix and malnutrition-inflammation cachexia syndrome (MICS) covariates.
Results
The cohort was composed of 50% whites, 34% African-Americans, and 16% Hispanics. Hispanics tended to have the highest baseline serum potassium levels (mean±SD: 4.58±0.55 mEq/L). Patients in our cohort were followed for a median of 1.3 (IQR: 0.6, 2.5) years In our cohort, associations between higher potassium (>5.5 mEq/L) and higher mortality risk were observed in African-American and white, but not Hispanic patients in models adjusted for case-mix and MICS covariates. While in Hispanics only, lower serum potassium (<3.6 mEq/L) levels were associated with higher mortality risk. Similar trends were for cardiovascular mortality.
Conclusions
Higher potassium levels were associated with higher mortality risk in white and African-American MHD patients, whereas lower potassium levels were linked with higher death risk in Hispanics. Further studies are needed to determine the underlying mechanisms for the differential association between potassium and mortality across race/ethnicity.
Keywords: Potassium, Hemodialysis, CKD, Mortality
Introduction
The kidneys play a primary role in the regulation of potassium homeostasis by balancing potassium intake with excretion.1 When kidney function declines, disruption in homeostasis and subsequent hyperkalemia are commonly observed, and the latter may be ameliorated by reduction of potassium intake and utilization of potassium-lowering medications that augment potassium excretion via the gastrointestinal tract.2 Despite these interventions, hyperkalemia is more frequently observed in chronic kidney disease (CKD) patients versus the non-CKD population.3 Dissecting the potassium-mortality relationship is of particular importance, given that maintenance hemodialysis (MHD) patients have an exceedingly high cardiovascular mortality (40% of all deaths) largely due to sudden cardiac arrest, and hyperkalemia may lead to life-threatening cardiac arrhythmias and higher death risk in this population.4–6
Several studies suggest that there may be differences in potassium homeostasis across racial/ethnic groups. For example, prior data has shown that dietary potassium intake and/or urinary potassium excretion used to determine serum potassium levels differs in whites and African-Americans.7,8 It is also well-established that there are racial/ethnic differences in the mortality of maintenance dialysis patients, such that African-Americans have a survival advantage compared to white patients, particularly in those of middle and older age groups.9–11 While there have been fewer studies in Hispanic MHD patients, emerging data suggests that Hispanics also have a survival advantage compared to non-Hispanic whites and African- Americans11–13, even after accounting for differences in socioeconomic factors and comorbid conditions.14 Whereas epidemiologic data show that serum potassium levels differ across race/ethnicity in the general population, there has not been prior study of the distribution of serum potassium levels and their associations with mortality across race/ethnicity in MHD patients.8 Therefore, to better inform the field, we examined racial/ethnic differences in serum potassium levels across whites, African-Americans, and Hispanics in a contemporary cohort of incident MHD patients who were followed up to 5 years. Moreover, we examined whether there is a differential association between serum potassium level and mortality risk across these racial/ethnic groups.
Materials and Methods
Source Population
We conducted analyses using administrative data from all incident hemodialysis (HD) patients who initiated dialysis over the period of January 1, 2007 to December 31, 2011 in the outpatient facilities of the large dialysis organization. The construction of the cohort has previously been described. From January 2007 to December 2011, there were 208,820 patients who initiated dialysis treatment. After excluding patients less than 18 years of age, patients who received less than 60 days total of dialysis treatment, or were ever treated with any other dialysis modality, 133,156 incident HD patients remained.
After excluding patients who were not of white, African-American, and Hispanic race/ethnicity and those with missing baseline serum potassium information, the final study population consisted of 102,241 incident HD patients (Appendix-Figure S1). The study was approved by the International Review Committees of the University of California Irvine, Los Angeles Biochemical Research Institute at Harbor-UCLA, and the University of Washington. Given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research, the requirement for written consent was exempted.
Socio-demographic and Clinical Measures
Over 99% of the cohort had information on race/ethnicity which was based on “self- identified” data based on definitions of the US Census Bureau and guidance of US Office of Management and Budget. Information on primary insurance, initial vascular access type and the presence of comorbidities at baseline were obtained from the large dialysis organization database. The following coexisting comorbidities were also considered: diabetes mellitus, hypertension, congestive heart disease (CHF), atherosclerotic heart disease, other cardiac disease (pericarditis and cardiac arrhythmia), cerebrovascular disease, chronic obstructive pulmonary disease, liver disease, dyslipidemia, malignancy, human immunodeficiency virus antibody positive status, substance abuse, and alcohol abuse.
Laboratory and Clinical Measures
Blood samples were drawn using standardized techniques in all dialysis clinics and were transported to a central laboratory in Deland, Florida, typically within 24 hours of collection. Most laboratory values were measured monthly, including serum potassium, creatinine, albumin, hemoglobin, platelet count, peripheral white blood cell count (WBC), lymphocyte percentage, total iron binding capacity (TIBC), calcium, phosphorus, bicarbonate, blood urea nitrogen (BUN) and alkaline phosphatase. Serum intact parathyroid hormone (iPTH) and ferritin levels were usually measured at least once during each calendar quarter. Most blood samples were collected before HD, except for post-dialysis BUN to calculate urea kinetics. The normalized protein catabolic rate (nPCR) was measured monthly as an indicator of daily protein intake. Dialysis dose was estimated by single pool Kt/V (spKt/V) using the urea kinetic model. The average serum urea concentration during the collection were assumed to be 90% of the pre-dialysis concentration according to the Daugirdas approach and thus residual renal function was calculated as follows.15
Residual renal function was adjusted for body surface area and expressed as mL/min/1.73m2.16,17 Ultrafiltration (UF) was calculated as the difference between pre-HD weights and post-HD weights that were measured every HD session. Seated pre-HD blood pressure (BP) values were measured every HD session. The median weekly erythropoiesis stimulating agent (ESA) dose was calculated by dividing the cumulative quarterly ESA dose by the total number of HD treatments over a 91-day period, and then multiplying the per-treatment dose by the number of median treatments per week. To minimize measurement variability, all repeated measures for each patient during any given patient quarter (i.e., 91-day intervals) were averaged, and summary estimates were used in all analyses. The 91-day averaged values during the first patient quarter of dialysis treatment were used as baseline values.
Outcome Measures
The recorded causes of death were obtained from the large dialysis organization data records, and cardiovascular death was defined as death due to cardiovascular reasons, including acute myocardial infarction, cardiac arrest, heart failure, cerebrovascular accident, and other cardiac disease. Patients were followed 91 days from the start of dialysis to death, kidney transplantation, transfer to a non-affiliated dialysis unit, recovery of renal function, dialysis discontinuation or end of the study period (December 31, 2011).
Statistical Methods
Baseline characteristics across race/ethnicity groups were summarized as proportions, means ± SD, or medians (interquartile ranges) depending on data type. We used logistic regression to estimate the association between various clinical characteristics with likelihood of hyperkalemia (serum potassium >5.0 vs >3.6 to ≤5.0 mEq/L) or hypokalemia (≤3.6 vs >3.6 to ≤5.0 mEq/L) across various racial/ethnic groups.
We examined the association between serum potassium as a continuous variable using restricted cubic spline analyses (with knots at 33rd and 66th percentile for each group’s potassium distribution) with all-cause mortality across racial/ethnic groups using Cox proportional hazards models. We then stratified patients according to the six groups of baseline serum potassium representing a combination of clinical reference ranges and the distribution of our cohort (≤3.6, >3.6 to ≤4.0, >4.0 to ≤4.5, >4.5 to ≤5.0, >5.0 to ≤5.5, and >5.5 mEq/L) and the three racial/ethnic groups (i.e., total of 18 categories) and examined their associations with mortality. We also separately examined associations of hyperkalemia and hypokalemia (reference: serum potassium >3.6 to ≤5.0 mEq/L) with all-cause and cardiovascular mortality in the overall cohort and across clinically relevant subgroups stratified by race/ethnicity, and p-values for interactions between serum potassium and subgroup covariates were assessed. Proportional hazards assumptions were assessed by graphical methods.
For each analysis, three levels of multivariable adjustment were used: (1) unadjusted models that included the main predictor, serum potassium; (2) case-mix adjusted models that included covariates in the unadjusted model as well as age, sex, diabetes mellitus, primary insurance, initial vascular access type, spKt/V, HD treatment time, UF, and cardiovascular risk factors including pre-HD systolic BP, pre-HD diastolic BP, body mass index (BMI), and previously mentioned comorbidities; (3) case-mix + malnutrition-inflammation cachexia syndrome (MICS) adjusted models that included covariates in the case-mix model as well as 12 surrogates of nutritional and/or inflammatory status: albumin, hemoglobin, peripheral WBC, lymphocyte percentage, ferritin TIBC, calcium, phosphorus, bicarbonate, BUN, iPTH, nPCR, and ESA dose.
To account for the competing risk of transplantation across race/ethnicity, in sensitivity analyses we conducted Fine and Gray competing risk regression to estimate sub-hazard ratios (SHRs) of death risk.18 To address potential residual confounding on the basis of residual renal function, sensitivity analyses that additionally adjusted for residual urine volume were conducted. While there were no missing data for age, data for sex, race/ethnicity and comorbidities, missing covariates <5% at study entry were imputed by mean or median values as appropriate. All analyses were conducted with STATA MP version 13.1 (StataCorp, College Station, TX).
Results
Study Population Description
The mean±SD age of the overall cohort was 63±15 years and mean serum potassium level was 4.4±0.5 mEq/L. Median duration of follow-up was 1.3 years (25th to 75th percentile, 0.6 to 2.5 years). The crude all-cause mortality rate of the overall cohort was 139 death events per 1000 patient-years of follow-up [95% CI: 138, 141 death events per 1000 patient-years]. Hispanics tended to have the highest baseline serum potassium levels (mean±SD: 4.58±0.55 mEq/L), whereas African-Americans had the lowest levels (mean±SD: 4.31±0.50 mEq/L) (p<0.001) (Figure 1). Baseline characteristics of the cohort stratified by race/ethnicity and baseline serum potassium level are presented in Table 1. Across all racial/ethnic groups, patients in the highest serum potassium category were more likely to be younger and to use Medicaid as their primary insurance. They were also more likely to have CHF and higher nPCR, albumin, creatinine, platelet, iPTH, phosphorus, BUN, pre-HD SBP and DBP, and UF levels; they had lower spKt/V and bicarbonate levels compared to those in the lowest potassium category.
Figure 1.
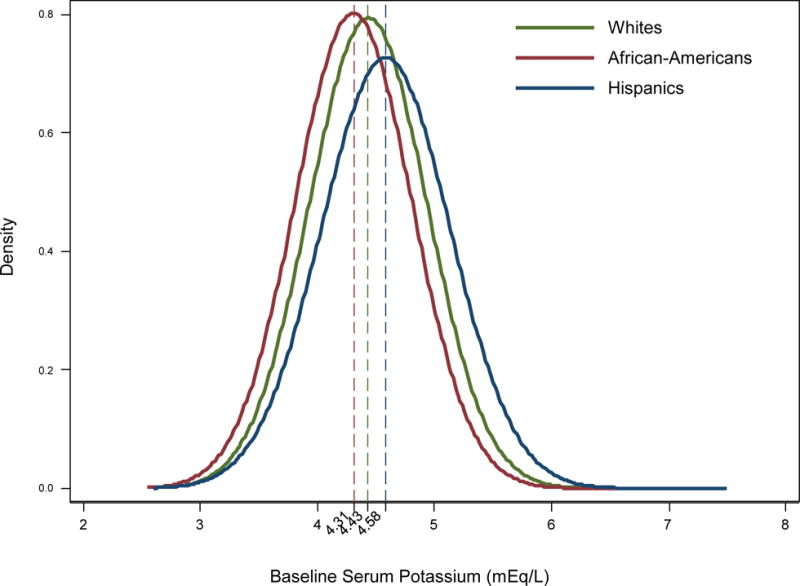
Distribution of baseline serum potassium levels across whites, African-Americans, and Hispanics in 102,241 patients.
Footnote: Dashed lines show the mean potassium level of each race
Table 1.
Baseline characteristics stratified by race/ethnicity and serum potassium in 102,241 incident hemodialysis patients
| Characteristics | Overall | Race |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White |
African-American |
Hispanic |
|||||||||||
| overall | Serum potassium (m Eq/L) | overall | Serum potassium (m Eq/L) | overall | Serum potassium (mEq/L) | ||||||||
|
|
|
|
|
||||||||||
| <3.6 | >3.6 to <5.0 |
>5.0 | <3.6 | >3.6 to <5.0 |
>5.0 | <3.6 | >3.6 to <5.0 |
>5.0 | |||||
|
|
|
|
|
||||||||||
| Patient number | 102,241 | 51297 | 1957 | 42908 | 6432 | 34574 | 2323 | 29358 | 2893 | 16370 | 431 | 12465 | 3474 |
| Age (yr) | 62.8±15.1 | 66.7±14.2 | 67.4±13.5 | 67.3±13.9 | 62.8±15.3 | 58.7±14.8 | 61.4±14.9 | 58.8±14.7 | 55.2±14.8 | 58.9±15.1 | 62.8±14.1 | 60.0±14.9 | 54.7±15.1 |
| Female (%) | 44 | 41 | 51 | 41 | 37 | 48 | 60 | 47 | 39 | 42 | 52 | 44 | 33 |
| DM (%) | 58 | 55 | 50 | 55 | 57 | 58 | 58 | 58 | 56 | 68 | 67 | 68 | 68 |
|
|
|
|
|
||||||||||
| Primary insurance (%) | |||||||||||||
| Medicare | 54 | 59 | 62 | 59 | 57 | 51 | 54 | 51 | 46 | 46 | 53 | 46 | 43 |
| Medicaid | 7 | 4 | 4 | 4 | 6 | 8 | 7 | 7 | 11 | 14 | 10 | 13 | 18 |
| Others | 39 | 37 | 35 | 37 | 37 | 42 | 38 | 42 | 43 | 40 | 37 | 41 | 39 |
|
|
|
|
|
||||||||||
| Access type (%) | |||||||||||||
| CVC | 75 | 74 | 75 | 74 | 76 | 75 | 77 | 74 | 76 | 79 | 83 | 78 | 81 |
| AVF | 15 | 17 | 13 | 18 | 14 | 13 | 10 | 13 | 11 | 13 | 8 | 14 | 11 |
| AVG | 4 | 3 | 4 | 3 | 2 | 6 | 6 | 7 | 4 | 3 | 2 | 3 | 2 |
| Others | <1 | <1 | <1 | <1 | 0 | <1 | <1 | <1 | <1 | <1 | 0 | <1 | <1 |
| Unknown | 6 | 6 | 8 | 6 | 8 | 6 | 7 | 6 | 9 | 5 | 7 | 5 | 6 |
|
|
|
|
|
||||||||||
| nPCR (g/kg/day) | 0.79±0.21 | 0.79±0.22 | 0.66±0.20 | 0.79±0.21 | 0.88±0.23 | 0.75±0.20 | 0.64±0.18 | 0.75±0.19 | 0.85±0.22 | 0.84±0.23 | 0.68±0.21 | 0.81±0.21 | 0.94±0.23 |
| spKt/V | 1.46±0.32 | 1.49±0.33 | 1.53±0.39 | 1.49±0.33 | 1.44±0.32 | 1.42±0.31 | 1.47±0.34 | 1.42±0.31 | 1.36±0.30 | 1.48±0.31 | 1.50±0.35 | 1.48±0.31 | 1.46±0.30 |
|
|
|
|
|
||||||||||
| Comorbidities (%) | |||||||||||||
| Hypertension | 52 | 48 | 51 | 48 | 46 | 61 | 61 | 60 | 62 | 45 | 46 | 44 | 46 |
| Cystic kidney disease | 2 | 3 | 2 | 3 | 3 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 |
| CHF | 37 | 37 | 33 | 37 | 40 | 36 | 35 | 36 | 40 | 38 | 37 | 37 | 39 |
| ASHD | 15 | 17 | 17 | 17 | 15 | 13 | 13 | 13 | 13 | 12 | 14 | 13 | 12 |
| Other cardiac disease | 15 | 18 | 18 | 19 | 17 | 13 | 13 | 13 | 12 | 12 | 13 | 12 | 10 |
| CVD | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| COPD | 5 | 7 | 7 | 7 | 7 | 4 | 4 | 5 | 4 | 3 | 5 | 3 | 2 |
| Liver disease | 2 | 2 | 3 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 4 | 1 | 1 |
| Dyslipidemia | 25 | 27 | 26 | 27 | 26 | 24 | 21 | 24 | 24 | 23 | 24 | 23 | 22 |
| Autoimmune disease | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 2 | 1 |
| Malignancy | 2 | 3 | 4 | 3 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| HIV Ab (+) status | 1 | <1 | <1 | <1 | <1 | 1 | 2 | 1 | 1 | <1 | 1 | <1 | <1 |
| Substance abuse | <1 | <1 | <1 | <1 | <1 | 1 | <1 | 1 | 1 | <1 | <1 | <1 | <1 |
| Alcohol abuse | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | 0 | <1 | <1 |
|
|
|
|
|
||||||||||
| BMI (kg/m2) | 28.3±7.4 | 28.3±7.4 | 28.1±7.6 | 28.4±7.3 | 27.7±7.4 | 28.6±7.8 | 27.5±7.4 | 28.8±7.8 | 27.7±7.8 | 27.4±6.4 | 27.5±6.2 | 27.8±6.6 | 26.2±5.5 |
| Potassium (mEq/L) | 4.41±0.52 | 4.43±0.50 | 3.44±0.15 | 4.35±0.35 | 5.31±0.28 | 4.31±0.50 | 3.43±0.16 | 4.28±0.35 | 5.31±0.28 | 4.58±0.55 | 3.46±0.15 | 4.40±0.35 | 5.38±0.30 |
| Albumin (g/dL) | 3.5±0.5 | 3.5±0.5 | 3.3±0.5 | 3.5±0.5 | 3.6±0.5 | 3.5±0.5 | 3.2±0.6 | 3.5±0.5 | 3.6±0.5 | 3.5±0.5 | 3.2±0.6 | 3.5±0.5 | 3.6±0.5 |
| Creatinine (mg/dL) | 5.9±2.4 | 5.2±2.0 | 4.3±1.5 | 5.1±1.8 | 6.4±2.3 | 6.7±2.6 | 5.3±2.0 | 6.6±2.5 | 8.3±3.1 | 6.1±2.5 | 4.4±1.7 | 5.8±2.2 | 7.7±2.7 |
| Bicarbonate (mEq/L) | 24±3 | 24±3 | 25±3 | 24±3 | 23±3 | 24±3 | 25±3 | 24±3 | 23±3 | 23±3 | 24±3 | 23±3 | 22±3 |
| Hemoglobin (g/dL) | 11.1±1.2 | 11.2±1.2 | 11.0±1.3 | 11.2±1.1 | 11.2±1.2 | 10.9±1.2 | 10.7±1.3 | 10.9±1.2 | 11.0±1.3 | 11.3±1.2 | 11.0±1.2 | 11.2±1.2 | 11.3±1.1 |
| WBC (mm3) | 7.8±2.7 | 8.1±2.9 | 8.1±3.2 | 8.0±2.8 | 8.4±3.3 | 7.4±2.5 | 7.5±2.8 | 7.4±2.5 | 7.6±2.8 | 7.8±2.3 | 7.9±2.6 | 7.9±2.3 | 7.8±2.3 |
| Lymphocyte (% of WBC) | 20.7±7.6 | 19.0±7.4 | 18.8±7.7 | 19.0±7.3 | 18.9±7.7 | 22.9±7.7 | 22.3±8.3 | 23.0±7.7 | 22.8±7.6 | 21.2±6.7 | 20.5±7.7 | 21.2±6.7 | 21.7±6.7 |
| Platelet (X109/L) | 251±88 | 243±90 | 228±91 | 241±88 | 262±99 | 262±88 | 250±92 | 262±87 | 271±92 | 254±84 | 222±95 | 252±83 | 268±86 |
| iPTH (pg/mL) | 314(196, 489) | 263(164, 402) | 225(138, 349) | 261(164, 395) | 295(176, 463) | 404(259, 614) | 335(205, 521) | 404(261, 610) | 472(297, 727) | 329(217, 489) | 254(157, 383) | 317(208, 467) | 385(265, 569) |
| Calcium (mg/dL) | 9.1±0.6 | 9.1±0.6 | 9.2±0.6 | 9.1±0.5 | 9.1±0.6 | 9.1±0.6 | 9.2±0.6 | 9.1±0.6 | 9.1±0.6 | 9.0±0.5 | 9.1±0.6 | 9.0±0.5 | 8.9±0.6 |
| Phosphorus (mg/dL) | 4.9±1.2 | 4.9±1.2 | 4.2±36.3 | 4.8±1.1 | 5.6±1.3 | 4.8±1.1 | 4.2±1.0 | 4.9±1.1 | 5.6±1.3 | 5.1±1.2 | 4.2±1.0 | 4.9±1.1 | 5.8±1.3 |
| Ferritin (ng/mL) | 280(163, 481) | 277(163, 474) | 321(179, 564) | 276(163, 472) | 270(155,4 59) | 297(170, 513) | 367(196, 666) | 293(169, 502) | 291(161, 504) | 259(150, 439) | 309(189, 582) | 262(151, 442) | 243(142, 414) |
| TIBC (mg/dL) | 225±49 | 227±51 | 208±58 | 228±51 | 230±49 | 220±47 | 198±54 | 221±50 | 226±45 | 227±47 | 200±56 | 226±47 | 231±42 |
| ISAT (%) | 23±9 | 23±9 | 25±12 | 23±9 | 22±9 | 23±9 | 25±11 | 23±9 | 23±10 | 23±9 | 27±12 | 23±9 | 23±9 |
| BUN (mg/dl) | 48±14 | 48±14 | 37±14 | 47±14 | 56±15 | 46±14 | 37±13 | 46±13 | 56±15 | 53±15 | 40±15 | 50±14 | 62±15 |
| 212(195, | 211(190, | 211(189, | 211(190, | 210(189, | 214(202, | 214(201, | 215(202, | 214(200, | 210(191, | 210(190, | 210(191, | 220(190, | |
| Pre-HD SBP | 231) | 229) | 230) | 230) | 227) | 235) | 234) | 235) | 233) | 225) | 228) | 226) | 220) |
| (mmHg) Pre-HDDBP | 147±19 | 143±19 | 139±20 | 143±19 | 146±19 | 151±19 | 147±21 | 151±19 | 152±19 | 150±18 | 142±20 | 149±18 | 152±18 |
| (mmHg) | 77±12 | 74±11 | 72±11 | 73±11 | 77±12 | 82±12 | 79±13 | 82±12 | 84±12 | 78±11 | 75±10 | 78±11 | 81±11 |
| Post-HD Weight | 80.7±22.4 | 81.5±22.4 | 79.6±22.9 | 81.8±22.4 | 80.2±22.0 | 82.8±23 | 77.7±21.3 | 83.4±23.4 | 81.3±22.9 | 73.9±18.7 | 72.8±18.5 | 74.7±19.3 | 71.1±16.4 |
| Ultrafiltration (kg) | 2.0±0.9 | 2.0±0.9 | 1.8±0.9 | 2.0±0.9 | 2.2±1.0 | 2.1±0.9 | 1.8±0.8 | 2.1±0.8 | 2.4±0.9 | 2.0±0.8 | 1.7±0.8 | 2.0±0.8 | 2.2±0.8 |
| Urine volume (ml) | 775(440, 1300) | 800(450, 1300) | 700(375. 1300) | 750(400, 1250) | 850(450, 1500) | 700(400, 1200) | 550(300, 1020) | 600(350, 1100) | 763(450, 1250) | 831(500, 1300) | 650(350, 1200) | 750(450, 1200) | 800(500, 1250) |
| ESA dose (unit/week) | 4687(1486, 11943) | 4676(1486, 12000) | 4999(1366, 12500) | 4665(1467, 11978) | 4731(1572, 12042) | 4714(1467, 11943) | 5000(1524, 12682) | 4681(1467, 11880) | 4654(1500, 11916) | 4668(1500, 11786) | 5566(2096, 13400) | 4659(1518, 11676) | 4576(1354, 11984) |
Note: continuous values are expressed as mean ± SD if normally distributed or median (interquartile range) if skewed.
Abbreviations: DM, Diabetes Mellitus; CVC, central venous catheter; AVF, arteriovenous fistula; AVG, arteriovenous graft; nPCR, normalized protein catabolic rate; spKt/V, single pool Kt/V; CHF, Congestive heart failure; ASHD, atherosclerotic heart disease; CVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease; HIV Ab, human immunodeficiency virus antibody; BMI, body mass index; WBC, white blood cell; iPTH, intact parathyroid hormone; TIBC, total iron binding capacity; ISAT, iron saturation; BUN, blood urea nitrogen; HD, hemodialysis; SBP, systolic blood pressure; DBP, diastolic blood pressure; ESA, erythopoiesis-stimulating agent.
Clinical Characteristics Associated with Potassium Level across Racial/Ethnic Groups
In logistic regression analyses in the overall cohort, compared to white patients, African-Americans had a 42% lower odds of hyperkalemia (OR: 0.58, 95%CI: 0.55–0.62) and a 63% higher odds of hypokalemia (OR: 1.63, 95%CI: 1.52–1.76), while Hispanic patients had a 32% higher odds of hyperkalemia (OR: 1.32, 95%CI: 1.25–1.39) and 11% lower odds of hypokalemia (OR: 0.89, 95%CI: 0.80–0.99) in case-mix + MICS adjusted models in the overall cohort. Table 2 shows the association between clinical characteristics with likelihood of hyperkalemia (reference: serum potassium >3.6 to ≤5.0 mEq/L) across the racial/ethnic groups in case-mix + MICS adjusted logistic regression models. Across all racial/ethnic groups, older age, higher baseline spKt/V, AV fistula access, and higher baseline BMI were associated with lower likelihood of hyperkalemia. Higher levels of nutritional status markers (higher albumin, phosphorus, and BUN), diabetes, higher UF, and shorter HD treatment time were associated with higher likelihood of hyperkalemia. Among both African-Americans and whites, females were more likely to have baseline hyperkalemia. In African-American patients only, Medicaid insurance type was associated with a higher likelihood of hyperkalemia.
Table 2.
Association of clinical characteristics with hyperkalemia (serum potassium >5.0 vs >3.6 to ≤5.0 mEq/l) among whites, African-Americans, and Hispanics in case-mix + MICS adjusted logistic regression models.
| Factors | Race |
|||||
|---|---|---|---|---|---|---|
| Whites, OR(95% CI) | African-Americans, OR(95% CI) | Hispanics, OR(95% CI) | ||||
|
Socio-demographic factors |
||||||
| Age,Δ 10-y | 0.96 | (0.94 – 0.99) | 0.96 | (0.92 – 0.99) | 0.96 | (0.92 – 0.99) |
| Female, vs Male | 1.15 | (1.08 – 1.23) | 1.14 | (1.03 – 1.26) | 0.94 | (0.85 – 1.04) |
| Comorbidity factors | ||||||
| DM | 1.13 | (1.07 – 1.21) | 1.09 | (1.00 – 1.19) | 1.24 | (1.12 – 1.37) |
| Hypertension | 0.91 | (0.86 – 0.96) | 1.01 | (0.93 – 1.10) | 0.97 | (0.89 – 1.05) |
| Congestive heart failure | 1.06 | (1.00 – 1.12) | 1.10 | (1.01 – 1.20) | 1.05 | (0.96 – 1.15) |
| BMI,Δ 1 kg/m2 | 0.97 | (0.96 – 0.97) | 0.96 | (0.96 – 0.97) | 0.95 | (0.94 – 0.95) |
| Socio-economic factors | ||||||
| Medicaid, vs Medicare | 1.07 | (0.93 – 1.22) | 1.31 | (1.13 – 1.51) | 1.09 | (0.96 – 1.23) |
| Other, vs Medicare | 0.86 | (0.81 – 0.92) | 1.01 | (0.93 – 1.10) | 0.86 | (0.79 – 0.95) |
| Dialysis related factors | ||||||
| Access type: AVF, vs CVC | 0.67 | (0.61 – 0.72) | 0.67 | (0.59 – 0.77) | 0.66 | (0.57 – 0.75) |
| Access type: AVG, vs CVC | 0.73 | (0.62 – 0.87) | 0.71 | (0.58 – 0.86) | 0.71 | (0.53 – 0.94) |
| Access type: Others, vs CVC | 0.46 | (0.11 – 1.97) | 0.34 | (0.08 – 1.54) | 0.81 | (0.23 – 2.79) |
| Access type: Unknown, vs CVC | 1.14 | (1.02 – 1.27) | 1.30 | (1.12 – 1.51) | 1.01 | (0.84 – 1.22) |
| spKt/V,Δ 0.1 unit | 0.97 | (0.96 – 0.98) | 0.97 | (0.95 – 0.99) | 0.97 | (0.95 – 0.99) |
| HD time,Δ 10 min/session | 0.99 | (0.98 – 1.00) | 0.97 | (0.96 – 0.99) | 0.97 | (0.96 – 0.99) |
| Ultrafiltration,Δ 1 kg | 1.26 | (1.21 – 1.30) | 1.32 | (1.25 – 1.39) | 1.39 | (1.31 – 1.48) |
| pre-HD SBP,Δ 10 mmHg | 0.98 | (0.96 – 1.00) | 0.99 | (0.95 – 1.02) | 0.99 | (0.95 – 1.02) |
| Nutritional factors | ||||||
| Albumin,Δ 0.1 g/dl | 1.02 | (1.01 – 1.03) | 1.02 | (1.01 – 1.03) | 1.02 | (1.01 – 1.03) |
| Phosphorus,Δ 0.1 mg/dl | 1.03 | (1.03 – 1.04) | 1.02 | (1.02 – 1.03) | 1.02 | (1.02 – 1.03) |
| BUN,Δ 1 mg/dl | 1.03 | (1.03 – 1.03) | 1.04 | (1.04 – 1.05) | 1.04 | (1.03 – 1.04) |
| nPCR,Δ 0.1 g/kg/day | 1.00 | (0.98 – 1.02) | 0.96 | (0.93 – 1.00) | 0.96 | (0.93 – 1.00) |
Case-mix + MICS models adjusted for age, sex, diabetes mellitus, primary insurance, vascular access type, spKt/V, HD treatment time, UF, and cardiovascular risk factors including pre-HD systolic BP, pre-HD diastolic BP, BMI, comorbidities, 13 surrogates of nutritional and/or inflammatory status (albumin, hemoglobin, peripheral WBC, lymphocyte percentage, ferritin, TIBC, calcium, phosphorus, bicarbonate, BUN, iPTH, nPCR, and ESA dose).
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; AVF, arteriovenous fistula; CVC, central venous catheter; AVG, arteriovenous graft; spKt/V, single pool Kt/V; HD, hemodialysis; SBP, systolic blood pressure; BUN, blood urea nitrogen; nPCR, normalized protein catabolic rate.
In case-mix + MICS adjusted logistic regression models, clinical characteristics associated with a higher likelihood of hypokalemia (reference: serum potassium >3.6 to ≤5.0 mEq/L) included lower serum albumin, phosphorus, and BUN across all racial/ethnic groups. Older age was a significant predictor of hypokalemia only among whites (OR [95% CI], 0.88 [0.85, 0.92]), while female sex was a predictor of hypokalemia (OR [95% CI], 1.18 [1.06, 1.31]) only in African-American patients (Appendix-Table S1). There was no association between HD treatment time and hypokalemia for any racial/ethnic group.
Serum Potassium and Mortality across Racial/Ethnic Groups
In case-mix +MICS adjusted cubic spline models, there was a J-shaped relationship between serum potassium and all-cause mortality in whites and African-Americans, such that higher serum potassium levels were associated with higher mortality risk, while lower serum potassium levels had no significant association with mortality (Table 3). In whites, serum potassium levels >4.5 mEq/L were associated with incrementally higher mortality risk in case-mix + MICS adjusted models (Figure 2(A)): HRs [95% CI], 1.05 [1.01, 1.09], 1.09 [1.04, 1.16], 1.17 [1.06, 1.30] for serum potassium levels >4.5 to ≤5.0, >5.0 to ≤5.5, and >5.5 mEq/L, respectively. African-Americans also demonstrated an association between higher serum potassium level and higher mortality risk, albeit at a higher serum potassium threshold of >5.5 mEq/L: HR [95% CI], 1.43 [1.21, 1.70] (Table 3).
Table 3.
The number of death, cardiovascular death, and transplanted patients across race/ethnicity and association between serum potassium with all-cause mortality according to race/ethnicity in case-mix and MICS adjusted Cox models.
| Serum potassium (mEq/L) | Total, N | whites |
||||
|---|---|---|---|---|---|---|
| All-cause mortality HR
(95% CI) |
||||||
| All cause death, N (%) | CV death, N (%) | transplant, N (%) | Unadjusted model | Case-mix + MICS models | ||
| Continuous value | 51297 | 16804(32.8) | 5700(11.1) | 2037(4.0) | 0.93 (0.90–0.96) | 1.08 (1.04–1.12) |
|
|
|
|||||
| <3.6 | 1957 | 737 (37.7) | 226 (11.6) | 50 (2.6) | 1.21 (1.12–1.31) | 1.00 (0.93–1.08) |
| >3.6 to <4.0 | 8467 | 2852 (33.7) | 929 (11.0) | 275 (3.2) | 1.07 (1.03–1.12) | 1.00 (0.95–1.04) |
| >4.0 to <4.5 | 19767 | 6340 (32.1) | 2,157 (11.0) | 743 (3.8) | 1 [reference] | 1 [reference] |
| >4.5 to <5.0 | 14674 | 4743 (32.3) | 1,639 (11.2) | 644 (4.4) | 0.98 (0.95–1.02) | 1.05 (1.01–1.09) |
| >5.0 to <5.5 | 5228 | 1706 (32.6) | 593 (11.3) | 249 (4.8) | 0.96 (0.91–1.01) | 1.09 (1.04–1.16) |
| >5.5 | 1204 | 426 (35.4) | 156 (13.0) | 76 (6.3) | 1.01 (0.91–1.11) | 1.17 (1.06–1.30) |
|
| ||||||
| African-Americans |
||||||
| Serum potassium (mEq/L) | All-cause mortality HR
(95% CI) |
|||||
| Total, N | All-cause death, N (%) | CV death, N (%) | transplant, N (%) | Unadjusted model | Case-mix + MICS models | |
|
| ||||||
| Continuous value | 34574 | 7573(21.9) | 2654(7.7) | 878(2.5) | 0.81 (0.77–0.85) | 1.00 (0.95–1.05) |
|
|
|
|||||
| <3.6 | 2323 | 707 (30.4) | 213 (9.2) | 42 (1.8) | 1.51 (1.39–1.64) | 1.07 (0.98–1.17) |
| >3.6 to <4.0 | 7642 | 1780 (23.3) | 628 (8.2) | 172 (2.3) | 1.13 (1.07–1.20) | 1.01 (0.95–1.07) |
| >4.0 to <4.5 | 13767 | 2884 (20.9) | 1,011 (7.3) | 340 (2.5) | 1 [reference] | 1 [reference] |
| >4.5 to <5.0 | 7949 | 1571 (19.8) | 563 (7.1) | 227 (2.9) | 0.92 (0.86–0.97) | 0.98 (0.92–1.04) |
| >5.0 to <5.5 | 2384 | 491 (20.6) | 192 (8.1) | 85 (3.6) | 0.93 (0.85–1.02) | 1.01 (0.92–1.12) |
| >5.5 | 509 | 140 (27.5) | 47 (9.2) | 12 (2.4) | 1.36 (1.14–1.61) | 1.43 (1.21–1.70) |
|
| ||||||
| Hispanics |
||||||
| Serum potassium (mEq/L) | All-cause mortality HR
(95% CI) |
|||||
| Total, N | All-cause death, N (%) | CV death, N (%) | transplant, N (%) | Unadjusted model | Case-mix + MICS models | |
|
| ||||||
| Continuous value | 16370 | 3084(18.8) | 1280(7.8) | 546(3.3) | 0.66 (0.62–0.70) | 0.95 (0.88–1.02) |
|
|
|
|||||
| <3.6 | 431 | 158 (36.7) | 49 (11.4) | 10 (2.3) | 2.05 (1.74–2.43) | 1.36 (1.15–1.62) |
| >3.6 to <4.0 | 1959 | 464 (23.7) | 193 (9.9) | 48 (2.5) | 1.27 (1.14–1.42) | 1.08 (0.97–1.21) |
| >4.0 to <4.5 | 5412 | 1049 (19.4) | 442 (8.2) | 161 (3.0) | 1 [reference] | 1 [reference] |
| >4.5 to <5.0 | 5094 | 861 (16.9) | 360 (7.1) | 183 (3.6) | 0.84 (0.76–0.91) | 1.00 (0.91–1.10) |
| >5.0 to <5.5 | 2572 | 417 (16.2) | 181 (7.0) | 114 (4.4) | 0.75 (0.67–0.84) | 1.05 (0.94–1.19) |
| >5.5 | 902 | 135 (15.0) | 55 (6.1) | 30 (3.3) | 0.66 (0.55–0.78) | 1.01 (0.84–1.22) |
Case-mix + MICS models adjusted for age, sex, diabetes mellitus, primary insurance, vascular access type, spKt/V, HD treatment time, UF, and cardiovascular risk factors including pre-HD systolic BP, pre-HD diastolic BP, BMI, comorbidities, 13 surrogates of nutritional and/or inflammatory status (albumin, hemoglobin, peripheral WBC, lymphocyte percentage, ferritin, TIBC, calcium, phosphorus, bicarbonate, BUN, iPTH, nPCR, and ESA dose).
Abbreviations: N, number; CV, cardiovascular; HR, hazard ratio; CI, confidence interval
Figure 2.
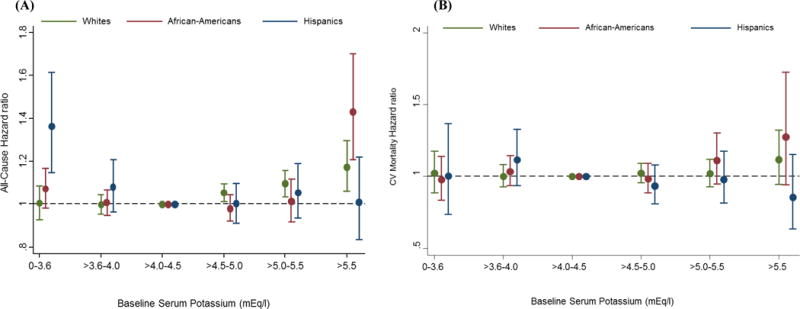
Association between serum potassium categories and (A) all-cause mortality and (B) cardiovascular mortality across race/ethnicity in case-mix + MICS models (reference: serum potassium >4.0 to 4.5 mEq/L).
Footnote: Case-mix + MICS models adjusted for age, sex, diabetes mellitus, primary insurance, vascular access type, spKt/V, HD treatment time, UF, and cardiovascular risk factors including pre-HD systolic BP, pre-HD diastolic BP, BMI, comorbidities, 13 surrogates of nutritional and/or inflammatory status (albumin, hemoglobin, peripheral WBC, lymphocyte percentage, ferritin, TIBC, calcium, phosphorus, bicarbonate, BUN, iPTH, nPCR, and ESA dose).
Conversely, among Hispanics, lower serum potassium levels were associated with higher mortality, whereas higher serum potassium levels had no association with mortality in the case-mix + MICS adjusted models. (Figure 2(A)). In categorical analyses, only very low serum potassium levels (≤3.6 mEq/L) were significantly associated with higher mortality risk in Hispanics: HR [95% CI], 1.36 [1.15, 1.62] (Table 3). These trends were similar in the case-mix + MICS adjusted cubic spline models (Appendix-Figure S2). While serum potassium levels >4.5 mEq/L were associated with incrementally higher cardiovascular mortality risk in whites and African-Americans, they were associated with lower cardiovascular mortality risk in Hispanics, although this was not statistically significant (Figure 2(B)).
Figure 3 shows the association between 18 categories of race/ethnicity and baseline serum potassium level with all-cause mortality. Compared to Hispanics with serum potassium levels >4.0 to ≤4.5 mEq/L as the reference category, all potassium categories were associated with higher mortality risk amongst whites in case-mix + MICS adjusted models. All potassium categories were associated with higher mortality risk amongst African-Americans compared to the reference group. Amongst Hispanics, patients with serum potassium >4.5 mEq/L had similar mortality risk compared to the reference group, whereas those with serum potassium ≤3.5mEq/L had higher mortality risk. After taking into consideration the impact of kidney transplantation as a competing event, Hispanics had lower mortality risk across serum potassium categories >4.0 mEq/l in case-mix + MICS adjusted models (Appendix-Figure S3).
Figure 3.
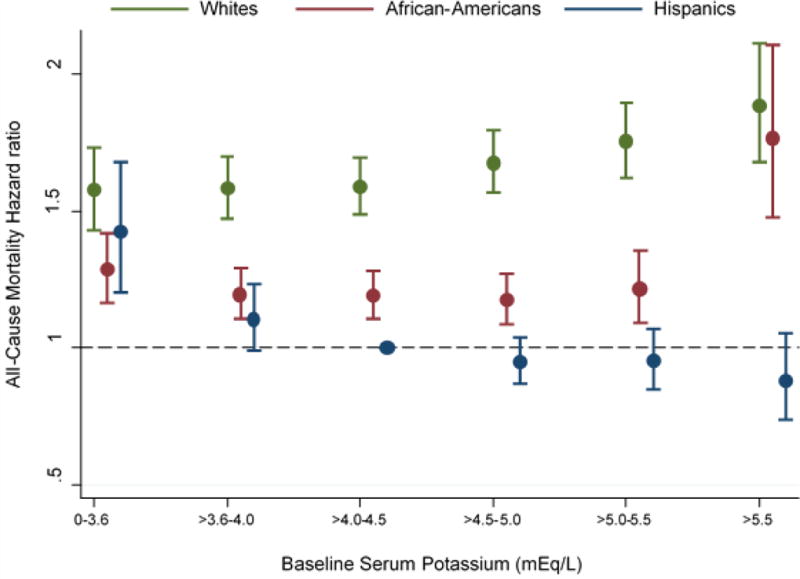
Association between 18 groups of race/ethnicity and baseline serum potassium with all-cause mortality in case-mix + MICS models (reference: Hispanics with serum potassium >4.0 to 4.5 mEq/l)
Footnote: Case-mix + MICS models adjusted for age, sex, diabetes mellitus, primary insurance, vascular access type, spKt/V, HD treatment time, UF, and cardiovascular risk factors including pre-HD systolic BP, pre-HD diastolic BP, BMI, comorbidities, 13 surrogates of nutritional and/or inflammatory status (albumin, hemoglobin, peripheral WBC, lymphocyte percentage, ferritin, TIBC, calcium, phosphorus, bicarbonate, BUN, iPTH, nPCR, and ESA dose).
Potassium and Mortality Across Subgroups
Figure 4 shows the case-mix + MICS adjusted associations between hyperkalemia and all-cause mortality (reference: potassium levels >3.6 to ≤5.0 mEq/L) across a priori selected subgroups stratified by race/ethnicity. Within white patients, there was effect modification on the basis of Medicare insurance type (p-interaction=0.007), and hypertension comorbidity (p-interaction=0.004). There was no significant effect modification across subgroups among African-American or Hispanic patients. Similarly associations of hypokalemia with mortality within each racial-ethnic group were persistent across most all subgroups. In white patients there was significant effect modification by diabetes comorbidity (p-interaction=0.001) and in African-American by hypertension comorbidity (p-interaction=0.025). In both Hispanic and white patients, significant effect modification on the association of hypokalemia with mortality was observed (p-interaction=<0.001 for each) (Appendix-Figure S4).
Figure 4.
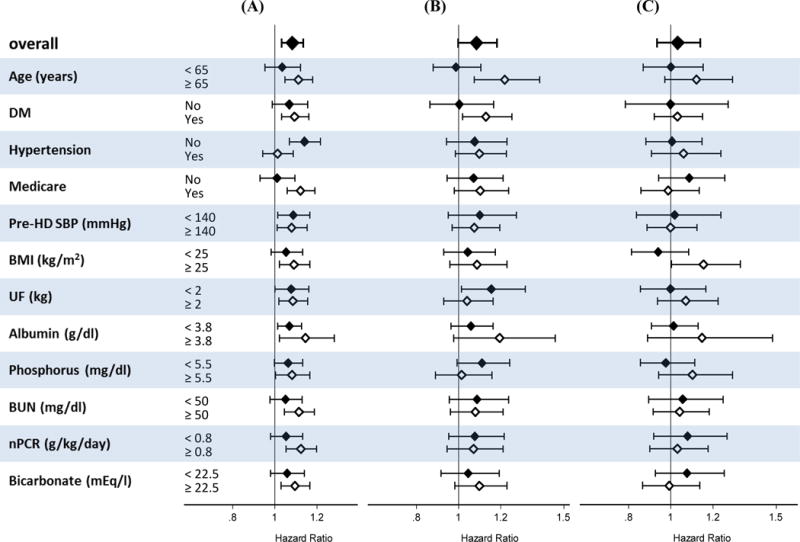
Association between hyperkalemia (serum potassium >5.0 mEq/l) with all-cause mortality (reference: normal serum potassium >3.6 to ≤5.0 mEq/l) across clinically relevant subgroups stratified by race/ethnicity in case-mix + MICS models. (A) whites, (B) African- Americans, (C) Hispanics.
Footnote: Case-mix + MICS models adjusted for age, sex, diabetes mellitus, primary insurance, vascular access type, spKt/V, HD treatment time, UF, and cardiovascular risk factors including pre-HD systolic BP, pre-HD diastolic BP, BMI, comorbidities, 13 surrogates of nutritional and/or inflammatory status (albumin, hemoglobin, peripheral WBC, lymphocyte percentage, ferritin, TIBC, calcium, phosphorus, bicarbonate, BUN, iPTH, nPCR, and ESA dose).
Abbreviations: DM, diabetes mellitus; HD, hemodialysis; SBP, systolic blood pressure; BMI, body mass index; UF, ultrafiltration; BUN, blood urea nitrogen; nPCR, normalized protein catabolic rate.
To account for confounding on the basis of residual renal function, in sensitivity analyses we incrementally adjusted for baseline urine volume within the case-mix + MICS adjusted models in a subcohort of 35,910 patients who had residual renal function data during the first patient quarter. Although the potassium-mortality associations were attenuated across all racial/ethnic groups, trends of higher serum potassium level and higher mortality risk in whites and African-Americans, but not in Hispanics were similar to that in our main analyses (Appendix-Figure S5).
Discussion
In this study we examined differences in the relationship between serum potassium level and all-cause mortality across three racial/ethnic groups of non-Hispanic whites, African- Americans, and Hispanics in a contemporary cohort of over 100,000 incident HD patients who were followed for up to five years. We found that Hispanic HD patients tended to be more hyperkalemic than whites and African-Americans. We also observed that hyperkalemia was associated with higher mortality risk in whites and African-American HD patients, but not in those who were Hispanic. Furthermore, in analyses that concurrently examined the association between serum potassium and race/ethnicity with mortality, compared with Hispanics with moderate range serum potassium levels (>4 to 4.5mEq/L), whites and African-Americans across all potassium categories had higher mortality risk, whereas Hispanics with serum potassium levels ≤3.5mEq/L had higher mortality risk.
To our knowledge, ours is the first study to examine differences in serum potassium distribution and associations with mortality in HD patients across racial/ethnic subgroups, including African-Americans, whites, and Hispanics. To date, there has been one study that has examined the association between serum potassium levels with mortality across race in predialysis CKD patients.19 In one study by Hayes et al., white CKD patients had higher baseline serum potassium levels compared to African-Americans, and associations of hyperkalemia with higher mortality risk were observed in white patients only, while associations of hypokalemia with higher mortality were found in both black and white patients.19 Notably, this study did not include dialysis patients or distinguish between Hispanics vs. non-Hispanics. Another previous study has also examined associations between potassium and mortality in pre-dialysis CKD patients, and found that African-Americans had significantly lower levels of serum potassium. However this study did not examine effect modification by race-ethnicity20.
While the sources of racial/ethnic differences in serum potassium levels remains unclear, it is possible that this may be explained by dietary differences across these groups. According to the National Health and Nutrition Examination Survey (NHANES) data from 1999–2002, African-Americans tended to consume less fruits and vegetables and more meat than whites and Hispanics, which may explain the lower serum potassium levels found in this racial/ethnic subgroup.21, 22 Despite differences seen in serum potassium levels, there were no significant differences between Hispanics and whites in consumption of fruit and vegetables.21 However, the primary sources of potassium intake in Hispanics were from eggs, whereas in whites this was obtained from legumes, nuts, seeds, dairy products, and beverages.23 In another study by Kalantar-Zadeh et al. characterizing food consumption in patients initiating hemodialysis, it was found that, after receiving dietary instructions, patients independently modified their diet and limited potassium intake in order to maintain appropriate serum potassium levels.24 Although Hispanics and non-Hispanics had identical adherence to potassium restriction recommendations,25 overall dietary modifications may have been more challenging in Hispanic patients due to the impact of cultural differences in food choice.26 Due to data limitations, we were unable to determine if the higher serum potassium distribution in Hispanics was due to higher intake of fruits and vegetables relating to cultural background.
Differences in serum potassium levels may also be impacted by potassium homeostasis. It is well established that African-Americans have lower serum potassium levels that may attributed to a blunted natriuretic response and lower Na+-K+ ATPase activity.27,28 These may also be related to differences in intracellular potassium content, differences in RAAS activity, and/or differences in intestinal potassium transport. However, since we cannot measure total body potassium or intracellular potassium in our cohort, we can only speculate what lower serum potassium levels in African-American hemodialysis patients represents, and we cannot conclude what effect this might have on membrane potential and excitability, and ultimately on clinical events in mortality. Increases in excretion or transcellular shifts and alterations in Na+-K+ ATPase activity may be additionally associated with diabetic comorbid conditions29 In our cohort, African-Americans had a significantly higher prevalence of diabetes comorbidity. Although, these mechanistic differences may explain why African-Americans had lower levels of serum potassium overall, in analyses adjusted for diabetes as well as across diabetes strata, we did not see significant differences in the associations of hyperkalemia with mortality outcomes.
To date, there is a lack of data examining differences in biological mechanisms of potassium levels in Hispanic vs. non-Hispanic patients. Additional information on factors that affect membrane excitability in this racial-ethnic group are needed to better understand if these factors can explain differences in mortality outcomes associated with the same serum potassium levels. Hispanics had the highest prevalence of diabetes comorbidity. Associations of hyperkalemia with mortality were attenuated in Hispanic diabetic patients; however there were no significant interactions between race and diabetes in the associations of hyperkalemia with mortality.
Racial/ethnic genetic differences may explain the differential association between hyperkalemia and mortality risk across racial/ethnic groups. Indeed, a mutational analysis study conducted in non-CKD patients has identified distinct cardiac potassium channel variants related to higher risk of sudden cardiac death across racial/ethnic groups, and further study in the kidney disease population is needed.30
Patients who chronically consume a high potassium diet may develop potassium tolerance, in which they are no longer susceptible to the adverse sequelae of hyperkalemia. In a large national cohort study of veterans, patients with CKD had a higher frequency of hyperkalemic events as compared with non-CKD patients. However, the mortality-predictability of hyperkalemia was inversely related to the CKD stage, presumably due to the development of potassium tolerance and less subsequent susceptibility to cardiac toxicity due to chronic exposure to high potassium levels.3 It is possible that Hispanic patients who are exposed to a high potassium diet over their lifetime may develop such potassium tolerance and thereby have a lower risk of mortality while in the hyperkalemic state. It also may explain why Hispanic patients with lower serum potassium have a higher mortality risk, since it may be a departure from a normative healthy state.
A systematic review has shown that Hispanics have lower cardiovascular mortality than non-Hispanic whites due to the differences in diet patterns that improve endothelial function and physical activity that modifies cardiovascular risk factors.31 Although Hispanics have a greater prevalence of cardiovascular risk factors such as diabetes, abdominal obesity, and hypertriglyceridemia,32 several studies have demonstrated that Hispanics with CKD and ESRD had lower incidence of cardiovascular events compared with non-Hispanic whites.12,33 It is possible that lower cardiovascular events account for lower cardiovascular mortality in Hispanics with higher serum potassium, but further study is needed to determine the mechanism. To our knowledge this is the first study to evaluate racial/ethnic differences in the relationship between serum potassium levels and mortality in a large nationally representative, contemporary cohort of incident HD patients with detailed capture of sociodemographic, comorbidity, and laboratory data. However, our study has several limitations. First, Hispanics may be heterogeneous in terms of cultural backgrounds and countries of origin, which may have implications of serum potassium concentrations.34,35 We were unable to distinguish Hispanic subpopulations which may be white or black race. It has been shown that Hispanic subgroups in the US originating from different countries including Mexico, the Caribbean, and Central and South America have differences in the consumption of fruits, vegetables, meat, and fats.36 Potassium intake also differed according to individual and/or neighboring socioeconomic status.37 We did not have data on dietary potassium intake. In addition, information on residual renal function was only available for one third of our cohort, analyses adjusting for potential confounding due to residual renal function may be inherent to selection biases and future studies investigating the impact of residual renal function on the racial-ethnic differences in serum potassium mortality associations are warranted. We also lacked data on certain dialysis treatment characteristics that may influence serum potassium concentrations such as use of RAAS inhibitors and diuretics and dialysate potassium concentration. While one observational study has shown that higher dialysate potassium bath was associated with higher mortality risk in HD patients with hyperkalemia,4 another study showed no difference in cardiac death between dialysate potassium bath and serum potassium levels even amongst hyperkalemic HD patients.38 Given the observational nature of this cohort analysis, our study is limited to adjusting for confounders that were measured in the dataset. We thereby cannot exclude the possibility of potential residual confounding.
In conclusion, our study has shown that Hispanic HD patients tend to have higher serum potassium levels as compared to African-American and white HD patients. While African- Americans and whites with higher potassium levels experienced higher death risk, this was not observed in Hispanic HD patients. Potential mechanisms underlying differences in serum potassium distributions and associations with mortality may be related to socio-cultural influences such as high potassium tolerance related to a chronically high potassium diet, or potassium adaptation related to racial/ethnic genetic differences. Further studies are needed to determine possible causal mechanisms underlying the survival advantage seen in hyperkalemic Hispanic HD patients.
Supplementary Material
Acknowledgments
The study was supported by Dr. Kalantar-Zadeh’s research grants from the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) of the National Institute of Health (NIH) (K24-DK09141, R01-DK078106 and R01-DK095668), and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Dr. Joseph Lee, and AVEO, Inc, Dr. Kovesdy’s NIH (NIDDK) grants R01-DK096920 and U01-DK102163, and Dr. Rhee’s NIH (NIDDK) grant K23-DK102903. Dr. Streja is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2-CX001266-01).
Footnotes
Disclosures
KKZ has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, ZS-Pharma, and was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA during 2007–2012. CPK has received honoraria from Abbott Nutrition, Sanofi-Aventis, Relypsa and ZS Pharma.
Author’s roles: Study design: TK, ES, CMR, CPK and KK-Z. Data analysis: TK and ES. Data Interpretation: TK, CMR, ES, YO, JC, AT, VR, MS, CPK, KK-Z. Drafting manuscript: TK, CMR, ES, YO, JC, AT, VR, MS, CPK, KK-Z. Approving final version of manuscript: TK, CMR, ES, YO, JC, AT, VR, MS, CPK, KK-Z. Each author contributed important intellectual content during manuscript drafting and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. KK-Z takes responsibility for the integrity of the data analysis.
Other authors have not declared any conflicts of interest.
References
- 1.Palmer BF. Regulation of Potassium Homeostasis. Clin JAm Soc Nephrol. 2015;10(6):1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pani A, Floris M, Rosner MH, Ronco C. Hyperkalemia in hemodialysis patients. Semin Dial. 2014;27(6):571–576. doi: 10.1111/sdi.12272. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Regidor DL, Mehrotra R, et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(5):999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 5.Genovesi S, Valsecchi MG, Rossi E, et al. Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24(8):2529–2536. doi: 10.1093/ndt/gfp104. [DOI] [PubMed] [Google Scholar]
- 6.Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011) 2015;5(1):2–7. doi: 10.1038/kisup.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turban S, Miller ER, 3rd, Ange B, Appel LJ. Racial differences in urinary potassium excretion. J Am Soc Nephrol. 2008;19(7):1396–1402. doi: 10.1681/ASN.2007101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavallari LH, Fashingbauer LA, Beitelshees AL, et al. Racial differences in patients’ potassium concentrations during spironolactone therapy for heart failure. Pharmacotherapy. 2004;24(6):750–756. doi: 10.1592/phco.24.8.750.36076. [DOI] [PubMed] [Google Scholar]
- 9.United States Renal Data System. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: 2014. 2014 USRDS annual data report: Epidemiology of kidney disease in the United States. [Google Scholar]
- 10.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. Jama. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan G, Norris KC, Yu AJ, et al. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol. 2013;8(6):953–961. doi: 10.2215/CJN.09180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy BV, Molony DA, Stack AG. Survival advantage of Hispanic patients initiating dialysis in the United States is modified by race. J Am Soc Nephrol. 2005;16(3):782–790. doi: 10.1681/ASN.2004080627. [DOI] [PubMed] [Google Scholar]
- 13.Frankenfield DL, Rocco MV, Roman SH, McClellan WM. Survival advantage for adult Hispanic hemodialysis patients? Findings from the end-stage renal disease clinical performance measures project. J Am Soc Nephrol. 2003;14(1):180–186. doi: 10.1097/01.asn.0000037400.83593.e6. [DOI] [PubMed] [Google Scholar]
- 14.Arce CM, Goldstein BA, Mitani AA, Winkelmayer WC. Trends in relative mortality between Hispanic and non-Hispanic whites initiating dialysis: a retrospective study of the US Renal Data System. Am J Kidney Dis. 2013;62(2):312–321. doi: 10.1053/j.ajkd.2013.02.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugirdas JT. Physiologic Principles and Urea Kinetic Modeling. In: Daugirdas JT, Blake PG, Ing TS, editors. Handbook of dialysis. 5th. Philadelphia: Lippincott Williams & Wilkins; 2014. p. 5960. [Google Scholar]
- 16.Clinical practice guidelines for hemodialysis adequacy, update 2006. American Journal of Kidney Diseases. 2006;48(Supple 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 17.Mosteller RD. Simplified calculation of body-surface area. The New England journal of medicine. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 19.Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120(1):c8–16. doi: 10.1159/000329511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakhoul GN, Huang H, Arrigain S, et al. Serum Potassium, End-Stage Renal Disease and Mortality in Chronic Kidney Disease. Am J Nephrol. 2015;41(6):456–463. doi: 10.1159/000437151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casagrande SS, Wang Y, Anderson C, Gary TL. Have Americans increased their fruit and vegetable intake? The trends between 1988 and 2002. Am J Prev Med. 2007;32(4):257–263. doi: 10.1016/j.amepre.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Chan KH, Chacko SA, Song Y, et al. Genetic variations in magnesium-related ion channels may affect diabetes risk among African American and Hispanic American women. J Nutr. 2015;145(3):418–424. doi: 10.3945/jn.114.203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neil CE, Nicklas TA, Keast DR, Fulgoni VL. Ethnic disparities among food sources of energy and nutrients of public health concern and nutrients to limit in adults in the United States: NHANES 2003–2006. Food Nutr Res. 2014;58:15784. doi: 10.3402/fnr.v58.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12(1):17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 25.Morales Lopez C, Burrowes JD, Gizis F, Brommage D. Dietary adherence in Hispanic patients receiving hemodialysis. J Ren Nutr. 2007;17(2):138–147. doi: 10.1053/j.jrn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd R. Influences on food choice and dietary behavior. Forum Nutr. 2005;57:36–43. doi: 10.1159/000083752. [DOI] [PubMed] [Google Scholar]
- 27.Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation. 1979;59(4):643–650. doi: 10.1161/01.cir.59.4.643. [DOI] [PubMed] [Google Scholar]
- 28.Suh A, DeJesus E, Rosner K, et al. Racial differences in potassium disposal. Kidney Int. 2004;66(3):1076–1081. doi: 10.1111/j.1523-1755.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- 29.Gennari FJ. Disorders of potassium homeostasis. Hypokalemia and hyperkalemia. Crit Care Clin. 2002;18(2):273–288. vi. doi: 10.1016/s0749-0704(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc. 2003;78(12):1479–1487. doi: 10.4065/78.12.1479. [DOI] [PubMed] [Google Scholar]
- 31.Cortes-Bergoderi M, Goel K, Murad MH, et al. Cardiovascular mortality in Hispanics compared to non-Hispanic whites: a systematic review and meta-analysis of the Hispanic paradox. Eur J Intern Med. 2013;24(8):791–799. doi: 10.1016/j.ejim.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 33.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 34.Daviglus ML, Talavera GA, Aviles-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. Jama. 2012;308(17):1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borrell LN, Crawford ND. All-cause mortality among Hispanics in the United States: exploring heterogeneity by nativity status, country of origin, and race in the National Health Interview Survey-linked Mortality Files. Ann Epidemiol. 2009;19(5):336–343. doi: 10.1016/j.annepidem.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Siega-Riz AM, Sotres-Alvarez D, Ayala GX, et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2014;99(6):1487–1498. doi: 10.3945/ajcn.113.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi SS, Ruff RR, Jung M, Waddell EN. Racial/ethnic residential segregation, neighborhood poverty and urinary biomarkers of diet in New York City adults. Soc Sci Med. 2014;122:122–129. doi: 10.1016/j.socscimed.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 38.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79(2):218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


