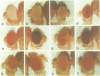
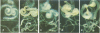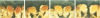Abstract
Constructions containing the Drosophila white gene and different amounts and arrangements of its regulatory region were introduced into the germ line of white mutant flies by P-mediated transformation. The results obtained with the different transposon constructions show that different parts of the 1.8-kb region preceding the transcription start are required for the expression of the gene in different tissues and at different developmental stages. Different sequences independently control the expression of the gene in the adult testes, in the larval and adult Malpighian tubules and in the eye. Another sequence located greater than 1080 bp upstream of the transcription start is the target of zeste interaction. The results also suggest that sequences required for dosage compensation are contained between -216 and the transcription start site. We show that at least some of these regulatory elements are equally functional if their distance from the promoter is varied or if their orientation is inverted. Their properties suggest that they act as enhancer-like elements to regulate the activity of the white promoter and, at least in the case of the zeste regulatory site, that they can act also in 'trans' on a white promoter locked in close physical proximity by homologous chromosome pairing.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. Gene activity dependent on chromosome synapsis in the polytene chromosomes of Drosophila melanogaster. Nature. 1967 Jun 10;214(5093):1159–1160. doi: 10.1038/2141159b0. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. 3. A comparison of the autosomal puffing patterns of the sibling species D. melanogaster and D. simulans. Chromosoma. 1969;27(1):64–85. doi: 10.1007/BF00326111. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Becker H J. Variegation in the Zeste Eye Color Alleles and Its Bearing on Gene Action during the Development of the Eye of Drosophila Melanogaster. Genetics. 1960 May;45(5):519–534. doi: 10.1093/genetics/45.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Zachar Z. Evidence that two mutations, wDZL and z1, affecting synapsis-dependent genetic behavior of white are transcriptional regulatory mutations. Cell. 1985 Apr;40(4):819–825. doi: 10.1016/0092-8674(85)90341-1. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Davison D., Chapman C. H., Wedeen C., Bingham P. M. Genetic and physical studies of a portion of the white locus participating in transcriptional regulation and in synapsis-dependent interactions in Drosophila adult tissues. Genetics. 1985 Jul;110(3):479–494. doi: 10.1093/genetics/110.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Ephrussi B, Herold J L. Studies of Eye Pigments of Drosophila. I. Methods of Extraction and Quantitative Estimation of the Pigment Components. Genetics. 1944 Mar;29(2):148–175. doi: 10.1093/genetics/29.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjose A., Polito L. C., Weber U., Gehring W. J. Developmental expression of the white locus of Drosophila melanogaster. EMBO J. 1984 Sep;3(9):2087–2094. doi: 10.1002/j.1460-2075.1984.tb02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- GREEN M. M. Further data on non-homologous pairing and crossing over in Drosophila melanogaster. Genetics. 1961 Nov;46:1555–1560. doi: 10.1093/genetics/46.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. J., Klemenz R., Weber U., Kloter U. Functional analysis of the white gene of Drosophila by P-factor-mediated transformation. EMBO J. 1984 Sep;3(9):2077–2085. doi: 10.1002/j.1460-2075.1984.tb02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart W. M. Synapsis-dependent allelic complementation at the decapentaplegic gene complex in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2636–2640. doi: 10.1073/pnas.79.8.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart W. M., Wu C. T. Interactions of zeste mutations with loci exhibiting transvection effects in Drosophila melanogaster. Genetics. 1982 Oct;102(2):179–189. doi: 10.1093/genetics/102.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrigg T., Levis R., Rubin G. M. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984 Feb;36(2):469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- Holmquist G. Transcription rates of individual polytene chromosome bands: effects of gene dose and sex in Drosophila. Chromosoma. 1972;36(4):413–452. doi: 10.1007/BF00336796. [DOI] [PubMed] [Google Scholar]
- JUDD B. H. Formation of duplication-deficiency products by asymmetrical exchange within a complex locus of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1961 Apr 15;47:545–550. doi: 10.1073/pnas.47.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. W., Judd B. H. Allelic pairing and gene regulation: A model for the zeste-white interaction in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1368–1372. doi: 10.1073/pnas.76.3.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd B. H., Shen M. W., Kaufman T. C. The anatomy and function of a segment of the X chromosome of Drosophila melanogaster. Genetics. 1972 May;71(1):139–156. doi: 10.1093/genetics/71.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman T. C., Tasaka S. E., Suzuki D. T. The interaction of two complex loci, zeste and bithorax in Drosophila melanogaster. Genetics. 1973 Oct;75(2):299–321. doi: 10.1093/genetics/75.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Direct correlation between a chromosome puff and the synthesis of a larval saliva protein in Drosophila melanogaster. Chromosoma. 1977 Jul 5;62(2):155–174. doi: 10.1007/BF00292637. [DOI] [PubMed] [Google Scholar]
- Korge G. Dosage compensation and effect for RNA synthesis in chromosome puffs of Drosophila melanogaster. Nature. 1970 Jan 24;225(5230):386–388. doi: 10.1038/225386a0. [DOI] [PubMed] [Google Scholar]
- Lefevre G., Jr, Green M. M. Genetic duplication in the white-split interval of the X chromosome in Drosophila melanogaster. Chromosoma. 1972;36(4):391–412. doi: 10.1007/BF00336795. [DOI] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985 Aug 9;229(4713):558–561. doi: 10.1126/science.2992080. [DOI] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M. Separable cis-acting control elements for expression of the white gene of Drosophila. EMBO J. 1985 Dec 16;4(13A):3489–3499. doi: 10.1002/j.1460-2075.1985.tb04108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschytz E., Green M. M. The zeste-white interaction: induction and genetic analysis of a novel class of zeste alleles. EMBO J. 1984 May;3(5):999–1002. doi: 10.1002/j.1460-2075.1984.tb01919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani C., Pirrotta V., Manet E. Isolation and characterization of the zeste locus of Drosophila. EMBO J. 1985 Aug;4(8):2045–2052. doi: 10.1002/j.1460-2075.1985.tb03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni G., Lucchesi J. C. X-chromosome transcription in Drosophila. Chromosoma. 1980;77(3):253–261. doi: 10.1007/BF00286051. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A. S., Beermann W. Synthesis of ribonucleic acid by the X-chromosomes of Drosophila melanogaster and the problem of dosage compensation. Nature. 1965 Aug 14;207(998):785–786. doi: 10.1038/207785a0. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Murphy C., Levis R., Rubin G. M. DNA sequence of the white locus of Drosophila melanogaster. J Mol Biol. 1984 Dec 15;180(3):437–455. doi: 10.1016/0022-2836(84)90021-4. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- O'hare K., Levis R., Rubin G. M. Transcription of the white locus in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6917–6921. doi: 10.1073/pnas.80.22.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce D. A., Lucchesi J. C. Dosage compensation of X-linked heat-shock puffs in Drosophila pseudoobscura. Chromosoma. 1980;76(3):245–254. doi: 10.1007/BF00327265. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Bröckl C. Transcription of the Drosophila white locus and some of its mutants. EMBO J. 1984 Mar;3(3):563–568. doi: 10.1002/j.1460-2075.1984.tb01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Hadfield C., Pretorius G. H. Microdissection and cloning of the white locus and the 3B1-3C2 region of the Drosophila X chromosome. EMBO J. 1983;2(6):927–934. doi: 10.1002/j.1460-2075.1983.tb01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976 Oct 15;53(2):217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Roehrdanz R. L., Kitchens J. M., Lucchesi J. C. Lack of dosage compensation for an autosomal gene relocated to the X chromosome in Drosophila melanogaster. Genetics. 1977 Mar;85(3):489–496. doi: 10.1093/genetics/85.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler J., Bowman J. T., Simmons J. R. Gene modulation in Drosophila: dosage compensation and relocated v + genes. Biochem Genet. 1971 Apr;5(2):111–117. doi: 10.1007/BF00485639. [DOI] [PubMed] [Google Scholar]
- Treisman R., Maniatis T. Simian virus 40 enhancer increases number of RNA polymerase II molecules on linked DNA. Nature. 1985 May 2;315(6014):73–75. doi: 10.1038/315072a0. [DOI] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Simian virus 40 enhancer increases RNA polymerase density within the linked gene. Nature. 1985 May 2;315(6014):75–77. doi: 10.1038/315075a0. [DOI] [PubMed] [Google Scholar]
- Wildeman A. G., Sassone-Corsi P., Grundström T., Zenke M., Chambon P. Stimulation of in vitro transcription from the SV40 early promoter by the enhancer involves a specific trans-acting factor. EMBO J. 1984 Dec 20;3(13):3129–3133. doi: 10.1002/j.1460-2075.1984.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]